Effect of Defatting and Extraction Solvent on the Antioxidant and Pancreatic Lipase Inhibitory Activities of Extracts from Hermetia illucens and Tenebrio molitor
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Defatting of Samples
2.3. Production of the Extracts
2.4. Antioxidant Activity of the Extracts by DPPH Assay
2.5. Pancreatic Lipase Inhibition Assay
2.6. Analysis of the H. illucens Extracts by GC-MS
2.7. Statistical Analysis
3. Results & Discussion
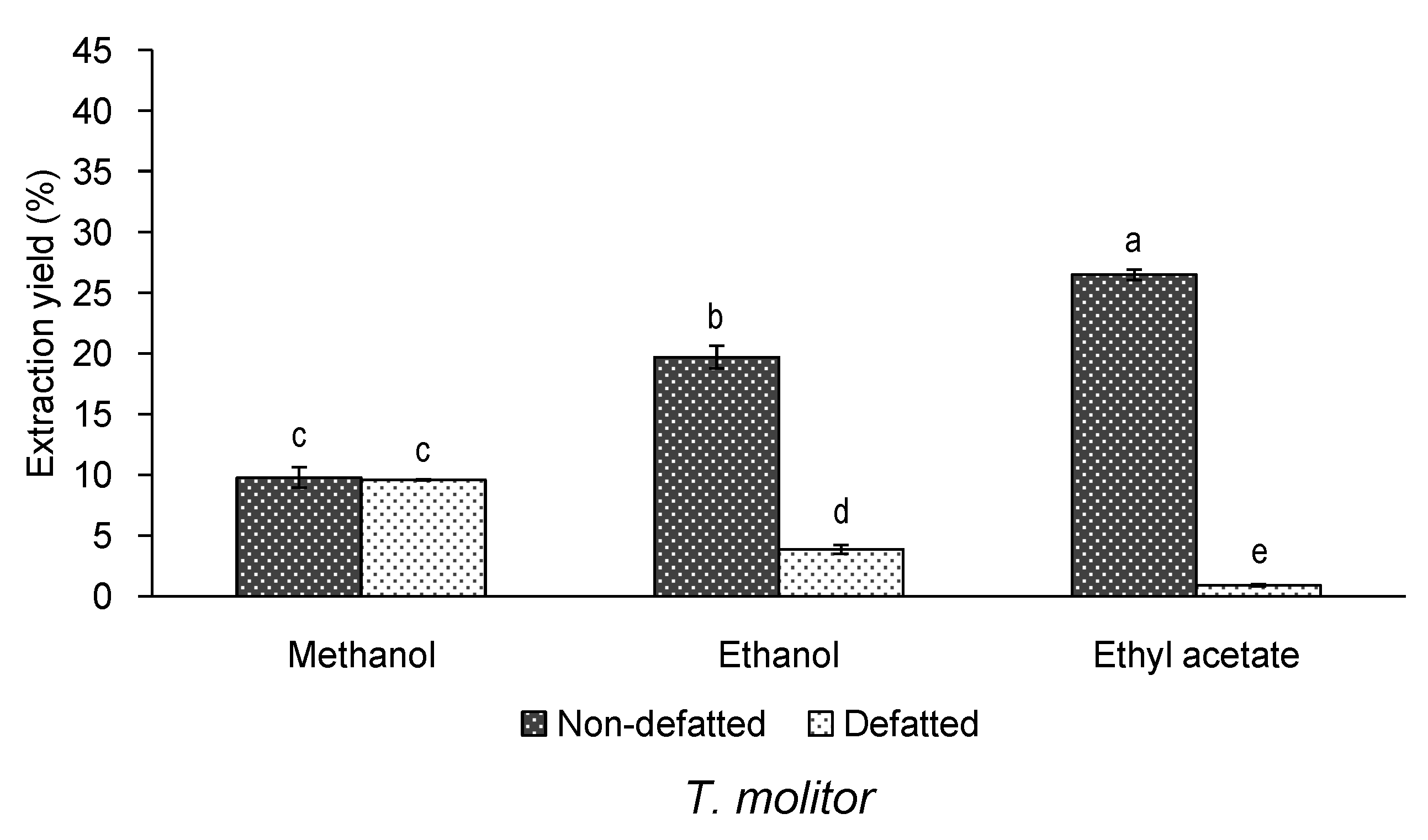
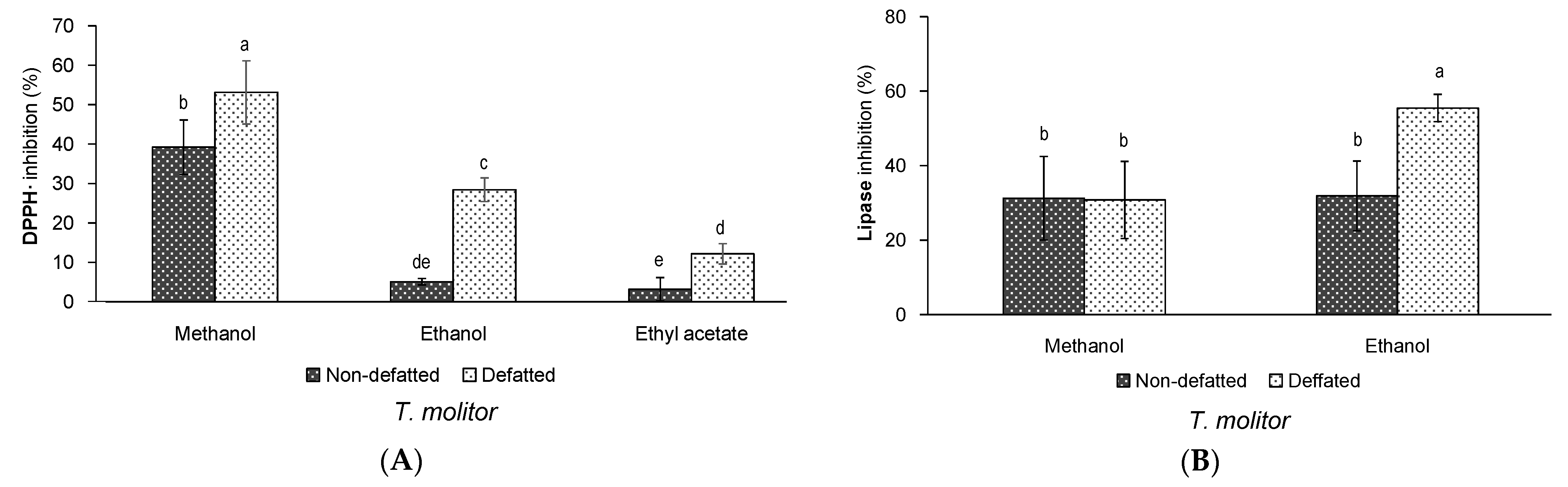
3.1. Effect of Defatting and Solvent of Extraction on the Yield and Bioactivity of Extracts of T. molitor
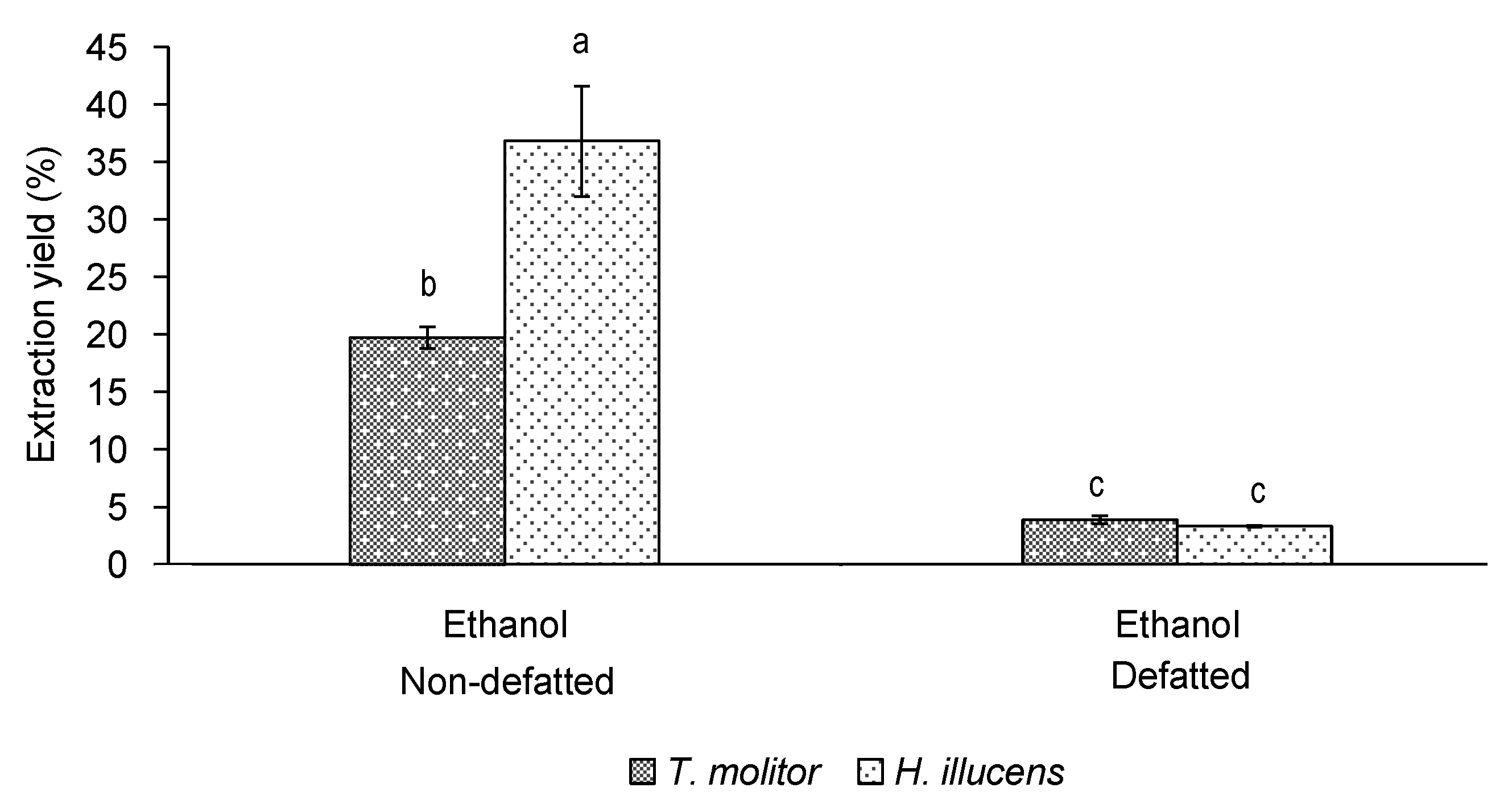
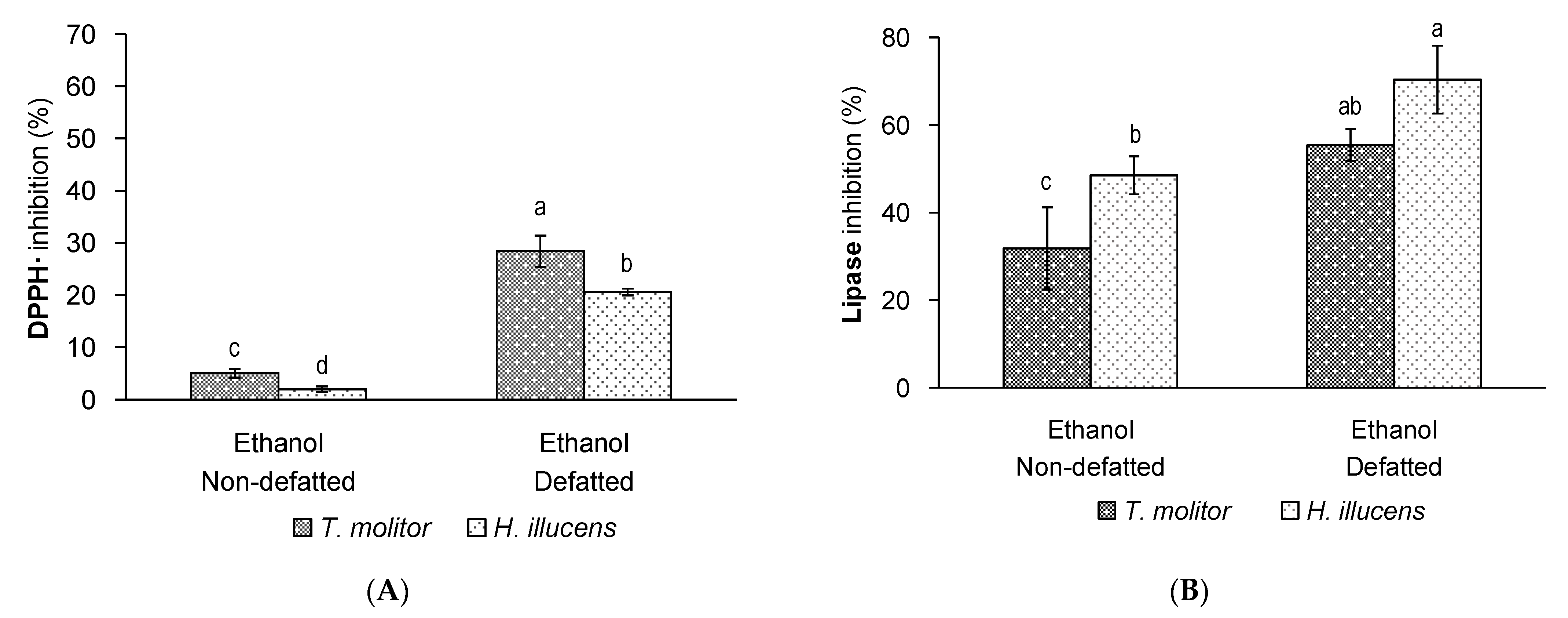
3.2. Effect of Defatting on the Yield and Bioactivity of Ethanol Extracts of T. molitor vs. H. illucens
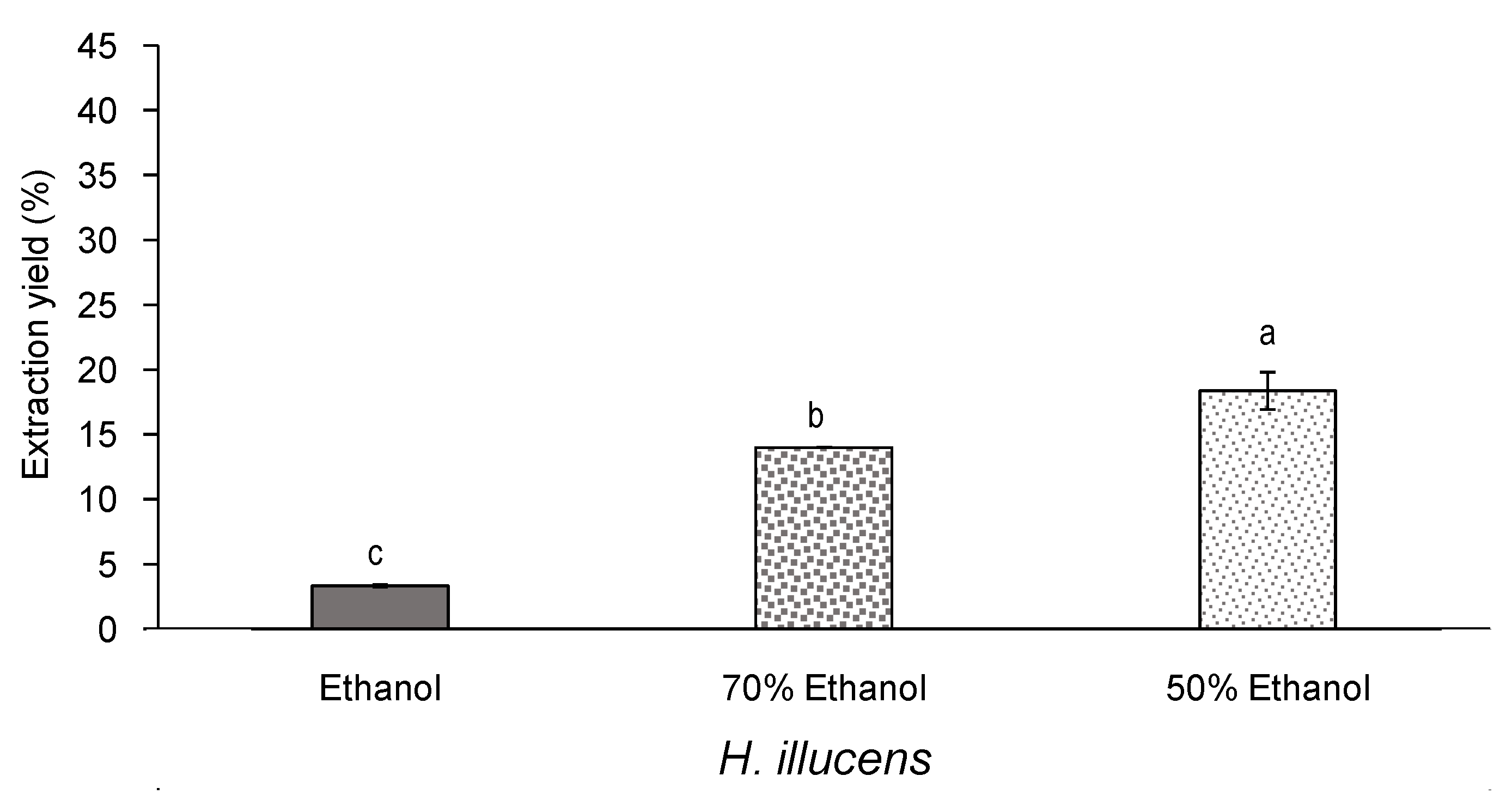
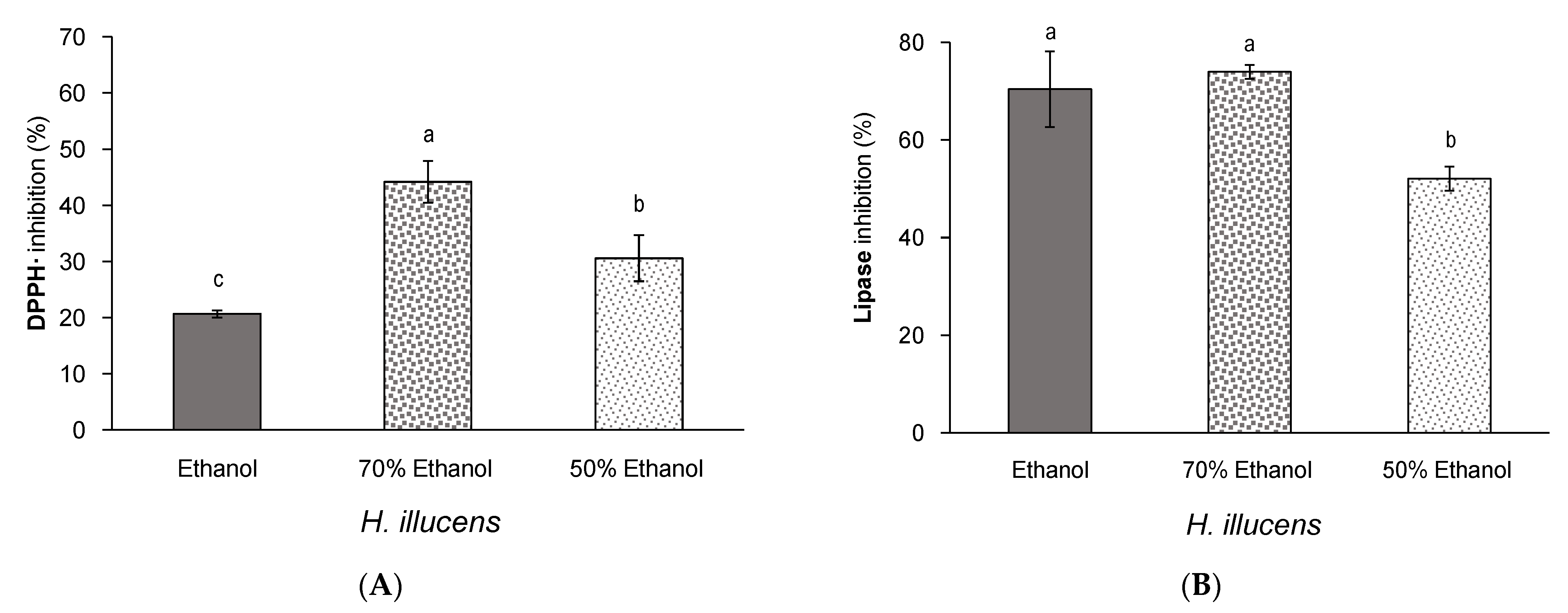
3.3. Effect of Aqueous Ethanol Mixtures on the Yield and Bioactivity of Extracts from Defatted H. illucens
3.4. Chemical Composition of Ethanol and Aqueous Ethanol Extracts from Defatted H. illucens
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- van Huis, A.; Oonincx, D.G. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef]
- European Commission. European Parliament and Council of the European Union Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliam. Off. J. Eur. Union 2015, L 327, 1–22. [Google Scholar]
- EFSA Scientific Committee. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef]
- Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar] [CrossRef]
- European Commission. European Parliament and Council of the European Union Commission Implementing Regulation (EU) 2021/882 of 1 June 2021 authorising the placing on the market of dried Tenebrio molitor larva as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and amending Commission Im. Off. J. Eur. Union 2021, L 194, 16–20. [Google Scholar]
- Seabrooks, L.; Hu, L. Insects: An underrepresented resource for the discovery of biologically active natural products. Acta Pharm. Sin. B 2017, 7, 409–426. [Google Scholar] [CrossRef]
