Age and Behavior-Dependent Differential miRNAs Expression in the Hypopharyngeal Glands of Honeybees (Apis mellifera L.)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honeybees and RNA Extraction
2.2. Small RNA Sequencing and Analysis
2.3. Differential Expression Analysis
2.4. Validation of Known DEMs by qPCR
3. Results
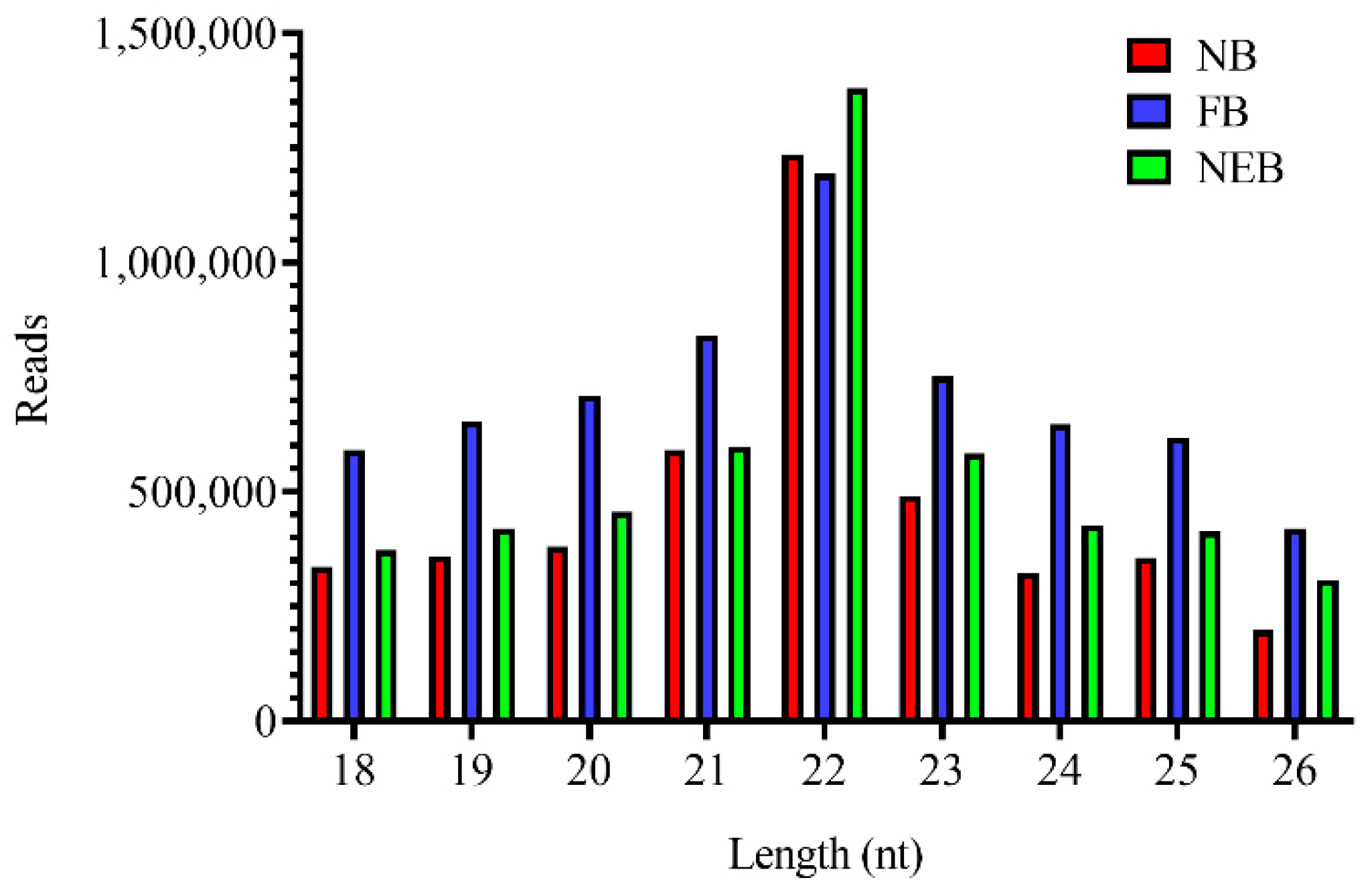
3.1. Small RNA Sequencing Analysis
3.2. Identification of Known and Novel miRNAs
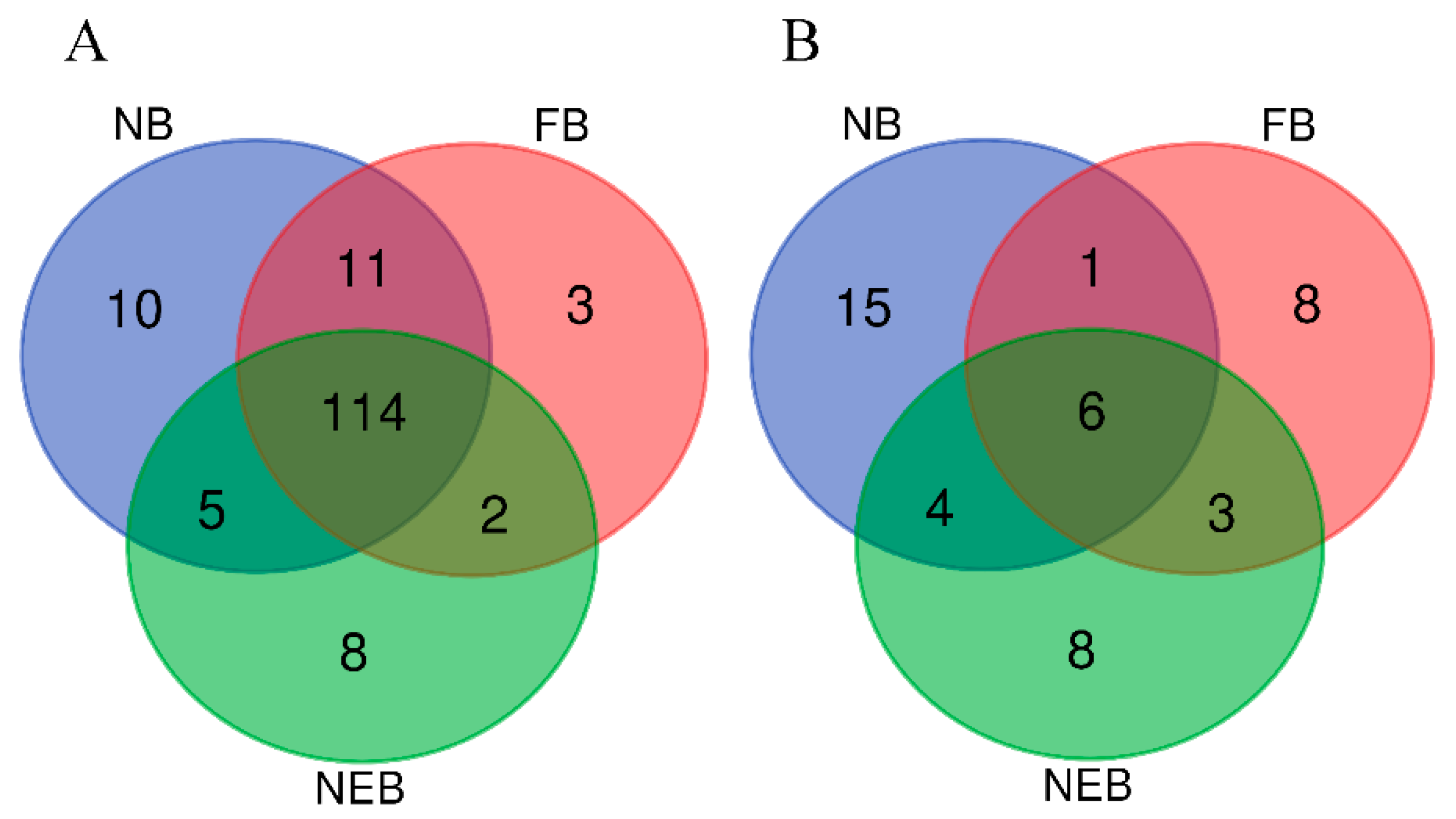
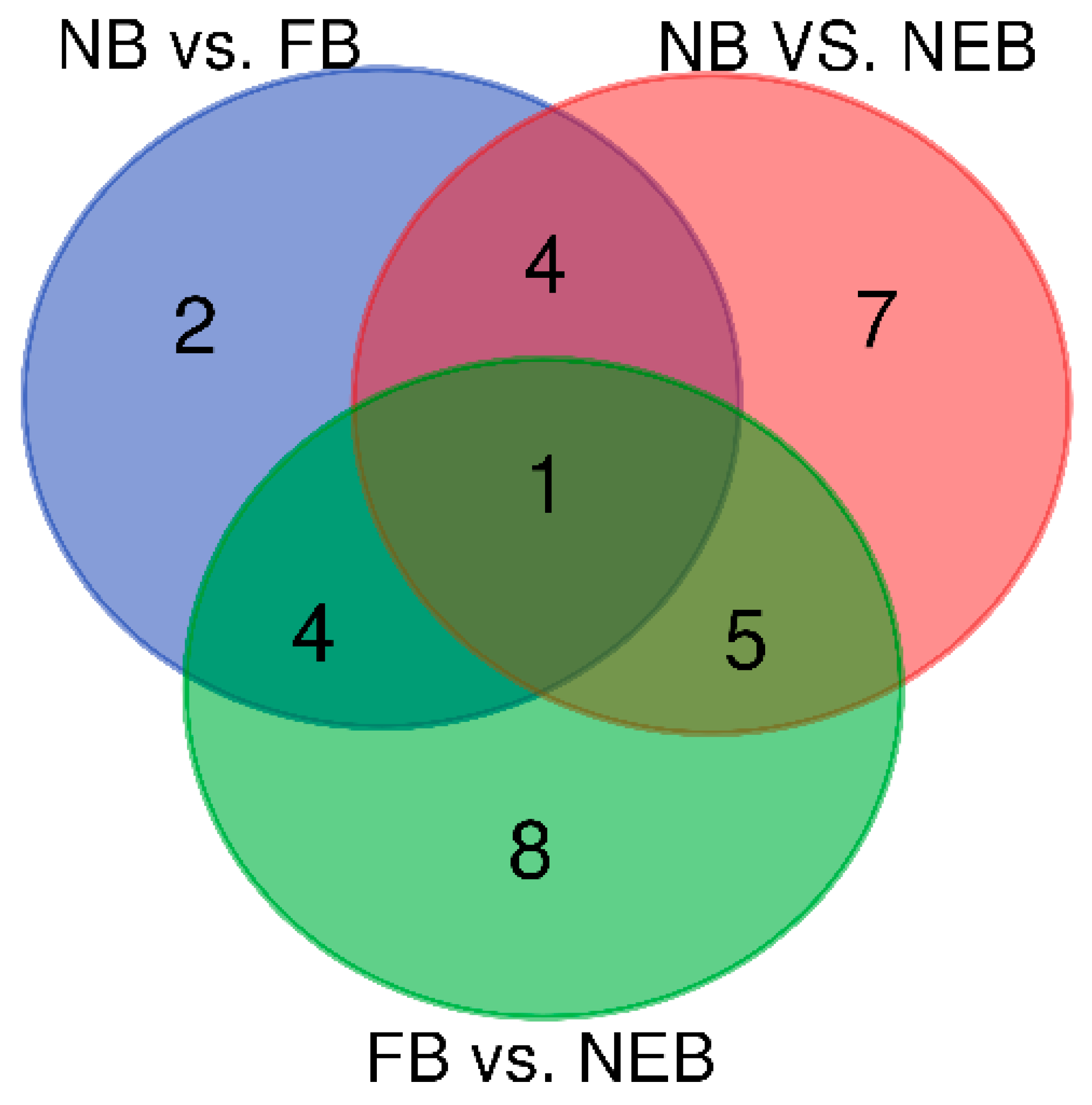
3.3. Differential Expression Analysis of miRNAs
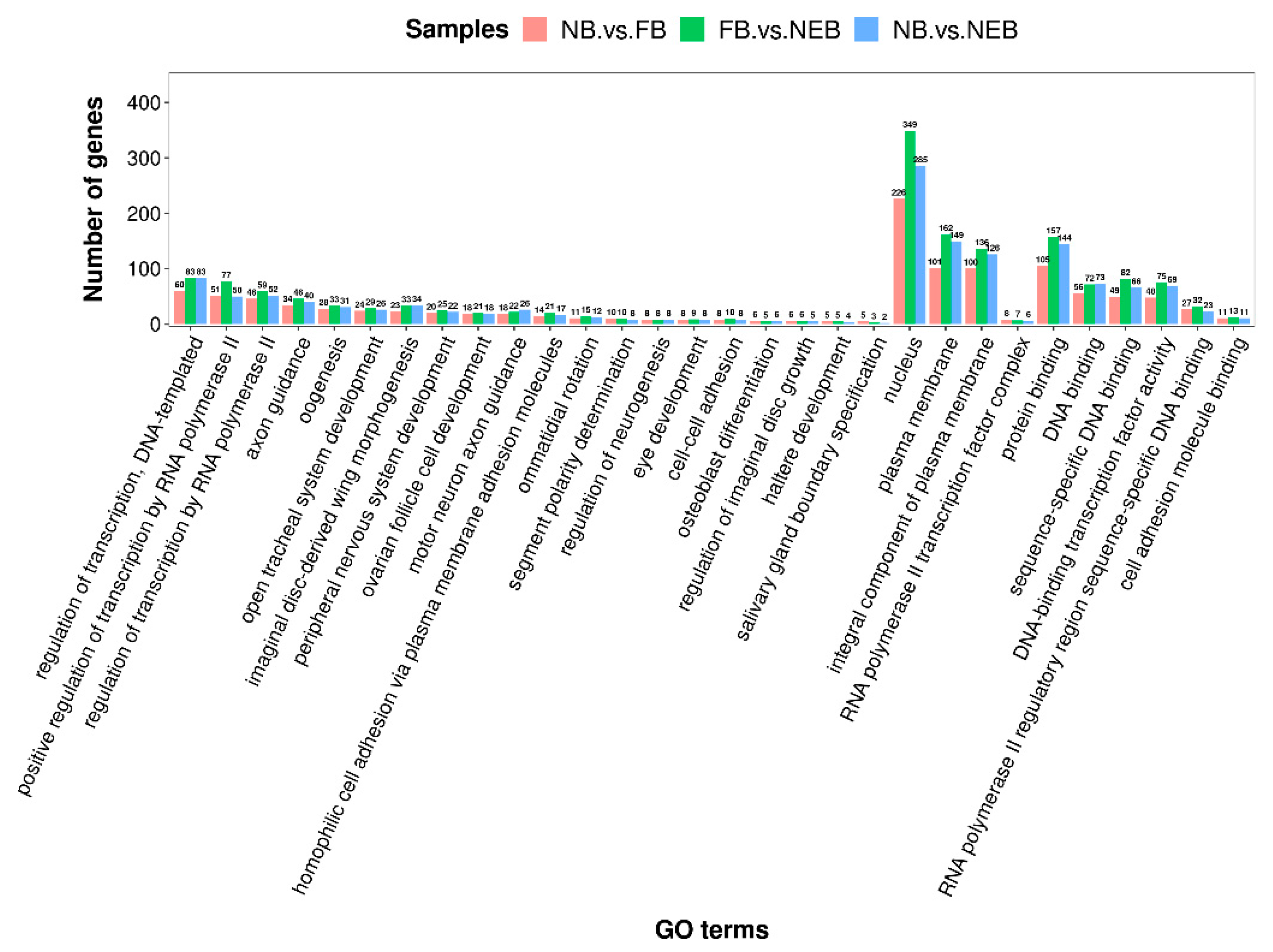
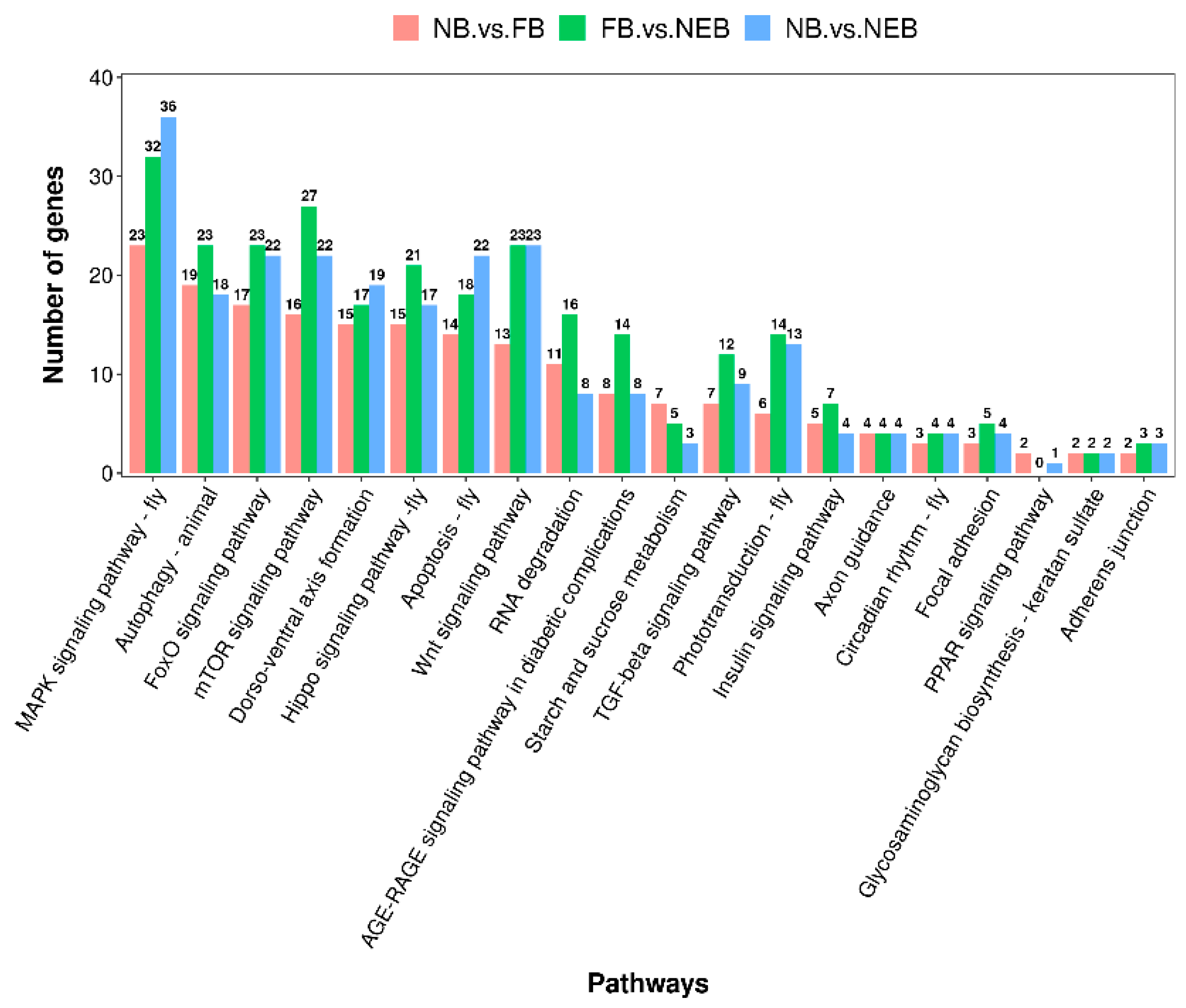
3.4. Prediction of the Target Genes of DEMs and Enrichment Analysis
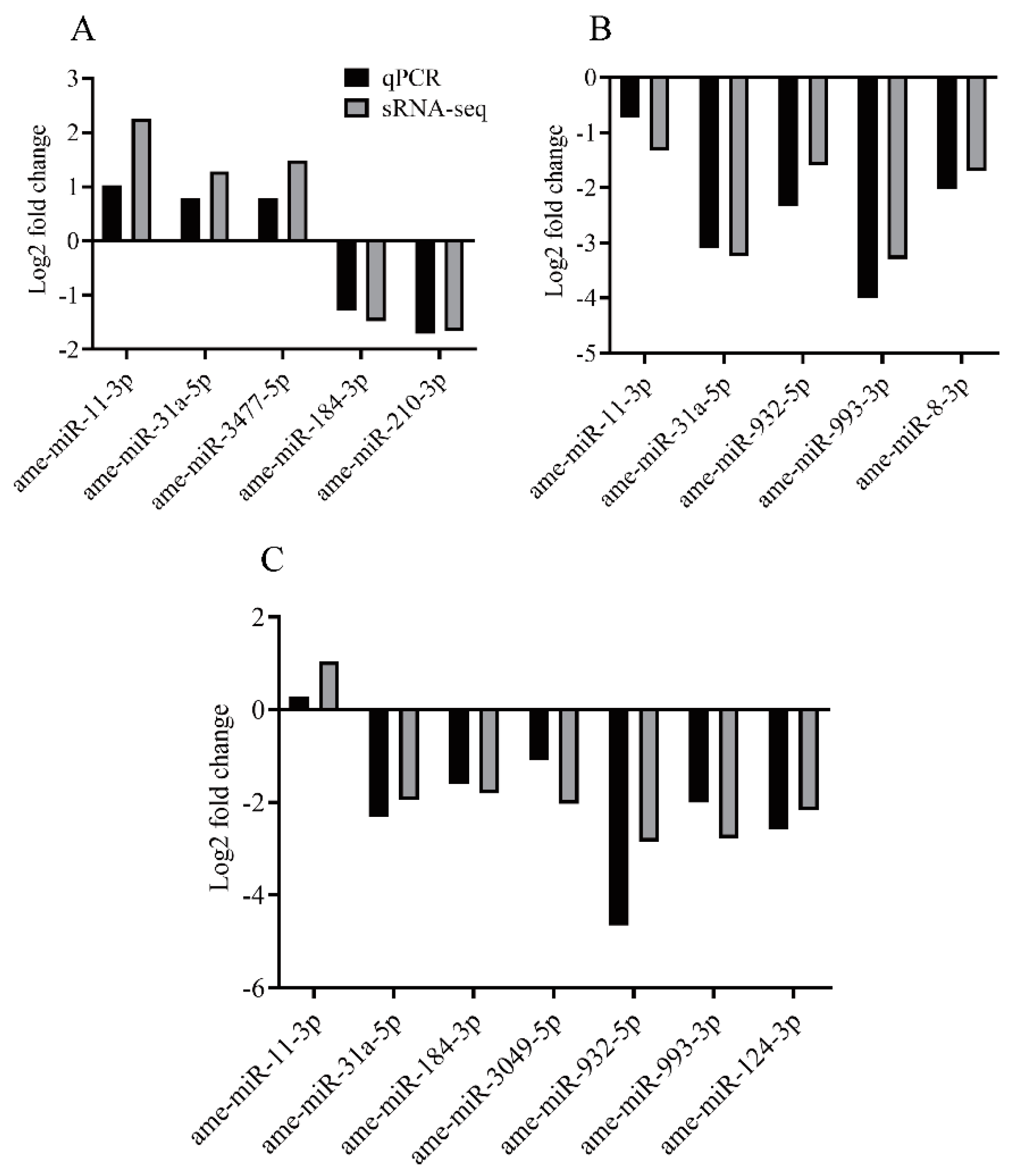
3.5. qPCR Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, CA, USA, 1987. [Google Scholar]
- Robinson, G.E. Regulation of division of labor in insect societies. Annu. Rev. Entomol. 1992, 37, 637–665. [Google Scholar] [CrossRef]
- Deseyn, J.; Billen, J. Age-dependent morphology and ultrastructure of the hypopharyngeal gland of Apis mellifera workers (Hymenoptera, Apidae). Apidologie 2005, 36, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Elaidy, W.K.M. Ultrastructural changes in the hypopharyngeal gland of Apis mellifera workers with age. J. Apicult. Res. 2014, 53, 377–384. [Google Scholar] [CrossRef]
- Huang, Z.-Y.; Otis, G. Factors determining hypopharyngeal gland activity of worker honey bees (Apis mellifera L.). Insect Soc. 1989, 36, 264–276. [Google Scholar]
- Kubo, T.; Sasaki, M.; Nakamura, J.; Sasagawa, H.; Ohashi, K.; Takeuchi, H.; Natori, S. Change in the expression of hypopharyngeal-gland proteins of the worker honeybees (Apis mellifera L.) with age and/or role. J. Biochem. 1996, 119, 291–295. [Google Scholar] [CrossRef]
- Ohashi, K.; Natori, S.; Kubo, T. Change in the mode of gene expression of the hypopharyngeal gland cells with an age-dependent role change of the worker honeybee Apis mellifera L. Eur. J. Biochem. 1997, 249, 797–802. [Google Scholar] [CrossRef]
- Hrassnigg, N.; Crailsheim, K. Adaptation of hypopharyngeal gland development to the brood status of honeybee (Apis mellifera L.) colonies. J. Insect Physiol. 1998, 44, 929–939. [Google Scholar] [CrossRef]
- Ohashi, K.; Sawata, M.; Takeuchi, H.; Natori, S.; Kubo, T. Molecular Cloning of cDNA and Analysis of Expression of the Gene for α-Glucosidase from the Hypopharyngeal Gland of the Honeybee Apis mellifera L. Biochem. Biophys. Res. Commun. 1996, 221, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Natori, S.; Kubo, T. Expression of amylase and glucose oxidase in the hypopharyngeal gland with an age-dependent role change of the worker honeybee (Apis mellifera L.). Eur. J. Biochem. 1999, 265, 127–133. [Google Scholar] [CrossRef]
