Antenna Cleaning Is Essential for Precise Behavioral Response to Alarm Pheromone and Nestmate–Non-Nestmate Discrimination in Japanese Carpenter Ants (Camponotus japonicus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect
2.2. Operation
2.3. NanoSuit Treatment and Scanning Electron Microscopy
2.4. Video Recording and Locomotion Analysis
2.5. Aggression Behavior Test
3. Results
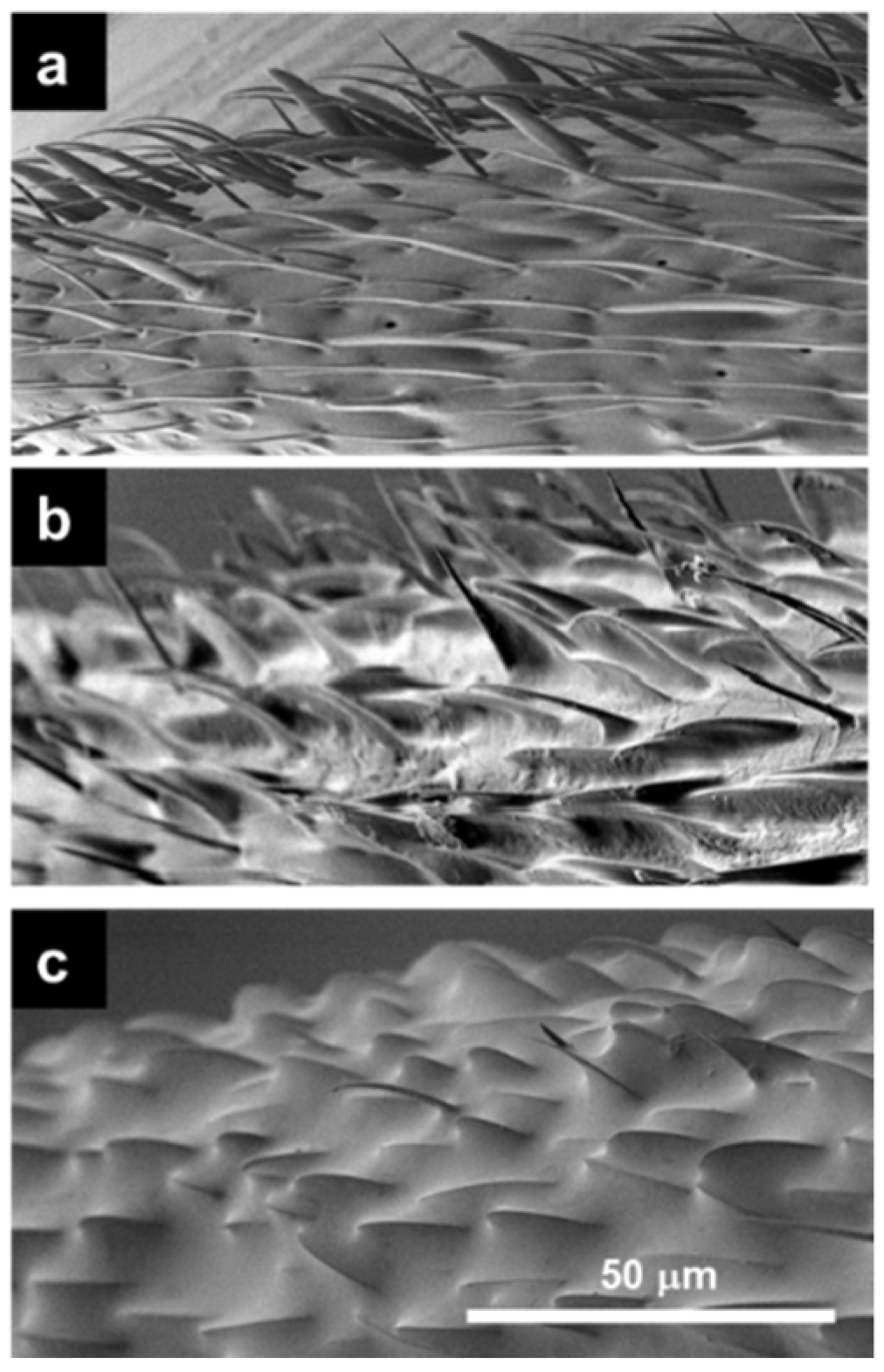
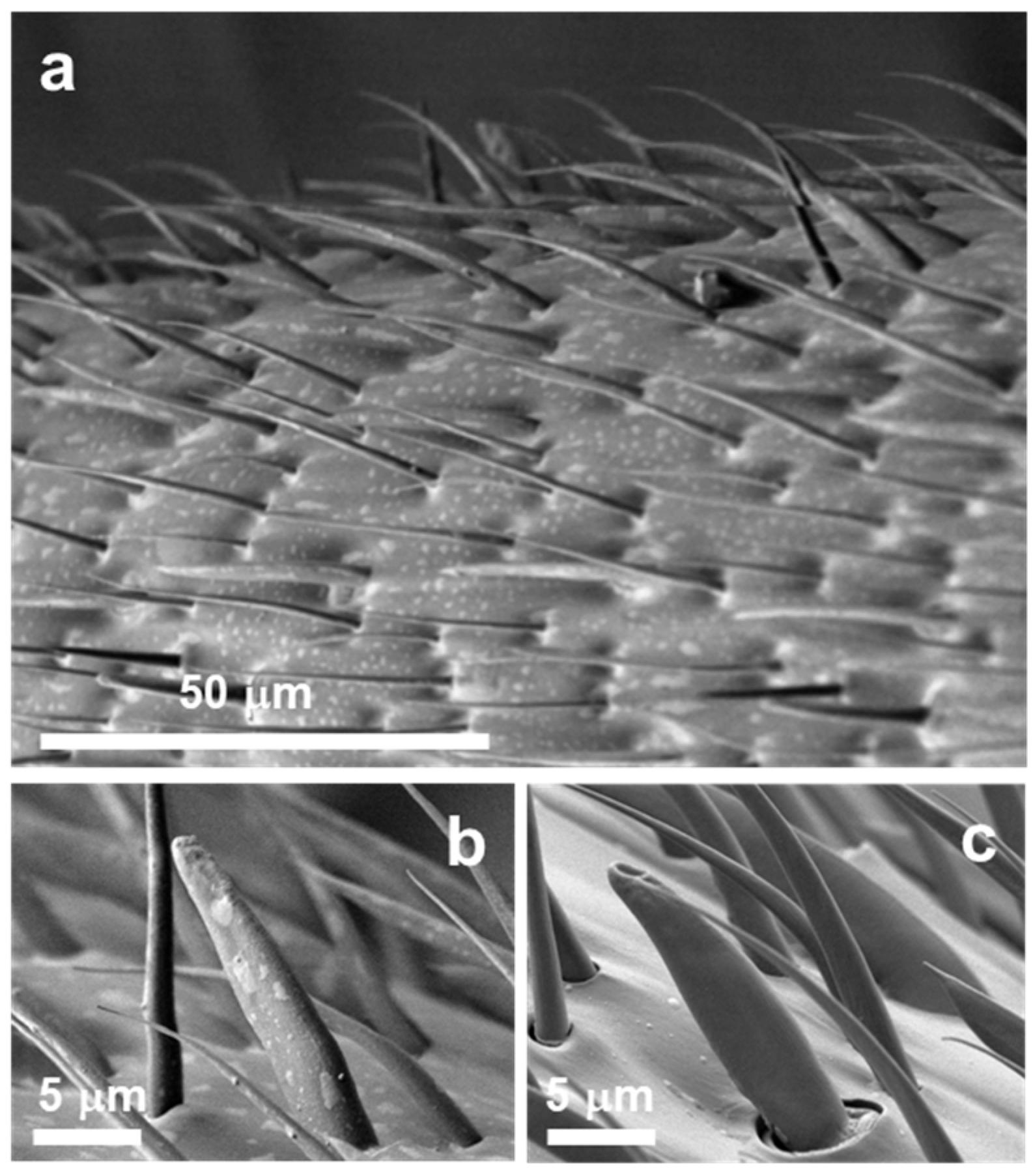
3.1. Accumulation of Antennal Surface Material
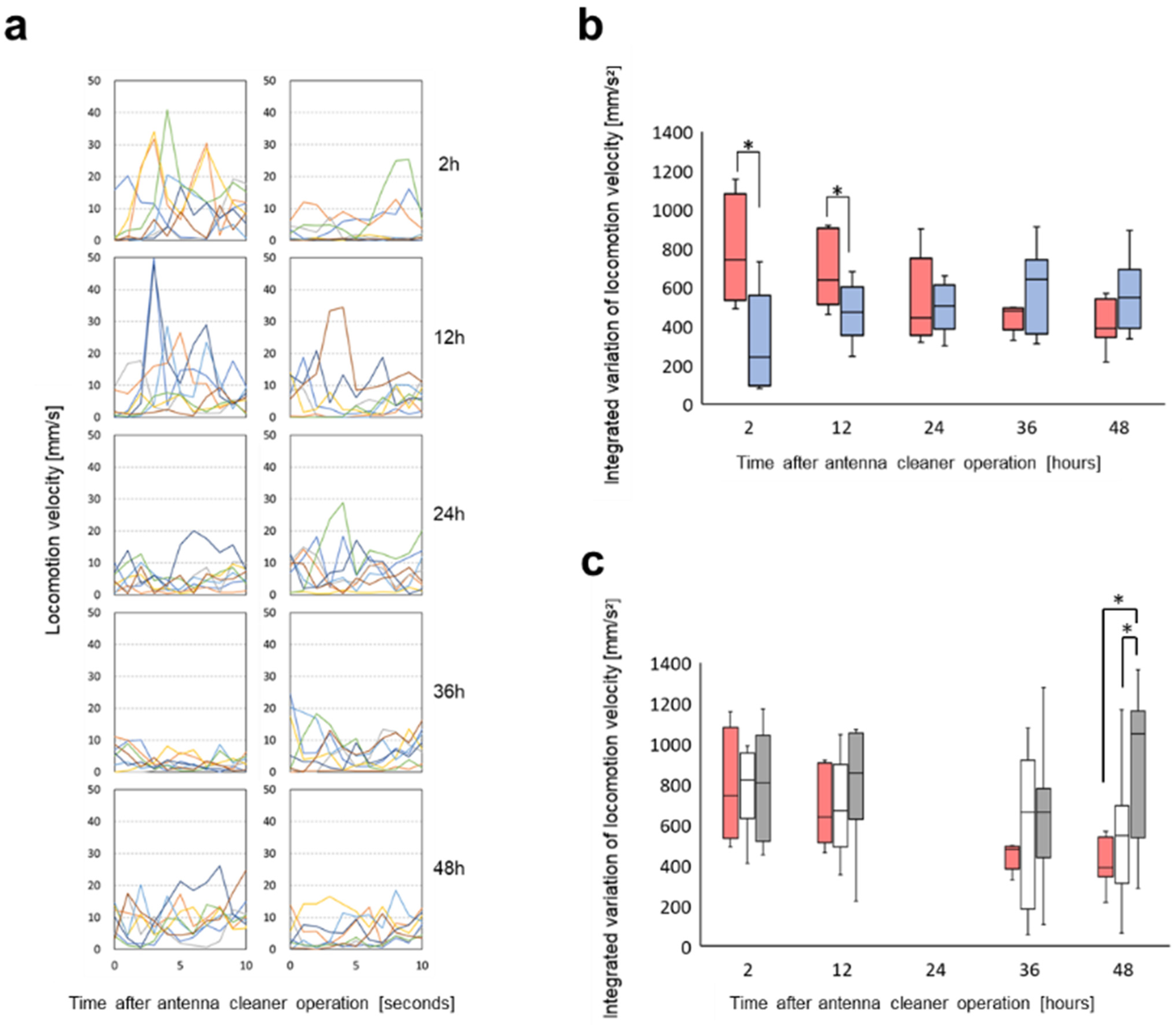
3.2. Change in Locomotion with the Alarm Pheromone
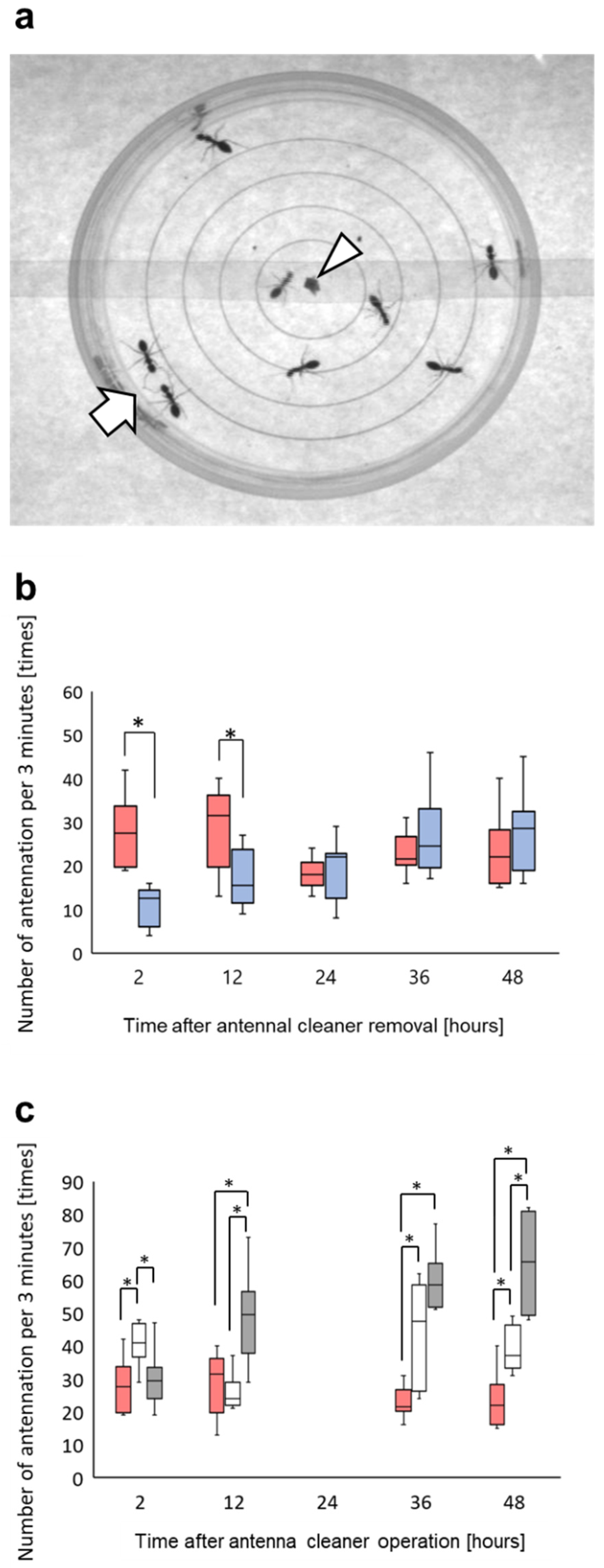
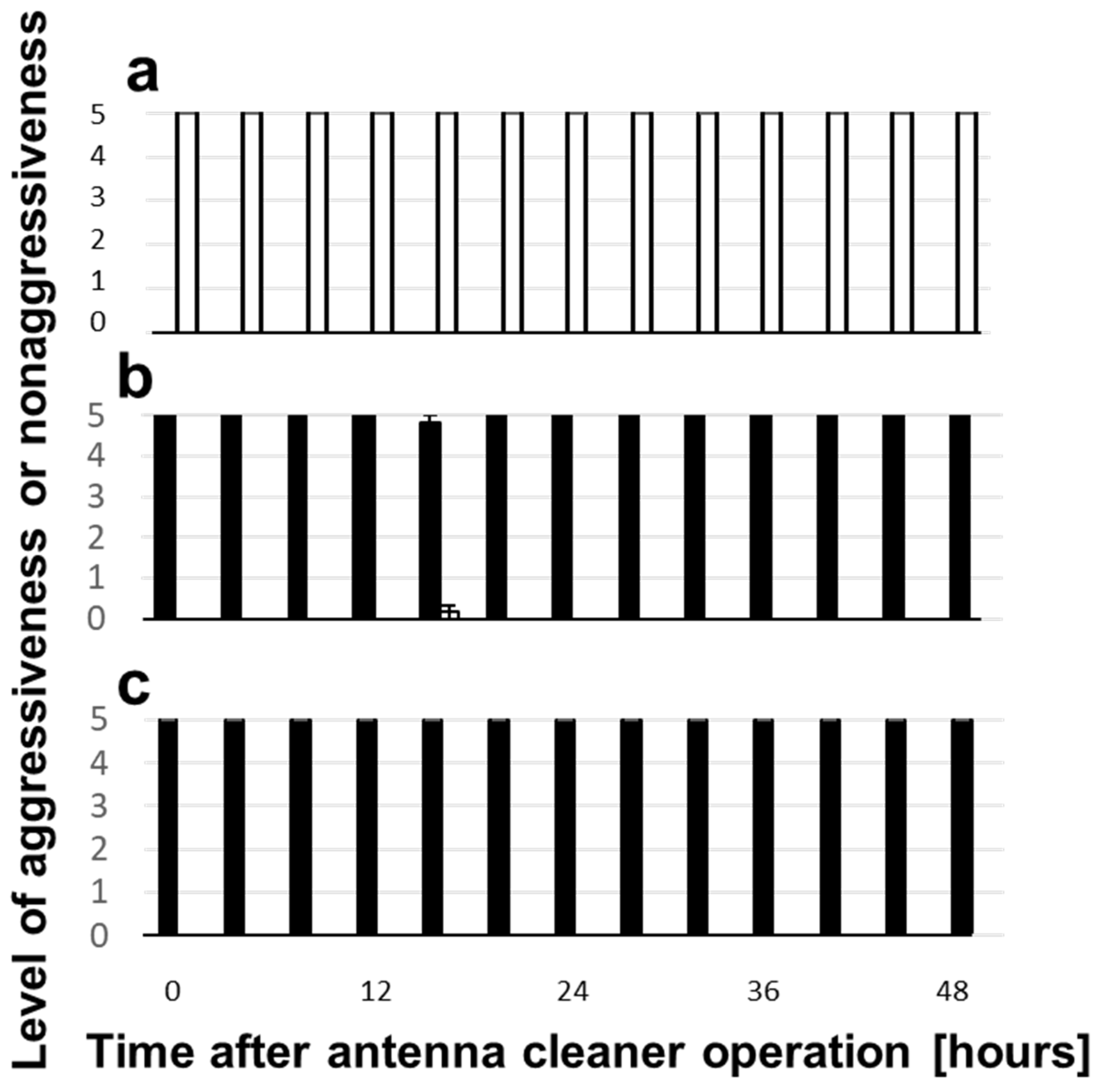
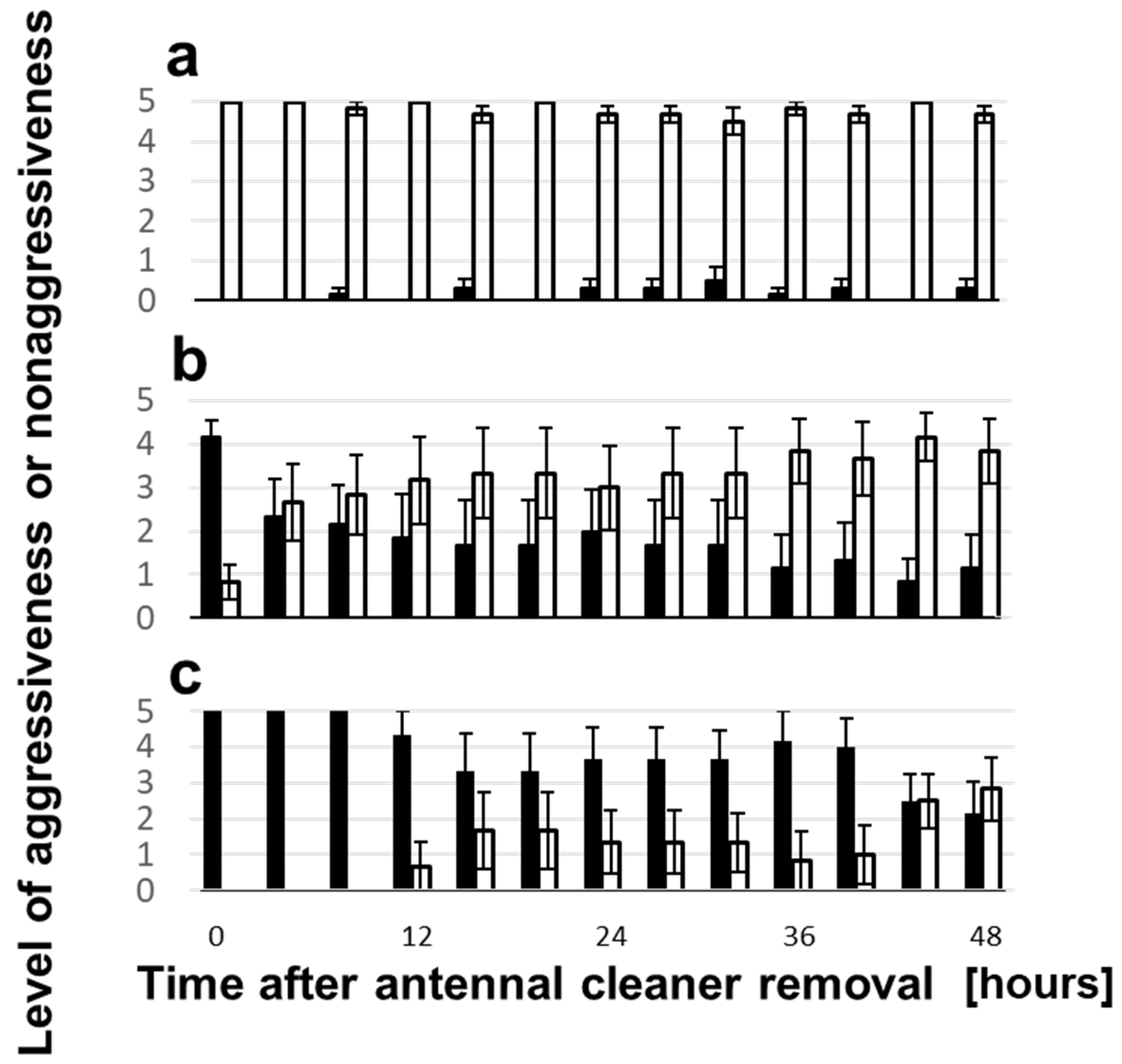
3.3. Change of Aggressiveness
4. Discussion
4.1. Improper Reaction to Alarm Pheromone
4.2. Ambiguous Nestmate–Non-Nestmate Discrimination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hlavac, T.F. Grooming systems of insects: Structure, mechanics. Ann. Entomol. Soc. Am. 1975, 68, 823–826. [Google Scholar] [CrossRef]
- Reingold, S.C.; Camhi, J.M. Abdominal grooming in the cockroach: Development of an adult behavior. J. Insect Physiol. 1978, 24, 101–110. [Google Scholar] [CrossRef]
- Vandervorst, P.; Ghysen, A. Genetic control of sensory connections in Drosophila. Nature 1980, 286, 65–67. [Google Scholar] [CrossRef]
- El-Awami, I.O.; Dent, D.R. The interaction of surface and dust particle size on the pick-up and grooming behaviour of the German cockroach Blattella germanica. Entomol. Exp. Appl. 1995, 77, 81–87. [Google Scholar] [CrossRef]
