Expression Patterns of Three Important Hormone Genes and Respiratory Metabolism in Antheraea pernyi during Pupal Diapause under a Long Photoperiod
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Respiratory Intensity of A. pernyi Pupae
2.3. Total RNA Extraction, cDNA Synthesis, and Gene Cloning
2.4. Sequence Alignment and Bioinformatics
2.5. Real-Time Quantitative PCR Analysis
2.6. 20-Hydroxyecdysone Titers in Diapausing Pupae under the Long Photoperiod
2.7. Data Analysis
3. Results
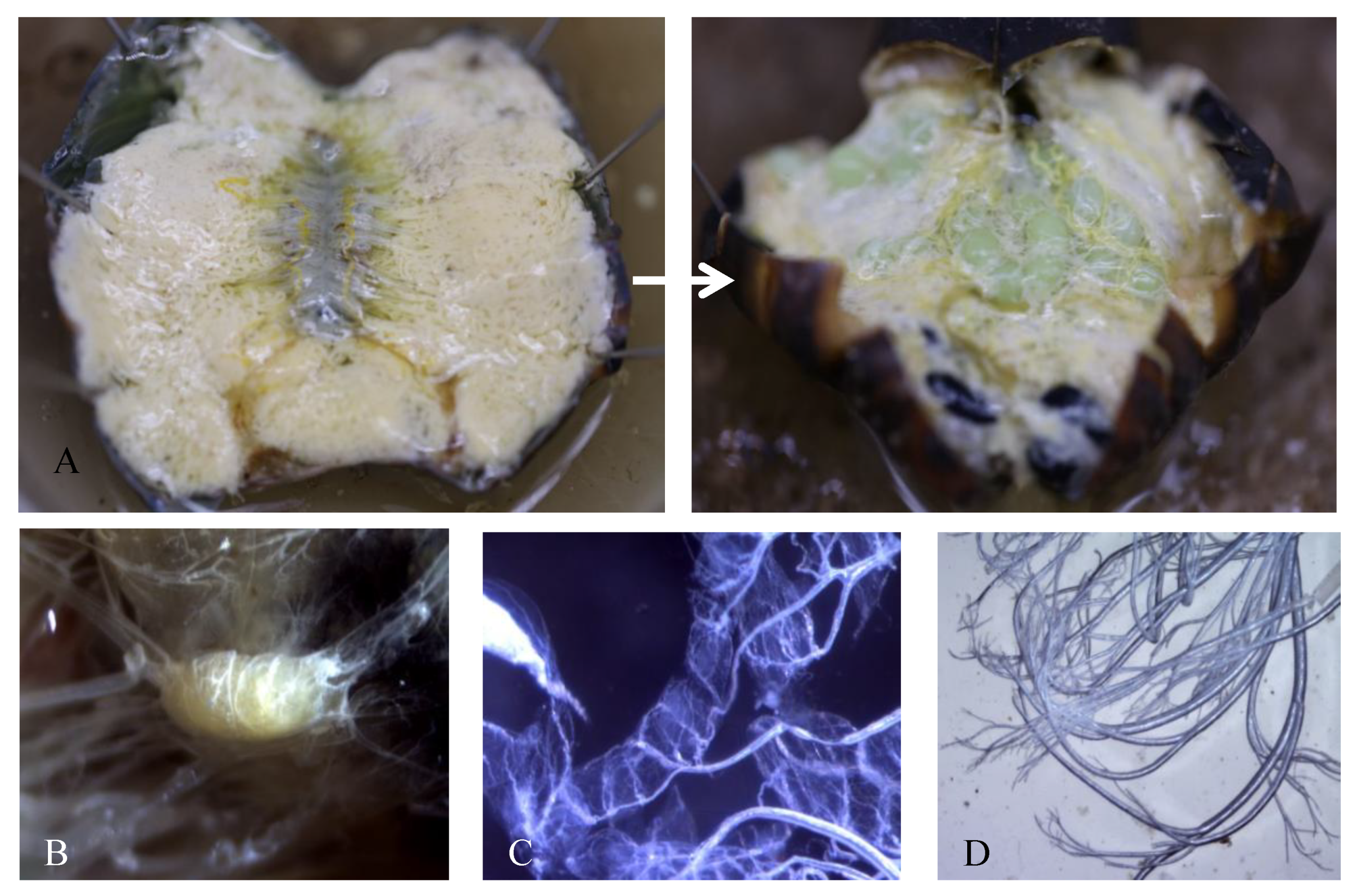
3.1. Morphological Changes during Pupal Development
3.2. Respiratory Intensity of A. pernyi Pupae
3.3. Sequence of ApPTTH, ApEH, and ApETH
3.4. Sequence Homology Alignment and Phylogenetic Relationships of ApPTTH, ApETH, and ApEH
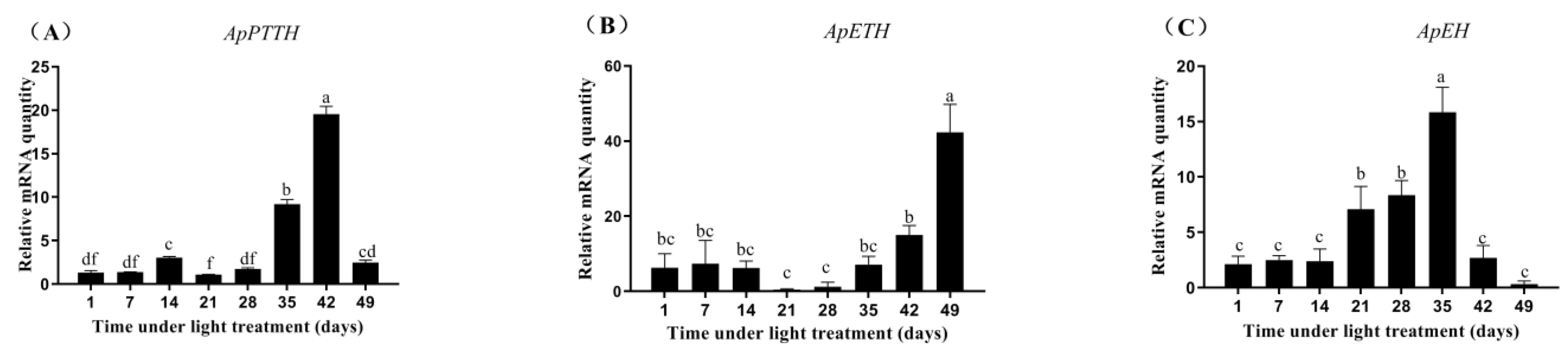
3.5. Expression Profiles of ApPTTH, ApETH, and ApEH from Diapause Termination to Eclosion
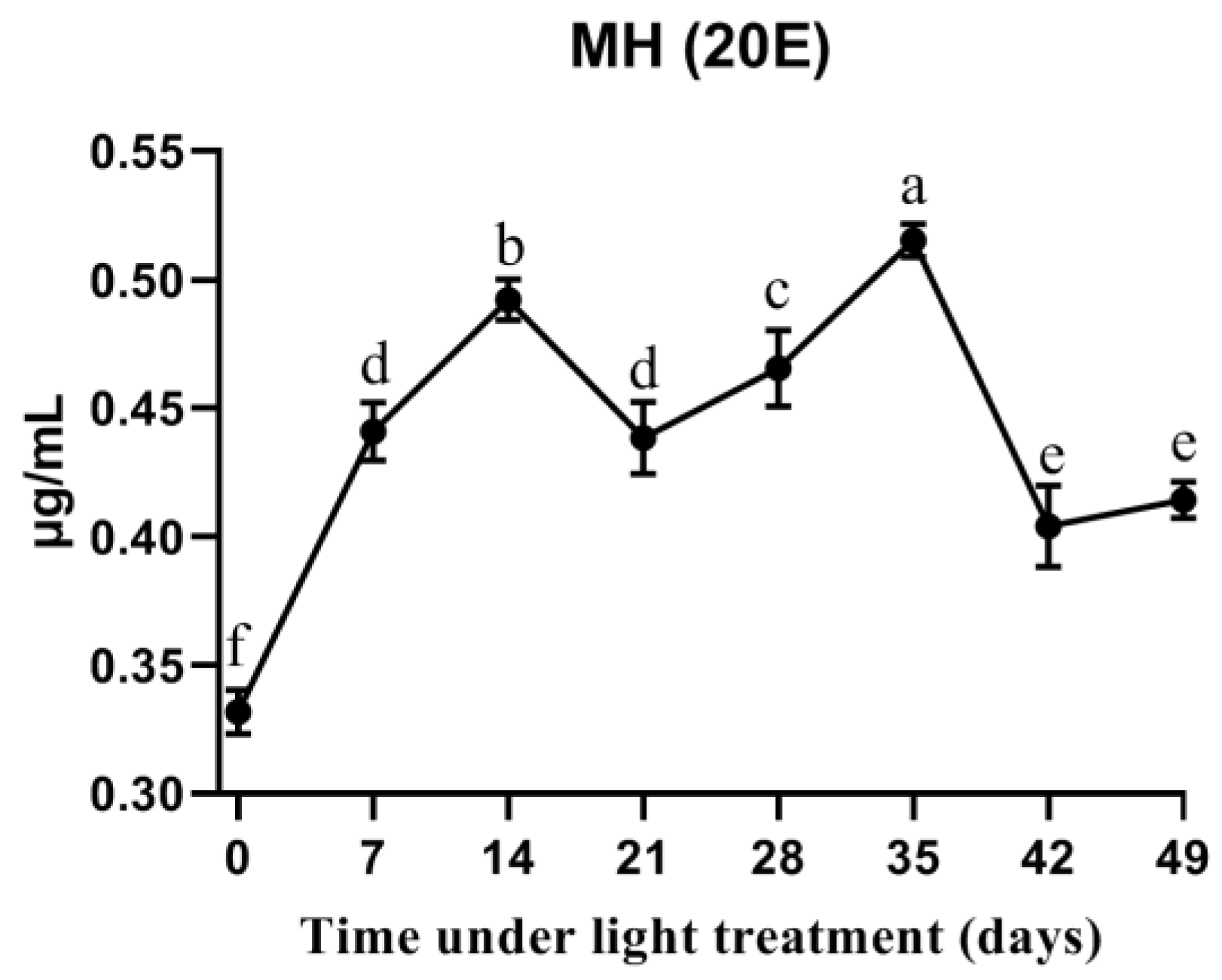
3.6. 20E Titers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denlinger, D.L.; Armbruster, P.A. Mosquito diapause. Annu. Rev. Entomol. 2014, 59, 73–93. [Google Scholar] [CrossRef]
- Danks, H.V. Studying insect photoperiodism and rhythmicity: Components, approaches and lessons. Eur. J. Entomol. 2003, 100, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, W.E.; Zani, P.A.; Holzapfel, C.M. Adaptation to temperate climates. Evolution 2004, 58, 1748–1762. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, N.; Rewitz, K.F.; O’Connor, M.B. Ecdysone control of developmental transitions: Lessons from Drosophila research. Annu. Rev. Entomol. 2013, 58, 497–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.M. Physiology of Insect Diapause. IV. The Brain and Prothoracic Glands as an Endocrine System in the Cecropia Silkworm. Biol. Bull. 1952, 103, 120–138. [Google Scholar] [CrossRef]
- Kawakami, A.; Kataoka, H.; Oka, T.; Mizoguchi, A.; Kimura-Kawakami, M.; Adachi, T.; Iwami, M.; Nagasawa, H.; Suzuki, A.; Ishizaki, H. Molecular cloning of the Bombyx mori prothoracicotropic hormone. Science 1990, 247, 1333–1335. [Google Scholar] [CrossRef]