- Ueno, T.; Nakaoka, T.; Takeuchi, H.; Kubo, T. Differential gene expression in the hypopharyngeal glands of worker honeybees (Apis mellifera L.) associated with an age-dependent role change. Zoolog. Sci. 2009, 26, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Ueno, T.; Takeuchi, H.; Kawasaki, K.; Kubo, T. Changes in the gene expression profiles of the hypopharyngeal gland of worker honeybees in association with worker behavior and hormonal factors. PLoS ONE 2015, 10, e0130206. [Google Scholar]
- Ahmad, S.; Khan, S.A.; Khan, K.A.; Li, J. Novel insight into the development and function of hypopharyngeal glands in honey bees. Front. Physiol. 2020, 11, 615830. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.L.; Tian, L.Q.; Qin, Q.H.; Wu, X.B.; Yan, W.Y.; Zeng, Z.J. Transcriptome differences in the hypopharyngeal gland between Western Honeybees (Apis mellifera) and Eastern Honeybees (Apis cerana). BMC Genom. 2014, 15, 744. [Google Scholar] [CrossRef] [Green Version]
- Nie, H.; Gao, Y.; Zhu, Y.; Liang, L.; Lin, Y.; Li, Q.; Li, Z.; Yang, D.; Li, Z.; Su, S. Comparative transcriptome analysis of hypopharyngeal glands from nurse and forager bees of Apis mellifera with the same age. Apidologie 2021, 52, 141–154. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillai, R.S.; Bhattacharyya, S.N.; Artus, C.G.; Zoller, T.; Cougot, N.; Basyuk, E.; Bertrand, E.; Filipowicz, W. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science 2005, 309, 1573–1576. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, X.; Luo, C.; Pu, Q.; Yin, Q.; Xu, L.; Peng, X.; Ma, S.; Xia, Q.; Liu, S. Let-7 microRNA is a critical regulator in controlling the growth and function of silk gland in the silkworm. RNA Biol. 2020, 17, 703–717. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Zheng, H.J.; Pan, Q.Z.; Wang, Z.L.; Zeng, Z.J. Differentially expressed microRNAs between queen and worker larvae of the honey bee (Apis mellifera). Apidologie 2015, 46, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Su, S.; Geir, S.; Li, W.; Li, Z.; Zhang, S.; Chen, S.; Chen, R. Differential expression of miRNAs related to caste differentiation in the honey bee, Apis mellifera. Apidologie 2016, 47, 495–508. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, J.K.; Xia, J.; Zhou, X.; Thatcher, S.R.; Gu, X.; Ament, S.A.; Newman, T.C.; Green, P.J.; Zhang, W.; Robinson, G.E.; et al. Behavioral plasticity in honey bees is associated with differences in brain microRNA transcriptome. Genes Brain Behav. 