Effects of Delayed Mating on the Reproductive Performance of Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Rearing of Beetles
2.2. Mating Experiments and Oviposition Assessment
2.3. Statistical Analysis
3. Results
3.1. Mating Success
3.2. Pre-Oviposition Period and Oviposition Period
3.3. Longevity of Females and Males
3.4. Reproductive Output and Egg Fertility
3.5. Correlations between the Reproductive Variables and Age at Mating
3.6. Life Tables and Population Growth Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sharma, A.; Thakur, A.; Kaur, S.; Pati, P.K. Effect of Alternaria alternata on the coccinellid pest Henosepilachna vigintioctopunctata and its implications for biological pest management. J. Pest Sci. 2012, 85, 513–518. [Google Scholar] [CrossRef]
- Zhou, L.; Xie, B.G.; Wang, X.P.; Li, C.R.; Wang, W.K. Population dynamic of Henosepilachna vigintioctopunctata in different host plants in Jianghan plain. China J. North. Hortic. 2015, 11, 103–105, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Wang, Z.L.; Li, C.R.; Yuan, J.J.; Li, S.X.; Wang, X.P.; Chi, H. Demographic comparison of Henosepilachna vigintioctopunctata reared on three cultivars of Solanum melongena L. and a wild hostplant Solanum nigrum L. J. Econ. Entomol. 2017, 110, 2084–2091. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K. Population dynamics of a phytophagous lady-beetle, Henosepilachna vigintioctopunctata (Fabricius), living in spatiotemporally heterogeneous habitats. I. Estimation of adult population parameters based on a capture-recapture census. Res. Popul. Ecol. 1985, 27, 159–170. [Google Scholar] [CrossRef]
- Araujo-Siqueira, M.; Almeida, L.M. Behavior and life cycle of Epilachna vigintioctopunctata (Fabricius) (Coleoptera, Coccinellidae) in Lycopersicum esculentum Mill. (Solanaceae). Rev. Bras. Zool. 2004, 21, 543–550. [Google Scholar] [CrossRef]
- Rajagopal, D.; Trivedl, T.P. Status, bioecology and management of Epilachna beetle, Epilachna vigintioctopunctata (Fab.) (Coleoptera: Coccinellidae) on potato in India: A review. Trop. Pest Manag. 1989, 35, 410–413. [Google Scholar] [CrossRef]
- Richards, A.M. The Epilachna vigintioctopunctata complex (Coleoptera: Coccinellidae). Int. J. Entomol. 1983, 25, 11–41. [Google Scholar]
- Richards, A.M.; Filewood, L.W. The effect of agricultural crops and weeds on the bionomics of the pest species comprising the Epilachna vigintioctopunctata complex (Col., Coccinellidae). J. Appl. Entomol. 1988, 105, 88–103. [Google Scholar] [CrossRef]
- Aguiar, R.L.; Furtado, G.M.; Assis, C.H.B.; Mulinario, P.M.; Cofler, T.P.; Holtz, A.M. Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae: Epilachninae) attacking cultivated and wild crops in Brazil. J. Exp. Agr. Int. 2019, 39, 1–4. [Google Scholar] [CrossRef]
- Mohanchander, P.; Reegan, A.D.; Rahman, M.A.; Raveen, R.S. Effect of Hadda beetle, Epilachna vigintioctopunctata Fab. (Coleoptera: Coccinellidae) infestation on eggplant leaf (Solanum melongena L.) and bio-control potential of essential oil formulations. Pak. J. Biol. Sci. 2013, 16, 991–997. [Google Scholar] [CrossRef][Green Version]
- Anam, M.; Ahmed, M.; Haque, M.A. Efficacy of neem oil on the biology and food consumption of epi laichna beetle, Epilachna dodecastigma (Wied.). J. Agr. Rural. Dev. 2006, 4, 132–136. [Google Scholar] [CrossRef]
- Rahaman, M.A.; Prodhan, M.D.H.; Maula, A.K.M. Effect of botanical and synthetic pesticides in controlling Epilachna beetle and the yield of bitter gourd. Int. J. Sustain. Crop. Prod. 2008, 3, 23–26. [Google Scholar]
- Li, Y.X.; Meng, Z.Q.; Liu, D.M.; Su, Z.F.; Song, J.H.; Li, A.L. Produce harm characteristics and control technology of Henosepilachna vigintioctopunctata. China Plant Prot. 2006, 3, 45–47, (In Chinese with English abstract). [Google Scholar]