- Matheson, T. Hindleg targeting during scratching in the locust. J. Exp. Biol. 1997, 200, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, J.; Stanimirovic, Z.; Lakic, N.; Djelic, N.; Radovic, I. Stimulating effect of sugar dusting on honey bee grooming behavior. Entomol. Exp. Appl. 2012, 143, 23–30. [Google Scholar] [CrossRef]
- Peng, Y.-S.; Fang, Y.; Xu, S.; Ge, L. The resistance mechanism of the Asian honey bee, Apis cerana Fabr., to an ectoparasitic mite, Varroa jacobsoni Oudemans. J. Invertebr. Pathol. 1987, 49, 54–60. [Google Scholar] [CrossRef]
- Kovac, D.; Maschwitz, U. Secretion-grooming in the water bug Plea minutissima: A chemical defense against microorganisms interfering with the hydrofuge properties of the respiratory region. Ecol. Entomol. 1989, 14, 403–411. [Google Scholar] [CrossRef]
- Lusebrink, I.; Dettner, K.; Seifert, K. Stenusine, an antimicrobial agent in the rove beetle genus Stenus (Coleoptera, Staphylinidae). Naturwissenschaften 2008, 95, 751–755. [Google Scholar] [CrossRef]
- Lusebrink, I.; Dettner, K.; Seifert, K. Resistance of the termite, Coptotermes formosanus Shiraki to Metarhizium anisopliae due to grooming. BioControl 2008, 52, 75–85. [Google Scholar]
- Vincent, C.M.; Bertram, S.M. Crickets groom to avoid lethal parasitoids. Anim. Behav. 2010, 79, 51–56. [Google Scholar] [CrossRef]
- Kovac, D.; Maschwitz, U. Secretion-grooming in aquatic beetles (Hydradephaga): A chemical protection against contamination of the hydrofuge respiratory region. Chem. Ecol. 1990, 1, 131–138. [Google Scholar] [CrossRef]
- Graystock, P.; Hughes, W.H.O. Disease resistance in a weaver ant, Polyrhachis dives, and the role of antibiotic- producing glands. Behav. Ecol. Sociobiol. 2011, 65, 2319–2327. [Google Scholar] [CrossRef]