2012, 11, 660–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Shi, T.; Yin, W.; Su, X.; Qi, L.; Huang, Z.Y.; Zhang, S.; Yu, L. The microRNA ame-miR-279a regulates sucrose responsiveness of forager honey bees (Apis mellifera). Insect Biochem. Mol. Biol. 2017, 90, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristino, A.S.; Barchuk, A.R.; Freitas, F.C.; Narayanan, R.K.; Biergans, S.D.; Zhao, Z.; Simoes, Z.L.; Reinhard, J.; Claudianos, C. Neuroligin-associated microRNA-932 targets actin and regulates memory in the honeybee. Nat. Commun. 2014, 5, 5529. [Google Scholar] [CrossRef] [Green Version]
- Michely, J.; Kraft, S.; Muller, U. miR-12 and miR-124 contribute to defined early phases of long-lasting and transient memory. Sci. Rep. (UK) 2017, 7, 7910. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ma, C.; Chen, C.; Lu, Q.; Shi, W.; Liu, Z.; Wang, H.; Guo, H. Integration of lncRNA–miRNA–mRNA reveals novel insights into oviposition regulation in honey bees. PeerJ 2017, 5, e3881. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fu, J. The microRNA miR-14 Regulates Egg-Laying by Targeting EcR in Honeybees (Apis mellifera). Insects 2021, 12, 351. [Google Scholar] [CrossRef]
- Chen, D.; Du, Y.; Chen, H.; Fan, Y.; Fan, X.; Zhu, Z.; Wang, J.; Xiong, C.; Zheng, Y.; Hou, C. Comparative Identification of MicroRNAs in Apis cerana cerana Workers’ Midguts in Response to Nosema ceranae Invasion. Insects 2019, 10, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Deng, Y.; Zhao, H.; Shen, S.; Hou, C. Citation: Identification of Immune Response to Sacbrood Virus Infection in Apis cerana Under Natural Condition. Front. Genet. 2020, 11, 587509. [Google Scholar] [CrossRef] [PubMed]
- Freitas, F.C.; Pires, C.V.; Claudianos, C.; Cristino, A.S.; Simões, Z.L. MicroRNA-34 directly targets pair-rule genes and cytoskeleton component in the honey bee. Sci. Rep. (UK) 2017, 7, 40884. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Chen, H.; Du, Y.; Zhu, Z.; Wang, J.; Geng, S.; Xiong, C.; Zheng, Y.; Hou, C.; Diao, Q. Systematic identification of circular RNAs and corresponding regulatory networks unveil their potential roles in the midguts of eastern honeybee workers. Appl. Microbiol. Biotechnol. 2020, 104, 257–276. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, R.; Bernhart, S.H.; Zu Siederdissen, C.H.; Tafer, H.; Flamm, C.; Stadler, P.F.; Hofacker, I.L. ViennaRNA Package 2.0. Algorithms Mol. Biol. 2011, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Betel, D.; Wilson, M.; Gabow, A.; Marks, D.S.; Sander, C. The microRNA. org resource: Targets and expression. Nucleic Acids Res. 2008, 36, D149–D153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, J.-W.; Rissland, O.S.; Koppstein, D.; Abreu-Goodger, C.; Jan, C.H.; Agarwal, V.; Yildirim, M.A.; Rodriguez, A.; Bartel, D.P. Global analyses of the effect of different cellular contexts on microRNA targeting. Mol. Cell 2014, 53, 1031–1043. [Google Scholar] [CrossRef] [Green Version]