- Tu, X.Y.; Wang, G.H. Research progress of bio-control of Henosepilachna vigintioctopunctata. China Plant Prot. 2010, 3, 13–16, (In Chinese with English abstract). [Google Scholar]
- Sharma, A.; Saxena, R. Bioactivity of some indigenous plants for the control of hadda beetle, Henosepilachna vigintioctopunctata infesting brinjal. J. Biopest. 2012, 5, 100–106. [Google Scholar]
- Xie, B.G.; Wang, X.P.; Li, C.R.; Wang, W.K. Effect of Starvation on the Survival Duration and Reproductivity and Longevity of Adults Females of Henosepilachna vigintioctopunctata (Fabricius). Int. J. Ecol. 2017, 6, 1–5, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Zhou, L.; Wang, X.P.; Li, C.R.; Gui, L.Y.; Zhang, Y.J. Life table of the laboratory population of Henosepilachna vigintioctopunctata at different temperatures. J. Environ. Entomol. 2014, 36, 494–500, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, H.X.; Wang, Z.L.; Li, C.R.; Wang, X.P. Study on the selectivity of Henosepilachna vigintioctopunctata adults to different host plants. J. Yangtze Univ. Nat. Sci. Ed. 2018, 15, 1–4, (In Chinese with English abstract). [Google Scholar]
- Walton, V.M.; Daane, K.M.; Pringle, K.L. Monitoring Planococcus ficus in South African vineyards with sex pheromone-baited traps. Crop. Prot 2004, 23, 1089–1096. [Google Scholar] [CrossRef]
- Guo, W.C.; Deng, C.S.; Li, G.Q.; Deng, J.Y.; Jiang, W.H.; Wu, J.H.; Wand, P.L.; Tan, W.Z.; Zhang, Q.W. Progress in biological control techniques of Colorado potato beetle in China. Xinjiang Agric. Sci. 2011, 48, 2217–2222, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Zhang, Y.M. Occurrence and integrated control techniques of Henosepilachna vigintioctomaculata in Yulin City. Agric. Eng. Technol. 2018, 38, 32–34, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Sankari, S.A.; Narayanasamy, P. Bioefficacy of flyash- based herbal pesticides against pest of rice and vegetable. Curr. Sci. India 2007, 92, 811–816. Available online: https://www.jstor.org/stable/24097813 (accessed on 25 March 2007).
- Lü, J.; Liu, Z.Q.; Guo, W.; Guo, M.J.; Chen, S.M.; Yang, C.X.; Zhang, Y.J.; Pan, H.P. Oral delivery of dsHvlwr is a feasible method for managing the pest Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae). Insect Sci. 2020, 28, 509–521. [Google Scholar] [CrossRef]
- Daane, K.; Bentley, W.; Walton, V.; Malakar-Kuenen, R.; Millar, J. New controls investigated for vine mealybug. Calif. Agr. 2006, 60, 31–38. [Google Scholar] [CrossRef]
- Lentini, A.; Mura, A.; Muscas, E.; Nuvoli, M.T.; Cocco, A. Effects of delayed mating on the reproductive biology of the vine mealybug, Planococcus ficus (Hemiptera: Pseudococcidae). Bull. Entomol. Res. 2018, 108, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Walton, V.M.; Daane, K.M.; Walter, J.; Bentley, W.J.; Millar, J.G.; Larsen, T.E.; Malakar-Kuenen, R. Pheromone based mating disruption of Planococcus ficus (Hemiptera: Pseudococcidae) in California vineyards. J. Econ. Entomol. 2006, 99, 1280–1290. [Google Scholar] [CrossRef]
- Amoah, B.A.; Mahroof, R.M.; Gerken, A.R.; Campbell, J.F. Effect of Delayed Mating on Longevity and Reproductive Performance of Lasioderma serricorne (Coleoptera: Anobiidae). J. Econ. Entomol. 2019, 112, 1011–1031. [Google Scholar] [CrossRef]
- Mahroof, R.M.; Phillips, T.W. Orientation of the Cigarette Beetle, Lasioderma serricorne (F.) (Coleoptera: Anobiidae) to Plant-Derived Volatiles. J. Insect Behav. 2007, 20, 99–115. [Google Scholar] [CrossRef]
- Mahroof, R.M.; Phillips, T.W. Mating disruption of Lasioderma serricorne (Coleoptera: Anobiidae) in stored product habitats using the synthetic pheromone serricornin. J. Appl. Entomol. 2014, 138, 378–386. [Google Scholar] [CrossRef]
- Yu, C. Susceptibility of Lasioderma serricorne (F.) Life Stages Exposed to Elevated Temperatures. Master’s Thesis, Kansas State University, Kansas, NY, USA, 2008. Available online: http://hdl.handle.net/2097/945 (accessed on 15 August 2008).