- Baek, S.-H.; Joung, O.; Lee, H.-Y.; Shin, J.-C.; Choi, W.-S.; Lee, T.H.; Hwang, J.-S.; Nam, S.-H.; Son, H.-U.; Lee, S.-H. Anti-oxidative Fraction of Lycorma delicatula Alleviates Inflammatory Indicators. Nat. Prod. Commun. 2018, 13, 431–434. [Google Scholar] [CrossRef]
- Hong, J.-H.; Kim, S.-H.; Lee, Y.-C. The Ethanol Extract of Holotrichia diomphalia Larvae, Containing Fatty acids and Amino acids, Exerts Anti-Asthmatic Effects through Inhibition of the GATA-3/Th2 Signaling Pathway in Asthmatic Mice. Molecules 2019, 24, 852. [Google Scholar] [CrossRef]
- Hwang, B.B.; Chang, M.H.; Lee, J.H.; Heo, W.; Kim, J.K.; Pan, J.H.; Kim, Y.J.; Kim, J.H. The Edible Insect Gryllus bimaculatus Protects against Gut-Derived Inflammatory Responses and Liver Damage in Mice after Acute Alcohol Exposure. Nutrients 2019, 11, 857. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Inoue, A.; Taniguchi, S.; Yukutake, T.; Suyama, K.; Nose, T.; Maeda, I. Multifunctional biological activities of water extract of housefly larvae (Musca domestica). PharmaNutrition 2017, 5, 119–126. [Google Scholar] [CrossRef]
- Tang, Y.; Debnath, T.; Choi, E.-J.; Kim, Y.W.; Ryu, J.P.; Jang, S.; Chung, S.U.; Choi, Y.-J.; Kim, E.-K. Changes in the amino acid profiles and free radical scavenging activities of Tenebrio molitor larvae following enzymatic hydrolysis. PLoS ONE 2018, 13, e0196218. [Google Scholar] [CrossRef]
- Seo, M.; Goo, T.-W.; Chung, M.; Baek, M.; Hwang, J.-S.; Kim, M.-A.; Yun, E.-Y. Tenebrio molitor Larvae Inhibit Adipogenesis through AMPK and MAPKs Signaling in 3T3-L1 Adipocytes and Obesity in High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2017, 18, 518. [Google Scholar] [CrossRef]
- Liu, C.; Masri, J.; Perez, V.; Maya, C.; Zhao, J. Growth Performance and Nutrient Composition of Mealworms (Tenebrio Molitor) Fed on Fresh Plant Materials-Supplemented Diets. Foods 2020, 9, 151. [Google Scholar] [CrossRef] [PubMed]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Otero, P.; Gutierrez-Docio, A.; Navarro del Hierro, J.; Reglero, G.; Martin, D. Extracts from the edible insects Acheta domesticus and Tenebrio molitor with improved fatty acid profile due to ultrasound assisted or pressurized liquid extraction. Food Chem. 2020, 314, 126200. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Gutiérrez-Docio, A.; Otero, P.; Reglero, G.; Martin, D. Characterization, antioxidant activity, and inhibitory effect on pancreatic lipase of extracts from the edible insects Acheta domesticus and Tenebrio molitor. Food Chem. 2020, 309, 125742. [Google Scholar] [CrossRef] [PubMed]
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and techno-functionality of flours and proteins from two edible insect species: Meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon 2016, 2, e00218. [Google Scholar] [CrossRef]
- Botella-Martínez, C.; Lucas-González, R.; Pérez-Álvarez, J.A.; Fernández-López, J.; Viuda-Martos, M. Assessment of chemical composition and antioxidant properties of defatted flours obtained from several edible insects. Food Sci. Technol. Int. 2020. [Google Scholar] [CrossRef]
- Flores, D.R.; Casados, L.E.; Velasco, S.F.; Ramírez, A.C.; Velázquez, G. Comparative study of composition, antioxidant and antimicrobial activity of two adult edible insects from Tenebrionidae family. BMC Chem. 2020, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-S.; Shelomi, M. Review of Black Soldier Fly (Hermetia illucens) as Animal Feed and Human Food. Foods 2017, 6, 91. [Google Scholar] [CrossRef]
- Grau, T.; Vilcinskas, A.; Joop, G. Sustainable farming of the mealworm Tenebrio molitor for the production of food and feed. Z. Naturforsch.-Sect. C J. Biosci. 2017, 72, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Harsányi, E.; Juhász, C.; Kovács, E.; Huzsvai, L.; Pintér, R.; Fekete, G.; Varga, Z.I.; Aleksza, L.; Gyuricza, C. Evaluation of Organic Wastes as Substrates for Rearing Zophobas morio, Tenebrio molitor, and Acheta domesticus Larvae as Alternative Feed Supplements. Insects 2020, 11, 604. [Google Scholar] [CrossRef] [PubMed]