- Borchelt, P.L. Care of the body surface (COBS). In Comparative Psychology: An Evolutionary Analysis of Animal Behavior; Denny, M.R., Ed.; John Wiley & Sons: New York, NY, USA, 1980; pp. 363–384. [Google Scholar]
- Sachs, B.D. The development of grooming and its expression in adult animals. Ann. N. Y. Acad. Sci. 1988, 525, 1–17. [Google Scholar] [CrossRef]
- Hefetz, A.; Soroker, V.; Dahbi, A.; Malherbe, M.C.; Fresneau, D. The front basitarsal brush in Pachycondyla apicalis and its role in hydrocarbon circulation. Chem. Ecol. 2001, 11, 17–24. [Google Scholar] [CrossRef]
- Scharf, I.; Stoldt, M.; Libbrecht, R.; Höpfner, A.L.; Jongepier, E.; Kever, M.; Foitzik, S. Social isolation causes downregulation of immune and stress response genes and behavioural changes in a social insect. Mol. Ecol. 2021, 10, 2378–2389. [Google Scholar] [CrossRef]
- Böröczky, K.; Wada-Katsumata, A.; Batchelor, D.; Zhukovskaya, M.; Schal, C. Insects groom their antennae to enhance olfactory acuity. Proc. Natl. Acad. Sci. USA 2013, 110, 3615–3620. [Google Scholar] [CrossRef] [Green Version]
- Wada-Katsumata, A.; Schal, C. Antennal grooming facilitates courtship performance in a group-living insect, the German cockroach Blattella germanica. Sci. Rep. 2019, 9, 2942. [Google Scholar] [CrossRef]
- Szebenyi, A.L. Cleaning behaviour in Drosophila melanogaster. Anim. Behav. 1969, 17, 641–651. [Google Scholar] [CrossRef]
- Jackson, L.L. Cuticular lipids of insects—IV. Hydrocarbons of cockroaches Periplaneta japonica and Periplaneta americana compared to other cockroach hydrocarbons. Comp. Biochem. Physiol. 1972, 41, 331–336. [Google Scholar] [CrossRef]