- Tran, V.D.T.; Moretti, S.; Coste, A.T.; Amorim-Vaz, S.; Sanglard, D.; Pagni, M. Condition-specific series of metabolic sub-networks and its application for gene set enrichment analysis. Bioinformatics 2019, 35, 2258–2266. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2007, 36, D480–D484. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101. [Google Scholar] [CrossRef]
- Liu, M.; Huang, J.; Zhang, G.; Liu, X.; An, J. Analysis of miRNAs in the heads of different castes of the bumblebee Bombus lantschouensis (Hymenoptera: Apidae). Insects 2019, 10, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hori, S.; Kaneko, K.; Saito, T.H.; Takeuchi, H.; Kubo, T. Expression of two microRNAs, ame-mir-276 and-1000, in the adult honeybee (Apis mellifera) brain. Apidologie 2011, 42, 89–102. [Google Scholar] [CrossRef] [Green Version]
- Behura, S.; Whitfield, C.W. Correlated expression patterns of microRNA genes with age-dependent behavioural changes in honeybee. Insect Mol. Biol. 2010, 19, 431–439. [Google Scholar] [CrossRef]
- Liu, F.; Peng, W.; Li, Z.; Li, W.; Li, L.; Pan, J.; Zhang, S.; Miao, Y.; Chen, S.; Su, S. Next-generation small RNA sequencing for microRNAs profiling in Apis mellifera: Comparison between nurses and foragers. Insect Mol. Biol. 2012, 21, 297–303. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, T.; Tang, G. Identification and characterization of conserved and novel miRNAs in different development stages of Atrijuglans hetaohei Yang (Lepidoptera: Gelechioidea). J. Asia-Pac. Entomol. 2018, 21, 9–18. [Google Scholar] [CrossRef]
- Zhang, Q.; Dou, W.; Song, Z.H.; Jin, T.J.; Yuan, G.R.; De Schutter, K.; Smagghe, G.; Wang, J.-J. Identification and profiling of Bactrocera dorsalis microRNAs and their potential roles in regulating the developmental transitions of egg hatching, molting, pupation and adult eclosion. Insect Biochem. Mol. Biol. 2020, 127, 103475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X. The Molecular Mechanisms of ame-miR-184 Regulating on Hypopharyngeal Gland Activity of Apis Mellifera Ligustica. Ph.D. Thesis, Shandong Agricultural University, Taian, China, 2020. [Google Scholar]
- Cruz-Landim, C.d.; Costa, R. Structure and function of the hypopharyngeal glands of Hymenoptera: A comparative approach. J. Comp. Biol. 1998, 3, 151–163. [Google Scholar]
- Hu, H.; Bezabih, G.; Feng, M.; Wei, Q.; Zhang, X.; Wu, F.; Meng, L.; Fang, Y.; Han, B.; Ma, C. In-depth Proteome of the Hypopharyngeal Glands of Honeybee Workers Reveals Highly Activated Protein and Energy Metabolism in Priming the Secretion of Royal Jelly. Mol. Cell. Proteom. 2019, 18, 606–621. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Shi, T.F. Expression difference of microRNAs ame-miR-31a and ame-miR-13b in brains of nurses and foragers of Apis mellifera ligustica (Hymenoptera: Apidae). Acta Entomol. Sin. 2019, 62, 1003–1008. [Google Scholar]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef]