- Firmansyah, M.; Abduh, M.Y. Production of protein hydrolysate containing antioxidant activity from Hermetia illucens. Heliyon 2019, 5, e02005. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Wolf, D.; Gutzeit, H.O. The black soldier fly, Hermetia illucens—A promising source for sustainable production of proteins, lipids and bioactive substances. Z. Naturforsch.-Sect. C J. Biosci. 2017, 72, 351–363. [Google Scholar] [CrossRef]
- Zhu, D.; Huang, X.; Tu, F.; Wang, C.; Yang, F. Preparation, antioxidant activity evaluation, and identification of antioxidant peptide from black soldier fly (Hermetia illucens L.) larvae. J. Food Biochem. 2020, 44, e13186. [Google Scholar] [CrossRef]
- Blois, M. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Herrera, T.; Navarro del Hierro, J.; Fornari, T.; Reglero, G.; Martin, D. Inhibitory effect of quinoa and fenugreek extracts on pancreatic lipase and α-amylase under in vitro traditional conditions or intestinal simulated conditions. Food Chem. 2019, 270, 509–517. [Google Scholar] [CrossRef]
- Herrera, T.; Navarro del Hierro, J.; Fornari, T.; Reglero, G.; Martin, D. Acid hydrolysis of saponin-rich extracts of quinoa, lentil, fenugreek and soybean to yield sapogenin-rich extracts and other bioactive compounds. J. Sci. Food Agric. 2019, 99, 3157–3167. [Google Scholar] [CrossRef]
- Seyedan, A.; Alshawsh, M.A.; Alshagga, M.A.; Koosha, S.; Mohamed, Z. Medicinal plants and their inhibitory activities against pancreatic lipase: A review. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Yoon, Y.-I.; Chung, M.Y.; Hwang, J.-S.; Han, M.S.; Goo, T.-W.; Yun, E.-Y. Allomyrina dichotoma (Arthropoda: Insecta) larvae confer resistance to obesity in mice fed a high-fat diet. Nutrients 2015, 7, 1978–1991. [Google Scholar] [CrossRef]
- Ali, M.; Arumugam, S. Effect of crude extract of Bombyx mori coccoons in hyperlipidemia and atherosclerosis. J. Ayurveda Integr. Med. 2011, 2, 72. [Google Scholar] [CrossRef]