- Kapitsky, S.V.; Gribakin, F.G. Electroantennogram of the American cockroach: Effect of oxygen and electrical model. J. Comp. Physiol. A 1992, 170, 651–663. [Google Scholar] [CrossRef]
- Gibbs, A.G. Lipid melting and cuticular permeability: New insights into an old problem. J. Insect Physiol. 2002, 48, 391–400. [Google Scholar] [CrossRef]
- Saïd, I.; Costagliola, G.; Leoncini, I.; Rivault, C. Cuticular hydrocarbon profiles aggregation in four Periplaneta species (Insecta: Dictyoptera). J. Insect Physiol. 2005, 51, 995–1003. [Google Scholar] [CrossRef]
- Zhukovskaya, M.I.; Kapitsky, S.V. Activity modulation in cockroach sensillum: The role of octopamine. J. Insect Physiol. 2006, 52, 76–86. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Bagnères, A.-G. Introduction: History and overview of insect hydrocarbons. In Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Blomquist, G.J., Bagnères, A.-G., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 3–18. [Google Scholar]
- Maitani, M.M.; Allara, D.L.; Park, K.C.; Lee, S.G.; Baker, T.C. Moth olfactory trichoid sensilla exhibit nanoscale-level heterogeneity in surface lipid properties. Arthropod Struct. Dev. 2010, 39, 1–16. [Google Scholar] [CrossRef]
- Schönitzer, K.; Lawitzky, G. A phylogenetic study of the antenna cleaner in Formicidae, Mutillidae, and Tiphiidae (Insecta, Hymenoptera). Zoomorphology 1987, 107, 273–285. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: Structure and Function, 4th ed.; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Hackmann, A.; Delacave, H.; Robinson, A.; Labonte, D.; Federle, W. Functional morphology and efficiency of the antenna cleaner in Camponotus rufifemur ants. R Soc. Open Sci. 2015, 2, 150129. [Google Scholar] [CrossRef] [Green Version]