- Shimell, M.; Pan, X.Y.; Martin, F.A.; Ghosh, A.C.; Leopold, P.; O’Connor, M.B.; Romero, N.M. Prothoracicotropic hormone modulates environmental adaptive plasticity through the control of developmental timing. Development 2018, 145, dev159699. [Google Scholar] [CrossRef] [Green Version]
- Mizoguchi, A.; Ohsumi, S.; Kobayashi, K.; Okamoto, N.; Yamada, N.; Tateishi, K.; Fujimoto, Y.; Kataoka, H. Prothoracicotropic Hormone Acts as a Neuroendocrine Switch between Pupal Diapause and Adult Development. PLoS ONE 2013, 8, e60824. [Google Scholar] [CrossRef] [Green Version]
- Denlinger, D.L.; Yocum, G.D.; Rinehart, J.P. Hormonal Control of Diapause. Compr. Mol. Insect Sci. 2005, 3, 615–650. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Ohashi, Y.; Hosoda, K.; Ishibashi, J.; Kataoka, H. Developmental profile of the changes in the prothoracicotropic hormone titer in hemolymph of the silkworm Bombyx mori: Correlation with ecdysteroid secretion. Insect Biochem. Mol. Biol. 2001, 31, 349–358. [Google Scholar] [CrossRef]
- Žitňan, D.; Kingan, T.G.; Hermesman, J.L.; Adams, M.E. Identification of ecdysis-triggering hormone from an epitracheal endocrine system. Science 1996, 271, 88–91. [Google Scholar] [CrossRef]
- Ewer, J.; Gammie, S.C.; Truman, J.W. Control of insect ecdysis by a positive-feedback endocrine system: Roles of eclosion hormone and ecdysis triggering hormone. J. Exp. Biol. 1997, 200, 869–881. [Google Scholar] [CrossRef]
- Roller, L.; Zitnanova, I.; Dai, L.; Simo, L.; Park, Y.; Satake, H.; Tanaka, Y.; Adams, M.E.; Zitnan, D. Ecdysis triggering hormone signaling in arthropods. Peptides 2010, 31, 429–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krüge, E.; Mena, W.; Lahr, E.C.; Johnson, E.C.; Ewer, J. Genetic analysis of Eclosion hormone action during Drosophila larval ecdysis. Development 2015, 142, 4279–4287. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.M. Photoperiodism and the endocrine aspects of insect diapause. Symp. Soc. Exp. Biol. 1969, 23, 285–300. [Google Scholar] [PubMed]