- Martin, A.M.; Festa-Bianchet, M.; Coltman, D.W.; Pelletier, F. Demographic drivers of age-dependent sexual selection. J. Evol. Biol. 2016, 29, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.D. The causes and evolutionary consequences of variation in female mate choice in insects: The effects of individual state, genotypes and environments. Curr. Opin. Insect Sci. 2018, 27, 1–8. [Google Scholar] [CrossRef]
- Hata, T.; Nishide, Y.; Tanaka, S.; Yasui1, H.; Fujiwara-Tsujii1, N.; Yasue, H.; Wakamura, S.; Nagayama, A.; Arakaki, N. Managing the white grub beetle Dasylepida ishigakiensis (Coleoptera: Scarabaeidae) in sugarcane fields on Miyako Island, Japan, using sex attractant Pheromone: Effects of mating delay on the reproductive ability of laboratory-reared and field-collected females. Int. J. Trop. Insect Sci. 2014, 34, 32–40. [Google Scholar] [CrossRef]
- Omkar; Pooja, P.; Shruti, R.; Geetanjali, M. Influence of age at mating on the reproductive performance of Parthenium beetle, Zygogramma bicolorata (Coleoptera: Chrysomelidae). Insect Sci. 2010, 17, 112–120. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Averill, A.L. Effects of delayed mating on reproductive output of female oriental beetle Anomala orientalis (Coleoptera: Scarabaeidae). Agr. For. Entomol. 2006, 8, 221–231. [Google Scholar] [CrossRef]
- Gerken, A.R.; Campbell, J.F. Life history changes in Trogoderma variabile and T. inclusum due to mating delay with implications for mating disruption as a management tactic. Ecol. Evol. 2018, 8, 2428–2439. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Averill, A.L. Mating disruption of oriental beetle (Coleoptera: Scarabaeidae) in cranberry using retrievable, point-source dispensers of sex pheromone. Environ. Entomol. 2006, 35, 458–464. [Google Scholar] [CrossRef]
- Unnithan, G.C.; Paye, S.O. Factors involved in mating, longevity, fecundity and egg fertility in the maize stem-borer, Busseola fusca (Fuller) (Lep., Noctuidae). J. Appl. Entomol. 1990, 109, 295–301. [Google Scholar] [CrossRef]
- Kruger, O. Age at first breeding and fitness in goshawk Accipiter gentilis. J. Anim. Ecol. 2005, 74, 266–273. [Google Scholar] [CrossRef]
- Saxena, S.; Mishra, G.; Omkar. Operational sex ratio and paternal age sway mating and reproductive performance in Menochilus sexmaculatus (Coleoptera: Coccinellidae). Can. Entomol. 2020, 152, 298–310. [Google Scholar] [CrossRef]