- Kalia, K.; Sharma, K.; Singh, H.P.; Singh, B. Effects of extraction methods on phenolic contents and antioxidant activity in aerial parts of potentilla atrosanguinea lodd. and quantification of its phenolic constituents by RP-HPLC. J. Agric. Food Chem. 2008, 56, 10129–10134. [Google Scholar] [CrossRef] [PubMed]
- Metrouh-Amir, H.; Duarte, C.M.M.; Maiza, F. Solvent effect on total phenolic contents, antioxidant, and antibacterial activities of Matricaria pubescens. Ind. Crop. Prod. 2015, 67, 249–256. [Google Scholar] [CrossRef]
- Zhu, K.X.; Lian, C.X.; Guo, X.N.; Peng, W.; Zhou, H.M. Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem. 2011, 126, 1122–1126. [Google Scholar] [CrossRef]
- Urbanek, A.; Szadziewski, R.; Stepnowski, P.; Boros-Majewska, J.; Gabriel, I.; Dawgul, M.; Kamysz, W.; Sosnowska, D.; Gołeogonekbiowski, M. Composition and antimicrobial activity of fatty acids detected in the hygroscopic secretion collected from the secretory setae of larvae of the biting midge Forcipomyia nigra (Diptera: Ceratopogonidae). J. Insect Physiol. 2012, 58, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bachhawat, A.K. Pyroglutamic acid: Throwing light on a lightly studied metabolite on JSTOR. Curr. Sci. 2012, 102, 288–297. [Google Scholar]
- Lalam, C.; Petla, N.; Tantravahi, S. Antiproliferative and Antibacterial Effects of Pyroglutamic Acid Isolated from Enterococcus Faecium (Mcc-2729). Ann. Rom. Soc. Cell Biol. 2021, 25, 7624–7628. [Google Scholar]
- Vesala, L.; Salminen, T.S.; Koštál, V.; Zahradníčková, H.; Hoikkala, A. Myo-inositol as a main metabolite in overwintering flies: Seasonal metabolomic profiles and cold stress tolerance in a northern drosophilid fly. J. Exp. Biol. 2012, 215, 2891–2897. [Google Scholar] [CrossRef][Green Version]
- Matthäus, B.; Piofczyk, T.; Katz, H.; Pudel, F. Renewable Resources from Insects: Exploitation, Properties, and Refining of Fat Obtained by Cold-Pressing from Hermetia illucens (Black Soldier Fly) Larvae. Eur. J. Lipid Sci. Technol. 2019, 121, 1800376. [Google Scholar] [CrossRef]
- Ravi, H.K.; Vian, M.A.; Tao, Y.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F. Alternative solvents for lipid extraction and their effect on protein quality in black soldier fly (Hermetia illucens) larvae. J. Clean. Prod. 2019, 238, 117861. [Google Scholar] [CrossRef]