- Liu, Y.; Li, Y.; Li, X.; Qin, L. The origin and dispersal of the domesticated Chinese oak silkworm, Antheraea pernyi, in China: A reconstruction based on ancient texts. J. Insect Sci. 2010, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.F.; Lei, R.C.; Cheng, Q.J.; Li, Q.S. Breeding of Antheraea pernyi variety Dabai I in bivoltine area in which breeding one generation for a year. North. Seric. 2011, 32, 32–36. [Google Scholar] [CrossRef]
- Huang, L.; Sun, L.Z.; Wang, Y.; Ru, Y.T.; Irfan, M.; Jiang, Y.R.; Shi, S.L.; Yang, R.S.; Li, X.S.; Qin, L. Changes in the expression of trehalose-6-phosphate synthase gene in Antheraea pernyi (Lepidoptera: Saturniidae) during pupal diapause termination. Acta Entomol. Sin. 2016, 59, 938–947. [Google Scholar] [CrossRef]
- Wang, D.Y.; Ru, Y.T.; Wang, Y.; Ma, Y.Y.; Na, S.; Sun, L.Z.; Jiang, Y.R.; Qin, L. Gene expression patterns and activities of trehalases in Antheraea pernyi (Lepidoptera: Saturniidae) pupae during diapause and diapause termination. Acta Entomol. Sin. 2018, 61, 784–794. [Google Scholar] [CrossRef]
- Sun, L.Z.; Wang, G.B.; Zhu, X.W.; Zhang, Y.T.; Jiang, Y.R.; Yang, R.S.; Qin, L. Effect of different light treatments on diapause termination and glycometabolism of Antheraea pernyi pupa. Sci. Seric. 2019, 45, 75–80. [Google Scholar] [CrossRef]
- Sauman, I.; Reppert, S.M. Molecular characterization of prothoracicotropic hormone (PTTH) from the giant silkmoth Antheraea pernyi: Developmental appearance of PTTH-expressing cells and relationship to circadian clock cells in central brain. Dev. Biol. 1996, 178, 418–429. [Google Scholar] [CrossRef]
- Li, X.S.; Wang, G.B.; Sun, Y.; Liu, W.; He, Y.Z.; Wang, F.C.; Jiang, Y.R.; Qin, L. Transcriptome Analysis of the Midgut of the Chinese Oak Silkworm Antheraea pernyi Infected with Antheraea pernyi Nucleopolyhedrovirus. PLoS ONE 2016, 11, e0165959. [Google Scholar] [CrossRef]
- Armenteros, J.J.A.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [Green Version]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Zhang, Q.R.; Denlinger, D.L. Imidazole derivative KK-42 boosts pupal diapause incidence and delays diapause termination in several insect species. J. Insect Sci. 2015, 74, 38–44. [Google Scholar] [CrossRef]