- İşci, M.; Atasay, A.; Kaymak, S. The efficacy of mating disruption against codling moth (Cydia pomonella (L.) Lep.: Tortricidae) under Isparta conditions. Fruit Sci. 2016, 3, 17–21. [Google Scholar]
- Athanassiou, C.G.; Kavallieratos, N.G.; Sciarretta, A.; Trematerra, P. Mating disruption of Ephestia kuehniella (Zeller) (Lepidoptera: Pyralidae) in a storage facility: Spatio-temporal distribution changed after long-term application. J. Stored Prod. Res. 2016, 67, 1–12. [Google Scholar] [CrossRef]
- Wu, C.X.; Liu, J.F.; Di, X.Y.; Yang, M.F. Delay in mating reduces reproductivity but increases life span in tobacco cutworm, Spodoptera litura Fabricius (Lepidoptera: Noctuidae). J. Econ. Entomol. 2018, 111, 1650–1657. [Google Scholar] [CrossRef]
- Edward, D.A.; Chapman, T. The evolution and significance of male mate choice. Trends Ecol. Evol. 2011, 26, 647–654. [Google Scholar] [CrossRef]
- Kaufmann, E.; Otti, O. Males increase their fitness by choosing large females in the common bedbug Cimex lectularius. Anim. Biol. 2018, 69, 17–32. [Google Scholar] [CrossRef]
- Puurtinen, M.; Fromhage, L. Evolution of male and female choice in polyandrous systems. Proc. R. Soc. B Biol. Sci. 2017, 284, 1851. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Holman, L. Evolution of female choice under intralocus sexual conflict and genotype-by-environment interactions. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 1757. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.M.; Troubridge, J.T.; Maurice, C. Pheromone released from polyvinyl chloride dispensers disrupts mate-fifinding and pheromone source location by Rhopobota naevana (Lepidoptera: Tortricidae) in cranberries. Can. Entomol. 2004, 136, 91–108. [Google Scholar] [CrossRef]
- Fadamiro, H.Y.; Baker, T.C. Pheromone puffs suppress mating by Plodia interpunctella and Sitotroga cerealella in an infested corn store. Entomol. Exp. Appl. 2002, 102, 239–251. [Google Scholar] [CrossRef]
- Fitzpatrick, S.M. Delayed mating reduces fecundity of blackheaded fireworm, Rhopobota naevana, on cranberry. Entomol. Exp. Appl. 2006, 120, 245–250. [Google Scholar] [CrossRef]