- Chiba, S. Molecular mechanism in α-glucosidase and glucoamylase. Biosci. Biotechnol. Biochem. 1997, 61, 1233–1239. [Google Scholar] [CrossRef]
- Feng, M.; Fang, Y.; Li, J. Proteomic analysis of honeybee worker (Apis mellifera) hypopharyngeal gland development. BMC Genom. 2009, 10, 645. [Google Scholar] [CrossRef] [Green Version]
- Halberstadt, K. Elektrophoretische untersuchungen zur sekretionstätigkeit der hypopharynxdrüse der honigbiene (Apis mellifera L.). Insect Soc. 1980, 27, 61–77. [Google Scholar] [CrossRef]
- Silva de Moraes, R.; Bowen, I.D. Modes of cell death in the hypopharyngeal gland of the honey bee (Apis mellifera L.). Cell Biol. Int. 2000, 24, 737–743. [Google Scholar]
- Mayer, C.; Grummt, I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene 2006, 25, 6384–6391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, S.; Liu, L.; Gan, W. The Roles of Post-Translational Modifications on mTOR Signaling. Int. J. Mol. Sci. 2021, 22, 1784. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Tumaneng, K.; Guan, K.-L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011, 13, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Halder, G.; Johnson, R.L. Hippo signaling: Growth control and beyond. Development 2011, 138, 9–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Accili, D.; Arden, K.C. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell Biol. Int. 2004, 117, 421–426. [Google Scholar]
- Gao, J.; Jin, S.S.; He, Y.; Luo, J.H.; Xu, C.Q.; Wu, Y.Y.; Hou, C.S.; Wang, Q.; Diao, Q.-Y. Physiological analysis and transcriptome analysis of Asian honey bee (Apis cerana cerana) in response to sublethal neonicotinoid imidacloprid. Insects 2020, 11, 753. [Google Scholar] [CrossRef]
- Liu, F.; Li, W.; Li, Z.; Zhang, S.; Chen, S.; Su, S. High-abundance mRNAs in Apis mellifera: Comparison between nurses and foragers. J. Insect Physiol. 2011, 57, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, Y.; Zheng, H.; Cao, L.; Niu, D.; Yu, D.; Sun, Y.; Hu, S.; Hu, F. Transcriptome comparison between honey bee queen-and worker-destined larvae. Insect Biochem. Mol. Biol. 2012, 42, 665–673. [Google Scholar] [CrossRef]
- He, X.J.; Wei, H.; Jiang, W.J.; Liu, Y.B.; Wu, X.B.; Zeng, Z. Honeybee (Apis mellifera) Maternal Effect Causes Alternation of DNA Methylation Regulating Queen Development. Sociobiology 2021, 68, 5935. [Google Scholar] [CrossRef]
| miR-Name | Primer Sequences (5′ to 3′) | Amplification Efficiencies |
|---|---|---|