- Yamagata, N.; Nishino, H.; Mizunami, M. Neural pathways for the processing of alarm pheromone in the ant brain. J. Comp. Neurol. 2007, 505, 424–442. [Google Scholar] [CrossRef]
- Mizunami, M.; Yamagata, N.; Nishino, H. Alarm pheromone processing in the ant brain: An evolutionary perspective. Front. Behav. Neurosci. 2010, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, M.; Wada-Katsumata, A.; Fujikawa, K.; Iwasaki, M.; Yokohari, F.; Satoji, Y.; Nisimura, T.; Yamaoka, R. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 2005, 309, 311–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeichi, Y.; Uebi, T.; Miyazaki, N.; Murata, K.; Yasuyama, K.; Inoue, K.; Suzaki, T.; Kubo, H.; Kajimura, N.; Takano, J.; et al. Putative neural network within an olfactory sensory unit for nestmate and non-nestmate discrimination in the Japanese carpenter ant: The ultra-structures and mathematical simulation. Front. Cell Neurosci. 2018, 12, 310. [Google Scholar] [CrossRef] [PubMed]
- Zube, C.; Kleineidam, C.J.; Kirschner, S.; Neef, J.; Rössler, W. Organization of the olfactory pathway and odor processing in the antennal lobe of the ant Camponotus floridanus. J. Comp. Neurol. 2008, 506, 425–441. [Google Scholar] [CrossRef] [Green Version]