- Kawazu, K.; Shintani, Y.; Tatsuki, S. Effect of increased male and female age at mating on the reproductive performance of Cnaphalocrocis medinalis (Crambidae: Lepidoptera). J. Econ. Entomol. 2014, 107, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.P.; Wiman, N.G.; Brunner, J.F. Comparison of delayed female mating on reproductive biology of codling moth and obliquebanded leafroller. Environ. Entomol. 2008, 37, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Angeli, G.; Rama, F.; Forti, D.; Montà, L.D.; Bellinazzo, S. Control of Cydia pomonella in walnuts by mating disruption. IOBC/WPRS Bull. 1999, 22, 83–89. [Google Scholar]
- Calkins, C.O.; Faust, R.J. Overview of areawide programs and the program for suppression of codling moth in the western USA directed by the United States Department of Agriculture?Agricultural Research Service. Pest Manag. Sci. 2003, 59, 601–604. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
- Carey, J.R. Applied Demography for Biologists with Special Emphasis on Insects; Oxford University Press: New York, NY, USA, 1993. [Google Scholar]
- Maia, H.N.M.; Luiz, A.J.; Campanhola, C. Statistical inference on associated fertility life table parameters using jackknife technique: Computational aspects. J. Econ. Entomol. 2000, 93, 511–518. [Google Scholar] [CrossRef]
- Omkar; Singh, S.K.; Singh, K. Effect of age on reproductive attributes of an aphidophagous ladybird, Cheilomenes sexmaculata. Insect Sci. 2006, 13, 301–308. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Wang, X. Effects of delayed mating on the reproductive performance and longevity of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Neotrop. Entomol. 2021. [Google Scholar] [CrossRef]
- Srivastava, S.; Omkar. Age specific mating and reproductive senescence in seven spotted ladybird, Coccinella septempunctata. J. Appl. Entomol. 2004, 128, 452–458. [Google Scholar] [CrossRef]
- Wang, X.P.; Fang, Y.L.; Zhang, Z.N. Effects of delayed mating on the fecundity, fertility and longevity of females of diamondback moth, Plutella xylostella. Insect Sci. 2011, 18, 305–310. [Google Scholar] [CrossRef]
- Song, W.; Liu, L.; Li, P.; Sun, H.; Qin, Y. Analysis of the mating and reproductive traits of Plutella xylostella (Lepidoptera: Plutellidae). J. Insect Sci. 2014, 14, 267. [Google Scholar] [CrossRef]
- Singh, K.; Omkar. Effect of parental ageing on offspring developmental and survival attributes in an aphidophagous ladybird, Cheilomenes sexmaculata. J. Appl. Entomol. 2009, 133, 500–504. [Google Scholar] [CrossRef]