| ame-miR-124-3p | F: TAAGGCACGCGGTGAATGC | 0.98 |
| ame-miR-184-3p | F: CCTTATCATTCTCCTGTCCGGT | 0.97 |
| ame-miR-210-3p | F: GCGTTGTGCGTGTGACA | 0.97 |
| ame-miR-3049-5p | F: CGTCGGGAAGGTAGTTGC | 0.95 |
| ame-miR-31a-5p | F: GGCAAGATGTCGGCATA | 0.93 |
| ame-miR-932-5p | F: CCGTCAATTCCGTAGTGCATTGCAG | 0.93 |
| ame-miR-993-3p | F: GCGAAGCTCGTCTCTACAGGTATCT | 0.94 |
| ame-miR-11-3p | F: CGCATCACAGGCAGAGTTCTAGTT | 0.96 |
| ame-miR-8-3p | F: CGCGCGTAATACTGTCAGGTAAAGATG | 1.05 |
| ame-miR-3477-5p | F: CGCTAATCTCATGCGGTAACTGTGAG | 0.97 |
| U6 (Reference) | F: GTTAGGCTTTGACGATTTCG | 1.02 |
| R: GGCATTTCTCCACCAGGTA |
| Rank | Nurse Bee | Forager Bee | Newly-Emerged Bee | |||
|---|---|---|---|---|---|---|
| miR-Name | Reads Count | miR-Name | Reads Count | miR-Name | Reads Count | |
| 1 | ame-miR-276-3p | 242,234 | ame-miR-276-3p | 238,540 | ame-miR-276-3p | 486,993 |
| 2 | ame-miR-375-3p | 221,939 | ame-miR-375-3p | 68,526 | ame-miR-375-3p | 75,545 |
| 3 | ame-miR-14-3p | 104,814 | ame-miR-14-3p | 42,487 | ame-miR-14-3p | 70,958 |
| 4 | ame-miR-3477-5p | 86,004 | ame-miR-317-3p | 28,086 | ame-miR-3477-5p | 38,165 |
| 5 | ame-miR-317-3p | 62,651 | ame-miR-3477-5p | 22,349 | ame-miR-275-3p | 33,428 |
| 6 | ame-miR-8-3p | 52,599 | ame-miR-275-3p | 18,213 | ame-miR-8-3p | 17,168 |
| 7 | ame-miR-306-5p | 38,992 | ame-miR-2796-3p | 16,675 | ame-miR-2796-3p | 16,052 |
| 8 | ame-miR-275-3p | 38,214 | ame-miR-100-5p | 13,408 | ame-miR-263a-5p | 14,172 |
| 9 | ame-miR-12-5p | 27,484 | ame-miR-34-5p | 7870 | ame-miR-100-5p | 11,417 |
| 10 | ame-miR-11-3p | 14,083 | ame-miR-252a-5p | 7759 | ame-miR-252a-5p | 11,363 |
| DEMs | NB vs. FB | NB vs. NEB | FB vs. NEB | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Log2 FC | p-Values | Regulated | Log2 FC | p-Values | Regulated | Log2 FC | p-Values | Regulated | |
| ame-miR-11-3p | 2.25 | 7.18 × 10−6 | up | - | - | - | −1.33 | 7.62 × 10−4 | down |
| ame-miR-281-3p | 1.23 | 1.90 × 10−3 | up | 1.7 | 3.18 × 10−3 | up | - | - | - |
| ame-miR-283-5p | 2.11 | 2.05 × 10−3 | up | - | - | - | −1.81 | 6.04 × 10−3 | down |
| ame-miR-31a-5p | 1.28 | 9.45 × 10−3 | up | −1.95 | 2.10 × 10−3 | down | −3.23 | 2.82 × 10−3 | down |
| ame-miR-3477-5p | 1.49 | 8.72 × 10−4 | up | 1.03 | 1.12 × 10−4 | up | - | - | - |
| ame-miR-184-3p | −1.48 | 2.45 × 10−3 | down | −1.81 | 6.45 × 10−4 | down | - | - | - |
| ame-miR-210-3p | −1.67 | 8.23 × 10−3 | down | - | - | - | 1.43 | 8.56 × 10−3 | up |
| ame-miR-252a-5p | −2.24 | 8.64 × 10−3 | down | −2.74 | 4.80 × 10−3 | down | - | - | - |
| ame-miR-2788-3p | −1.57 | 7.12 × 10−4 | down | - | - | - | - | - | - |
| ame-miR-3791-3p | −1.66 | 7.49 × 10−3 | down | - | - | - | 4.91 | 8.03 × 10−4 | up |
| ame-miR-6047a-5p | −1.15 | 5.72 × 10−3 | down | - | - | - | 3.36 | 2.22 × 10−3 | up |