- Takeda, M.; Matsumoto, M.; Tohno, Y. Diapause intensity and ecdysiotroph: Comparisons between two Antheraea species (Lepidoptera: Saturniidae) having summer and winter diapause at pupae. Eur. J. Entomol. 1997, 94, 67–73. [Google Scholar] [CrossRef]
- Marchal, E.; Vandersmissen, H.P.; Badisco, L.; Van de Velde, S.; Verlinden, H.; Iga, M.; Van Wielendaele, P.; Huybrechts, R.; Simonet, G.; Smagghe, G.; et al. Control of ecdysteroidogenesis in prothoracic glands of insects: A review. Peptides 2010, 31, 506–519. [Google Scholar] [CrossRef]
- Warren, J.T.; Petryk, A.; Marques, G.; Jarcho, M.; Parvy, J.P.; Dauphin-Villemant, C.; O’Connor, M.B.; Gilbert, L.I. Molecular and biochemical characterization of two P450 enzymes in the ecdysteroidogenic pathway of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2002, 99, 11043–11048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petryk, A.; Warren, J.T.; Marqués, G.; Jarcho, M.P.; Gilbert, L.I.; Kahler, J.; Parvy, J.; Li, Y.T.; Dauphin-Villemant, C.; O’Connor, M.B. Shade is the Drosophila P450 enzyme that mediates the hydroxylation of ecdysone to the steroid insect molting hormone 20-hydroxyecdysone. Proc. Natl. Acad. Sci. USA 2003, 100, 13773–13778. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.J.; Wei, H.Y.; Hong, G.Y.; Juan, L.; Jiang, S.T.; Luo, J.P. Molecular cloning and tissue expression of the cDNA of the gene encoding eclosion hormone in the Asian corn borer, Ostrinia furnacalis (Lepidoptera: Pyralidae). Acta Entomol. Sin. 2007, 50, 323–329. [Google Scholar] [CrossRef]
- Truman, J.W. The eclosion hormone system of insects. Prog. Brain Res. 1992, 92, 361–374. [Google Scholar] [CrossRef]