- Simmons, L.W. Sperm Competition and Its Evolutionary Consequences in the Insects, 3rd ed.; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Himuro, H.; Fujisaki, K. Effects of mating duration on female reproductive traits of the seed bug Togo hemipterus (Heteroptera: Lygaeidae). Appl. Entomol. Zool. 2015, 50, 491–496. [Google Scholar] [CrossRef]
- Eberhard, W.G.; Cordero, C. Sexual selection by cryptic female choice on male seminal products—A new bridge between sexual selection and reproductive physiology. Trends Ecol. Evol. 1995, 10, 493–496. [Google Scholar] [CrossRef]
- Sharon, R.; Zahavi, T.; Sokolsky, T.; Sofer-Arad, C.; Tomer, M.; Kedoshim, R.; Harari, A.R. Mating disruption method against the vine mealybug, Planococcus Ficus: Effect of sequential treatment on infested vines. Entomol. Exp. Appl. 2016, 161, 65–69. [Google Scholar] [CrossRef]
- Cotter, S.C.; Ward, R.J.S.; Kilner, R.M. Age-specific reproductive investment in female burying beetles: Independent effects of state and risk of death. Funct. Ecol. 2010, 25, 652–660. [Google Scholar] [CrossRef]
- Fox, C.W.; Bush, M.L.; Wallin, W.G. Maternal age affects offspring lifespan of the seed beetle, Callosobruchus maculatus. Funct. Ecol. 2003, 17, 811–820. [Google Scholar] [CrossRef]
- Mbata, N.G. Some physical and biological factors affecting oviposition by Plodia interpunctella (Hübner) (Lepidoptera: Phycitidae). Insect Sci. Appl. 1985, 6, 597–604. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Q. Male moths undertake both pre-and in-copulation mate choice based on female age and weight. Behav. Ecol. Sociobiol. 2009, 63, 801–808. [Google Scholar] [CrossRef]
- Jones, V.P.; Aihara-Sasaki, M. Demographic analysis of delayed mating in mating Disruption: A case study with Cryptophlebia illepida (Lepidoptera: Tortricidae). J. Econ. Entomol. 2001, 94, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.I.; Ramaswamy, S.B.; Srinivasas, A. Spermatophore formation and regulation of egg maturation and oviposition in female Heliothis virescens by the male. J. Insect Physiol. 1998, 44, 903–908. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, J.; Wu, X.; Peng, L.; Liu, X. A meta analysis of the effect of delayed mating on female reproductive fitness in moths. Acta Agric. Univ. Jiangxiensis 2016, 38, 113–123, (In Chinese with English abstract). [Google Scholar]
- Dhillon, M.K.; Tanwar, A.K.; Hasan, F. Fitness consequences of delayed mating on reproductive performance of Chilo partellus (Swinhoe). J. Exp. Zool. 2018, 1–7. [Google Scholar] [CrossRef]