| ame-miR-279c-3p | - | - | - | 1.38 | 1.26 × 10−3 | up | - | - | - |
| ame-miR-6040-3p | - | - | - | 3.63 | 9.35 × 10−3 | up | - | - | - |
| ame-miR-6044-5p | - | - | - | 1.7 | 2.27 × 10−4 | up | - | - | - |
| ame-miR-124-3p | - | - | - | −2.17 | 3.82 × 10−4 | down | - | - | - |
| ame-miR-125-5p | - | - | - | −1.33 | 3.02 × 10−3 | down | - | - | - |
| ame-miR-276-3p | - | - | - | −1.16 | 7.17 × 10−3 | down | - | - | - |
| ame-miR-3049-5p | - | - | - | −2.03 | 4.30 × 10−3 | down | - | - | - |
| ame-miR-3785-3p | - | - | - | −2.13 | 1.14 × 10−5 | down | −1.54 | 3.05 × 10−3 | down |
| ame-miR-92b-3p | - | - | - | −2.36 | 3.75 × 10−4 | down | −1.56 | 2.18 × 10−3 | down |
| ame-miR-92c-3p | - | - | - | −1.41 | 8.15 × 10−3 | down | - | - | |
| ame-miR-932-5p | - | - | - | −2.85 | 1.80 × 10−3 | down | −1.59 | 3.34 × 10−3 | down |
| ame-miR-993-3p | - | - | - | −2.77 | 6.31 × 10−3 | down | −3.29 | 5.74 × 10−3 | down |
| ame-miR-263b-5p | - | - | - | - | - | - | 2.32 | 3.44 × 10−3 | up |
| ame-miR-3770-5p | - | - | - | - | - | - | - | 3.46 × 10−3 | up |
| ame-miR-6052-5p | - | - | - | - | - | - | 1.55 | 2.58 × 10−3 | up |
| ame-miR-12-5p | - | - | - | - | - | - | −1.12 | 3.28 × 10−3 | down |
| ame-miR-305-5p | - | - | - | - | - | - | −2.08 | 2.54 × 10−5 | down |
| ame-miR-3715-5p | - | - | - | - | - | - | - | 2.75 × 10−3 | down |
| ame-miR-79-3p | - | - | - | - | - | - | −1.23 | 2.34 × 10−3 | down |
| ame-miR-8-3p | - | - | - | - | - | - | −1.7 | 8.68 × 10−5 | down |
| miR-Name | Potential Target Gene Symbol | Target Gene Description | KEGG Pathways | Regulation of miRNAs |
|---|---|---|---|---|
| ame-miR-252a-5p | STT3B | STT3, subunit of the oligosaccharyltransferase complex, homolog B [Apis mellifera] | ko04141 (Protein processing in endoplasmic reticulum) | Downregulated in hypopharyengeal glands of nurse |
| GstZ1 | glutathione S-transferase Z1 [Apis mellifera] | ko00350 (Tyrosine metabolism) | ||
| LOC551016 | midasin [Apis mellifera] | ko03008 (Ribosome biogenesis in eukaryotes) | ||
| LOC413650 | nuclear RNA export factor 1 [Apis mellifera] | ko03008 (Ribosome biogenesis in eukaryotes) | ||
| SdhA | succinate dehydrogenase A [Apis mellifera] | ko00020 (Citrate cycle); ko00190 (Oxidative phosphorylation) | ||
| LOC113218834 | cytochrome b-c1 complex subunit 8-like [Apis mellifera] | ko00190 (Oxidative phosphorylation) | ||
| LOC724652 | uncharacterized protein LOC724652 [Apis mellifera] | ko00020 (Citrate cycle); ko00190 (Oxidative phosphorylation) | ||
| Ilp-2 | insulin-like peptide 2 [Apis mellifera] | ko04150 (mTOR signaling pathway) | ||
| Pten | phosphatase and tensin-like [Apis mellifera] | ko04150 (mTOR signaling pathway) | ||