- Mysore, K.; Subramanian, K.A.; Sarasij, R.C.; Suresh, A.; Shyamala, B.V.; VijayRaghavan, K.; Rodrigues, V. Caste and sex specific olfactory glomerular organization and brain architecture in two sympatric ant species Camponotus sericeus and Camponotus compressus (Fabricius, 1798). Arthropod Struct. Dev. 2009, 38, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, A.; Nishino, H.; Watanabe, H.; Yokohari, F.; Nishikawa, M. Sex-specific antennal sensory system in the ant Camponotus japonicus: Structure and distribution of sensilla on the flagellum. Cell Tissue Res. 2009, 338, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Watanabe, H.; Yokohari, F. Higher brain centers for social tasks in worker ants, Camponotus japonicus. J. Comp. Neurol. 2012, 520, 1584–1598. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.R.; Enzmann, B.L.; Schmidt, Y.; Moore, D.; Jones, G.R.; Parker, J.; Berger, S.L.; Reinberg, D.; Zwiebel, L.J.; Breit, B.; et al. Cuticular hydrocarbon pheromones for social behavior and their coding in the ant antenna. Cell Rep. 2015, 12, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Dussutour, A.; Simpson, S.J. Description of a simple synthetic diet for studying nutritional responses in ants. Insectes Sociaux 2008, 55, 329–333. [Google Scholar] [CrossRef]
- Takaku, Y.; Suzuki, H.; Ohta, I.; Ishii, D.; Muranaka, Y.; Shimomura, M.; Hariyama, T. A thin polymer membrane, nano-suit, enhancing survival across the continuum between air and high vacuum. Proc. Natl. Acad. Sci. USA 2013, 110, 7631–7635. [Google Scholar] [CrossRef] [Green Version]
- Takaku, Y.; Takehara, S.; Suzuki, C.; Suzuki, H.; Shimomura, M.; Hariyama, T. In situ elemental analysis of living biological specimens using ‘NanoSuit’ and EDS methods in FE-SEM. Sci. Rep. 2020, 10, 14574. [Google Scholar] [CrossRef]