- Caligiani, A.; Marseglia, A.; Sorci, A.; Bonzanini, F.; Lolli, V.; Maistrello, L.; Sforza, S. Influence of the killing method of the black soldier fly on its lipid composition. Food Res. Int. 2019, 116, 276–282. [Google Scholar] [CrossRef] [PubMed]
- De Pradhan, I.; Dutta, M.; Choudhury, K.; De, B. Metabolic diversity and in vitro pancreatic lipase inhibition activity of some varieties of Mangifera indica L. fruits. Int. J. Food Prop. 2017, 20, S3212–S3223. [Google Scholar] [CrossRef]
| Rt | Compound | Ethanol | 70% Ethanol | 50% Ethanol | |||
|---|---|---|---|---|---|---|---|
| Area 1 | g/100 g | Area 1 | g/100 g | Area 1 | g/100 g | ||
| NITROGEN COMPOUNDS | 6.98 | 12.31 | 0.27 | ||||
| 8.452 | 1-Aminocyclopropane-1-carboxylic acid | 247,215 | 0.060 ± 0.000 a | 441,580 | 0.109 ± 0.018 a | n.d. | n.d. |
| 8.941 | L-Valine | 1,318,699 | 0.318 ± 0.008 a | 1,356,182 | 0.335 ± 0.020 a | n.d. | n.d. |
| 9.704 | L-Proline | 5,984,738 | 1.443 ± 0.034 a | 7,246,143 | 1.790 ± 0.158 a | 125,239 | 0.030 ± 0.013 b |
| 10.265 | Serine | 361,032 | 0.087 ± 0.001 a | 1,130,996 | 0.279 ± 0.025 b | 17,938 | 0.004 ± 0.003 c |
| 10.503 | Threonine | 499,614 | 0.120 ± 0.000 a | 867,255 | 0.214 ± 0.012 b | 16,597 | 0.004 ± 0.003 c |
| 11.016 | L-Homoserine | 220,070 | 0.053 ± 0.006 a | 308,851 | 0.076 ± 0.009 a | n.d. | n.d. |
| 11.664 | Pyroglutamic acid | 11,601,355 | 2.797 ± 0.050 a | 21,341,912 | 5.271 ± 0.464 b | 584,034 | 0.140 ± 0.055 c |
| 11.824 | L-Norvaline | 661,970 | 0.160 ± 0.004 a | 1,042,372 | 0.257 ± 0.020 b | n.d. | n.d. |
| 12.317 | Ornithine | 98,721 | 0.024 ± 0.000 a | 587,180 | 0.145 ± 0.021 b | n.d. | n.d. |
| 12.358 | Glutamine | 125,877 | 0.030 ± 0.000 a | 1,476,601 | 0.365 ± 0.031 b | n.d. | n.d. |
| 12.411 | Phenylalanine | 180,696 | 0.044 ± 0.001 a | 192,340 | 0.047 ± 0.000 b | n.d. | n.d. |
| 12.748 | Asparagine | n.d. | n.d. | 304,685 | 0.075 ± 0.016 | n.d. | n.d. |
| 13.117 | L-Lysine | 79,662 | 0.019 ± 0.002 a | 701,187 | 0.173 ± 0.014 b | n.d. | n.d. |