| Dad | daughters against dpp [Apis mellifera] | ko04350 (TGF-beta signaling pathway) | ||
| ame-miR-184-3p | LOC410870 | proton channel OtopLc [Apis mellifera] | ko03010 (Ribosome) | |
| InRS | insulin receptor substrate 1-B [Apis mellifera] | ko04150 (mTOR signaling pathway) | ||
| LOC551830 | ral GTPase-activating protein subunit alpha-1 [Apis mellifera] | ko04150 (mTOR signaling pathway); ko04115 (p53 signaling pathway); ko04910 (Insulin signaling pathway) | ||
| LOC100577028 | insulin-like growth factor I [Apis mellifera] | ko04150 (mTOR signaling pathway) | ||
| ame-miR-11-3p | LOC410744 | glucose dehydrogenase [FAD, quinone] [Apis mellifera] | - | Downregulated in hypopharyengeal glands of forager |
| LOC413098 | glucose dehydrogenase [FAD, quinone] [Apis mellifera] | - | ||
| LOC552747 | glucosidase 2 subunit beta [Apis mellifera] | - | ||
| LOC551303 | hexosaminidase D [Apis mellifera] | ko00511 (Other glycan degradation) | ||
| LOC412161 | broad-complex core protein isoforms 1/2/3/4/5 isoform X1 [Apis mellifera] | ko04214 (Apoptosis-fly) | ||
| Mblk-1 | transcription factor mblk-1-like [Apis mellifera] | ko04214 (Apoptosis-fly) | ||
| E74 | ecdysteroid-regulated gene E74 [Apis mellifera] | ko04214 (Apoptosis-fly) | ||
| LOC100577393 | epidermal growth factor receptor-like [Apis mellifera] | ko04214 (Apoptosis-fly) | ||
| ame-miR-283-5p | LOC726210 | myogenesis-regulating glycosidase [Apis mellifera] | ko00052 (Galactose metabolism); ko00500 (Starch and sucrose metabolism) | |
| LOC726547 | protein abrupt [Apis mellifera] | ko04214 (Apoptosis-fly) | ||
| ame-miR-31a-5p | LOC726547 | protein abrupt [Apis mellifera] | ko04214 (Apoptosis-fly) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.; Zhu, Y.; Liu, P.; Ye, L.; Jiang, X.; Cao, H.; Yu, L. Age and Behavior-Dependent Differential miRNAs Expression in the Hypopharyngeal Glands of Honeybees (Apis mellifera L.). Insects 2021, 12, 764. https://doi.org/10.3390/insects12090764
Shi T, Zhu Y, Liu P, Ye L, Jiang X, Cao H, Yu L. Age and Behavior-Dependent Differential miRNAs Expression in the Hypopharyngeal Glands of Honeybees (Apis mellifera L.). Insects. 2021; 12(9):764. https://doi.org/10.3390/insects12090764
Chicago/Turabian StyleShi, Tengfei, Yujie Zhu, Peng Liu, Liang Ye, Xingchuan Jiang, Haiqun Cao, and Linsheng Yu. 2021. "Age and Behavior-Dependent Differential miRNAs Expression in the Hypopharyngeal Glands of Honeybees (Apis mellifera L.)" Insects 12, no. 9: 764. https://doi.org/10.3390/insects12090764
APA StyleShi, T., Zhu, Y., Liu, P., Ye, L., Jiang, X., Cao, H., & Yu, L. (2021). Age and Behavior-Dependent Differential miRNAs Expression in the Hypopharyngeal Glands of Honeybees (Apis mellifera L.). Insects, 12(9), 764. https://doi.org/10.3390/insects12090764