- Suzuki, C.; Takaku, Y.; Suzuki, H.; Ishii, D.; Shimozawa, T.; Nomura, S.; Shimomura, M.; Hariyama, T. Hydrophobic-hydrophilic crown-like structure enables aquatic insects to reside effectively beneath the water surface. Commun. Biol. 2021, 4, 708. [Google Scholar] [CrossRef]
- Kimura, T.; Ohashi, M.; Crailsheim, K.; Schmickl, T.; Okada, R.; Radspieler, G.; Ikeno, H. Development of a new method to track multiple honey bees with complex behaviors on a flat laboratory arena. PLoS ONE 2014, 9, e84656. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Cardona, A. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2019, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Sakai, T.; Hölldobler, B.; Millar, J.G.; Pratt, S.C. A context-dependent alarm signal in the ant Temnothorax rugatulus. Exp. Biol. 2014, 217, 3229–3236. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara-Tsujii, N.; Yamagata, N.; Takeda, T.; Mizunami, M.; Yamaoka, R. Behavioral responses to the alarm pheromone of the ant Camponotus obscuripes (Hymenoptera: Formicidae). Zoolog Sci. 2006, 23, 353–358. [Google Scholar] [CrossRef] [PubMed]
- O’Fallon, S.; Suarez, A.V.; Smith, A.A. A comparative analysis of rapid antennation behavior in four species of Odontomachus trap-jaw ants. Insect Soc. 2016, 63, 265–270. [Google Scholar] [CrossRef]
- Bos, N.; d’Ettorre, P. Recognition of social identity in ants. Front. Psychol. 2012, 3, 83. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, M.; Hefetz, A. Neural mechanisms and information processing in recognition systems. Insects 2014, 5, 722. [Google Scholar] [CrossRef] [Green Version]