| 14.641 | L-Tyrosine | 5,214,872 | 1.258 ± 0.077 a | 10,036,215 | 2.478 ± 0.159 b | 368,497 | 0.089 ± 0.081 c |
| 14.510 | L-Histidine | 547,412 | 0.132 ± 0.001 a | 1,222,452 | 0.302 ± 0.047 b | n.d. | n.d. |
| 16.346 | L-Tryptophan | 1,819,631 | 0.439 ± 0.039 a | 1,583,493 | 0.392 ± 0.082 a | n.d. | n.d. |
| CARBOHYDRATES | 5.77 | 11.72 | 0.43 | ||||
| 15.344 | Inositol | 1,181,332 | 0.638 ± 0.002 a | 2,148,282 | 1.190 ± 0.155 b | n.d. | n.d. |
| 18.328 | Disaccharide n.i. | n.d. | n.d. | 5,546,267 | 1.020 ± 0.045 | 171,438 | 0.031 ± 0.014 |
| 18.603 | D-Turanose | 2,770,474 | 0.498 ± 0.016 a | 15,594,801 | 2.881 ± 0.972 b | 460,240 | 0.083 ± 0.041 c |
| 19.067 | Disaccharide n.i. | n.d. | n.d. | 1,615,927 | 0.297 ± 0.012 a | 93,107 | 0.017 ± 0.001 b |
| 19.193 | Disaccharide n.i. | 4,100,860 | 0.737 ± 0.030 a | 12,577,917 | 2.318 ± 0.376 b | 994,620 | 0.178 ± 0.044 c |
| 19.279 | Sucrose | 21,682,632 | 3.895 ± 0.120 a | 21,782,315 | 4.015 ± 0.799 a | 708,107 | 0.130 ± 0.056 b |
| LIPIDS | 1.89 | 1.29 | 0.76 | ||||
| 9.794 | Butanedioic acid | 171,695 | 0.023 ± 0.001 a | 659,873 | 0.090 ± 0.008 b | 25,606 | 0.003 ± 0.001 c |
| 12.518 | Dodecanoic acid | 1,200,162 | 0.159 ± 0.005 a | 373,726 | 0.051 ± 0.003 b | 138,970 | 0.018 ± 0.003 c |
| 13.909 | Tetradecanoic acid | 1,287,237 | 0.171 ± 0.006 a | 573,640 | 0.078 ± 0.010 b | 178,096 | 0.024 ± 0.004 c |
| 15.337 | Hexadecanoic acid | 3,113,733 | 0.413 ± 0.030 a | 1,554,915 | 0.211 ± 0.016 b | 863,370 | 0.114 ± 0.041 b |
| 16.351 | Linoleic acid | 737,090 | 0.098 ± 0.001 a | n.d. | n.d. | 87,952 | 0.012 ± 0.002 b |
| 16.499 | Oleic acid | 2,712,012 | 0.360 ± 0.011 a | 1,541,780 | 0.209 ± 0.035 b | 610,728 | 0.081 ± 0.003 c |
| 18.211 | 2-Monopalmitin | n.d. | n.d. | n.d. | n.d. | 262,082 | 0.056 ± 0.000 |
| 18.380 | 1-Monopalmitin | 2,849,658 | 0.579 ± 0.014 a | 3,141,554 | 0.651 ± 0.119 a | 1,933,553 | 0.400 ± 0.021 a |
| 19.141 | 2-Monostearin | n.d. | n.d. | n.d. | n.d. | 243,208 | 0.052 ± 0.002 |
| 19.044 | Monoglyceride n.i. | 335,835 | 0.072 ± 0.003 | n.d. | n.d. | n.d. | n.d. |