- Kono, T.; Nagasawa, H.; Kataoka, H.; Isogai, A.; Fugo, H.; Suzuki, A. Eclosion hormone of the silkworm Bombyx mori. Expression in Escherichia coli and location of disulfide bonds. FEBS Lett. 1990, 263, 358–360. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, H.; Li, J.P.; Lui, A.S.; Kramer, S.J.; Schooley, D.A. Complete structure of eclosion hormone of Manduca sexta. Assignment of disulfide bond location. Int. J. Pept. Protein Res. 1992, 39, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Ye, Q.Y.; Lyu, Z.H.; Lin, T. RNA interference of eclosion hormone gene reveals its roles in the control of ecdysis behavior in Heortia vitessoides Moore. Arch. Insect Biochem. Physiol. 2020, 105, e21726. [Google Scholar] [CrossRef]
- Kingan, T.G.; Adams, M.E. Ecdysteroids regulate secretory competence in Inka cells. J. Exp. Biol. 2000, 203, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Hewes, R.S.; Truman, J.W. The roles of central and peripheral eclosion hormone release in the control of ecdysis behavior in Manduca sexta. J. Comp. Physiol. A 1991, 168, 697–707. [Google Scholar] [CrossRef]
- Meiselman, M.; Lee, S.S.; Tran, R.T.; Dai, H.; Ding, Y.; Rivera-Perez, C.; Wijesekera, T.P.; Dauwalder, B.; Noriega, F.G.; Adams, M.E. Endocrine network essential for reproductive success in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2017, 114, E3849–E3858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagata, S.; Namiki, T.; Ko, R.; Kataoka, H.; Suzuki, A. A novel type of receptor cDNA from the prothoracic glands of the silkworm, Bombyx mori. Biosci. Biotechnol. Biochem. 2006, 70, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Rewitz, K.F.; Yamanaka, N.; Gilbert, L.I.; O’Connor, M.B. The insect neuropeptide PTTH activates receptor tyrosine kinase torso to initiate metamorphosis. Science 2009, 326, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.X.; Chen, D.B.; Zheng, X.X.; Ma, H.F.; Li, Y.P.; Li, Q.; Xia, R.X.; Wang, H.; Jiang, Y.R.; Liu, Y.Q.; et al. Transcriptomic analysis of the prothoracic gland from two lepidopteran insects, domesticated silkmoth Bombyx mori and wild silkmoth Antheraea pernyi. Sci. Rep. 2019, 9, 5313. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Primer Name | Primer Sequence (5′–3′) | For Purpose |
|---|---|---|---|
| ApPTTH | ApPTTH-F | TCCTGGAGGTTCAAACAACACAT | for RT-PCR |