- Neupert, S.; Hornung, M.; Millar, J.G.; Kleineidam, C.J. Learning distinct chemical labels of nestmates in ants. Front. Behav. Neurosci. 2018, 12, 191. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizutani, H.; Tagai, K.; Habe, S.; Takaku, Y.; Uebi, T.; Kimura, T.; Hariyama, T.; Ozaki, M. Antenna Cleaning Is Essential for Precise Behavioral Response to Alarm Pheromone and Nestmate–Non-Nestmate Discrimination in Japanese Carpenter Ants (Camponotus japonicus). Insects 2021, 12, 773. https://doi.org/10.3390/insects12090773
Mizutani H, Tagai K, Habe S, Takaku Y, Uebi T, Kimura T, Hariyama T, Ozaki M. Antenna Cleaning Is Essential for Precise Behavioral Response to Alarm Pheromone and Nestmate–Non-Nestmate Discrimination in Japanese Carpenter Ants (Camponotus japonicus). Insects. 2021; 12(9):773. https://doi.org/10.3390/insects12090773
Chicago/Turabian StyleMizutani, Hitomi, Kazuhiro Tagai, Shunya Habe, Yasuharu Takaku, Tatsuya Uebi, Toshifumi Kimura, Takahiko Hariyama, and Mamiko Ozaki. 2021. "Antenna Cleaning Is Essential for Precise Behavioral Response to Alarm Pheromone and Nestmate–Non-Nestmate Discrimination in Japanese Carpenter Ants (Camponotus japonicus)" Insects 12, no. 9: 773. https://doi.org/10.3390/insects12090773
APA StyleMizutani, H., Tagai, K., Habe, S., Takaku, Y., Uebi, T., Kimura, T., Hariyama, T., & Ozaki, M. (2021). Antenna Cleaning Is Essential for Precise Behavioral Response to Alarm Pheromone and Nestmate–Non-Nestmate Discrimination in Japanese Carpenter Ants (Camponotus japonicus). Insects, 12(9), 773. https://doi.org/10.3390/insects12090773