| 20.238 | Hexacosanoic acid | 94,318 | 0.013 ± 0.002 | n.d. | n.d. | n.d. | n.d. |
| ACIDS | 0.00 | 1.31 | 0.04 | ||||
| 11.337 | Malic acid | n.d. | n.d. | 313,005 | 0.140 ± 0.022 | n.d. | n.d. |
| 13.855 | Isocitric acid | n.d. | n.d. | 1,661,187 | 0.743 ± 0.141 a | 101,811 | 0.044 ± 0.013 b |
| 15.139 | Gluconic acid | n.d. | n.d. | 951,216 | 0.425 ± 0.049 | n.d. | n.d. |
| STEROLS | 0.40 | 0.09 | - | ||||
| 21.381 | Ergosterol | 136,147 | 0.091 ± 0.006 | n.d. | n.d. | n.d. | n.d. |
| 21.487 | Campesterol | 121,618 | 0.081 ± 0.006 | n.d. | n.d. | n.d. | n.d. |
| 21.687 | Stigmasterol | 48,518 | 0.032 ± 0.002 a | 17,156 | 0.012 ± 0.004 b | n.d. | n.d. |
| 22.036 | β-Sitosterol | 296,052 | 0.197 ± 0.015 a | 10,9096 | 0.075 ± 0.015 b | n.d. | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro del Hierro, J.; Cantero-Bahillo, E.; Fornari, T.; Martin, D. Effect of Defatting and Extraction Solvent on the Antioxidant and Pancreatic Lipase Inhibitory Activities of Extracts from Hermetia illucens and Tenebrio molitor. Insects 2021, 12, 789. https://doi.org/10.3390/insects12090789
Navarro del Hierro J, Cantero-Bahillo E, Fornari T, Martin D. Effect of Defatting and Extraction Solvent on the Antioxidant and Pancreatic Lipase Inhibitory Activities of Extracts from Hermetia illucens and Tenebrio molitor. Insects. 2021; 12(9):789. https://doi.org/10.3390/insects12090789
Chicago/Turabian StyleNavarro del Hierro, Joaquín, Emma Cantero-Bahillo, Tiziana Fornari, and Diana Martin. 2021. "Effect of Defatting and Extraction Solvent on the Antioxidant and Pancreatic Lipase Inhibitory Activities of Extracts from Hermetia illucens and Tenebrio molitor" Insects 12, no. 9: 789. https://doi.org/10.3390/insects12090789
APA StyleNavarro del Hierro, J., Cantero-Bahillo, E., Fornari, T., & Martin, D. (2021). Effect of Defatting and Extraction Solvent on the Antioxidant and Pancreatic Lipase Inhibitory Activities of Extracts from Hermetia illucens and Tenebrio molitor. Insects, 12(9), 789. https://doi.org/10.3390/insects12090789