| ApPTTH-R | CTACCAAAAACAAAGCATTAGTATCAT | for RT-PCR | |
| ApETH | ApETH-F | ACGTTTCGAAAGTTTTAATCATGAGT | for RT-PCR |
| ApETH-R | TGTACCGGTTTTCCGAATCATAAT | for RT-PCR | |
| ApEH | ApEH-F | ATGGCCGGCAAGATCTTCG | for qPCR |
| ApEH-R | TTAGAGTTTGTTGAGGAAGGCTGAG | for qPCR | |
| q-ApPTTH | q-ApPTTH-F | ACAATCATCGTGTCTCTTTCCGT | for qPCR |
| q-ApPTTH-R | TTTTCAGCAATCCACCTAAACTTC | for qPCR | |
| q-ApETH | q-ApETH-F | CAACATACTGCCTACTCTGCGTCC | for qPCR |
| q-ApETH-R | CAAAGCCTCGTTGCTCCGTC | for qPCR | |
| q-ApEH | q-ApEH-F | ATCTTCGCTGTTTTCGTTGTTTT | for qPCR |
| q-ApEH-R | TTCCAGAGGATCGTAGCCCAT | for qPCR | |
| q-β-actin | q-β-actin -F | ACCAACTGGGACGACATGGAGAAA | for qPCR |
| q-β-actin-R | TCTCTCTGTTGGCCTTTGGGTTGA | for qPCR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Luo, Y.-T.; Wang, Y.; Wang, D.-Y.; Duan, X.-X.; Zhang, Y.-T.; Bian, Y.-M.; Liu, W.; Qin, L. Expression Patterns of Three Important Hormone Genes and Respiratory Metabolism in Antheraea pernyi during Pupal Diapause under a Long Photoperiod. Insects 2021, 12, 699. https://doi.org/10.3390/insects12080699
Wang Q, Luo Y-T, Wang Y, Wang D-Y, Duan X-X, Zhang Y-T, Bian Y-M, Liu W, Qin L. Expression Patterns of Three Important Hormone Genes and Respiratory Metabolism in Antheraea pernyi during Pupal Diapause under a Long Photoperiod. Insects. 2021; 12(8):699. https://doi.org/10.3390/insects12080699
Chicago/Turabian StyleWang, Qi, Yu-Tong Luo, Yong Wang, De-Yi Wang, Xiao-Xia Duan, Yao-Ting Zhang, Yu-Meng Bian, Wei Liu, and Li Qin. 2021. "Expression Patterns of Three Important Hormone Genes and Respiratory Metabolism in Antheraea pernyi during Pupal Diapause under a Long Photoperiod" Insects 12, no. 8: 699. https://doi.org/10.3390/insects12080699
APA StyleWang, Q., Luo, Y.-T., Wang, Y., Wang, D.-Y., Duan, X.-X., Zhang, Y.-T., Bian, Y.-M., Liu, W., & Qin, L. (2021). Expression Patterns of Three Important Hormone Genes and Respiratory Metabolism in Antheraea pernyi during Pupal Diapause under a Long Photoperiod. Insects, 12(8), 699. https://doi.org/10.3390/insects12080699