- Mishra, G.; Omkar. Influence of parental age on reproductive performance of an aphidophagous ladybird, Propylea dissecta (Mulsant). J. Appl. Entomol. 2004, 128, 605–609. [Google Scholar] [CrossRef]
- Pervez, A.; Omkar; Richmond, A.S. The influence of age on reproductive performance of a predatory ladybird beetle, Propylea dissecta. J. Insect Sci. 2004, 4, 1–8. [Google Scholar] [CrossRef][Green Version]
- Bell, W.J.; Bohm, M.K. Oosorption in insects. Biol. Rev. 1975, 50, 373–396. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.J.; Sharma, S. A delay in age at first mating results in the loss of future reproductive potential via apoptosis. Evol. Dev. 2005, 7, 216–222. [Google Scholar] [CrossRef]
- Maklakov, A.A.; Kremer, N.; Arnqvist, G. The effects of age at mating on female life-history traits in a seed beetle. Behav. Ecol. 2007, 18, 551–555. [Google Scholar] [CrossRef]
- Jiao, X.; Xuan, W.; Sheng, C. Effects of delayed mating and male mating history on longevity and reproductive performance of the rice stem borer, Chilo suppressalis (Walker) (Lep., Pyralidae). J. Appl. Entomol. 2006, 130, 108–112. [Google Scholar] [CrossRef]
- Burke, N.W.; Bonduriansky, R. The fitness effects of delayed switching to sex in a facultatively asexual insect. Ecol. Evol. 2018, 8, 2698–2711. [Google Scholar] [CrossRef]
- Bakker, A.C.; Campos Louçã, J.; Roessingh, P.; Menken, S.B.J. The cost of mating: Influences of life history traits and mating strategies on lifespan in two closely related Yponomeuta species. Int. J. Zool. 2011, 2011, 1–8. [Google Scholar] [CrossRef][Green Version]
- Mahroof, R.; Amoah, B.; Gerken, A.; Campbell, J. Effect of delayed mating on reproductive performance of Lasioderma serricorne (F.) (Coleoptera: Anobiidae). Julius-Kühn-Archiv 2018, 463, 117–122. [Google Scholar] [CrossRef]
- Proshold, F.I.; Karpenko, C.P.; Graham, C.K. Egg production and oviposition in the tobacco Budworm: Effect of age at mating. Ann. Entomol. Soc. Am. 1982, 75, 51–55. [Google Scholar] [CrossRef]
- Nischala, A.; Hari, P.K.V. Construction of life table parameters of cigarette beetle, Lasioderma serricorne on different varieties of dry turmeric. J. Entomol. Zool. Stud. 2017, 5, 118–124. [Google Scholar]
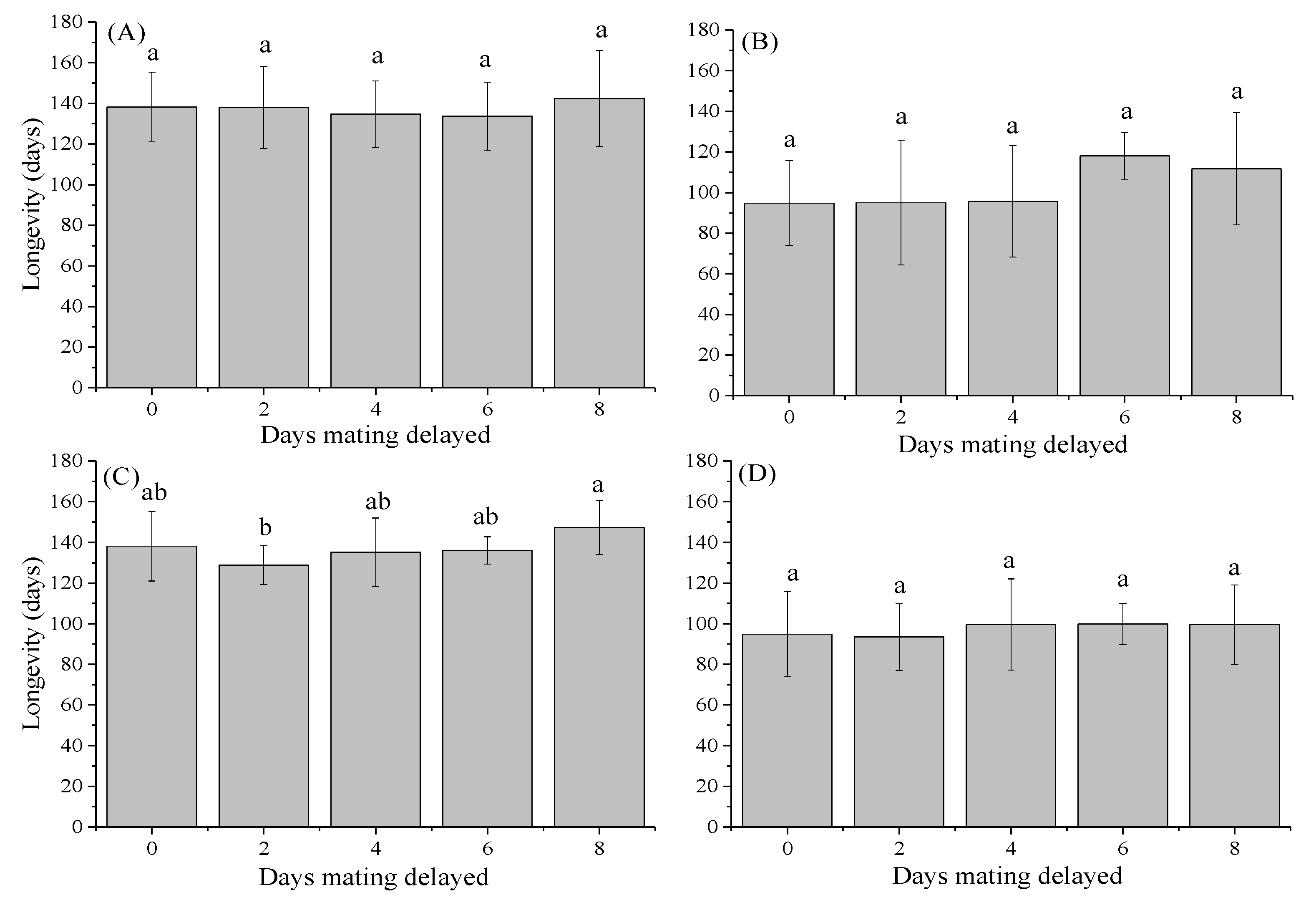
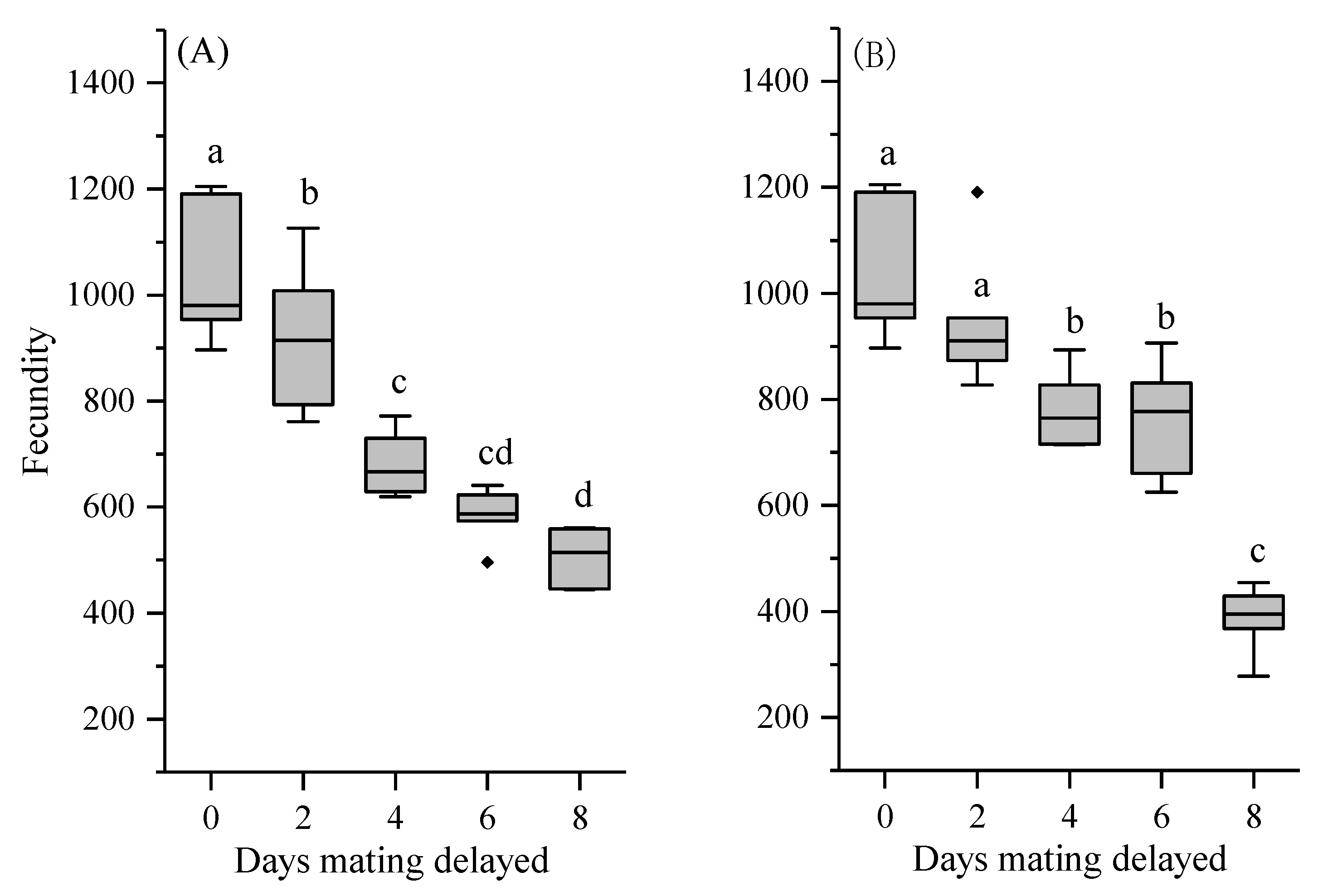
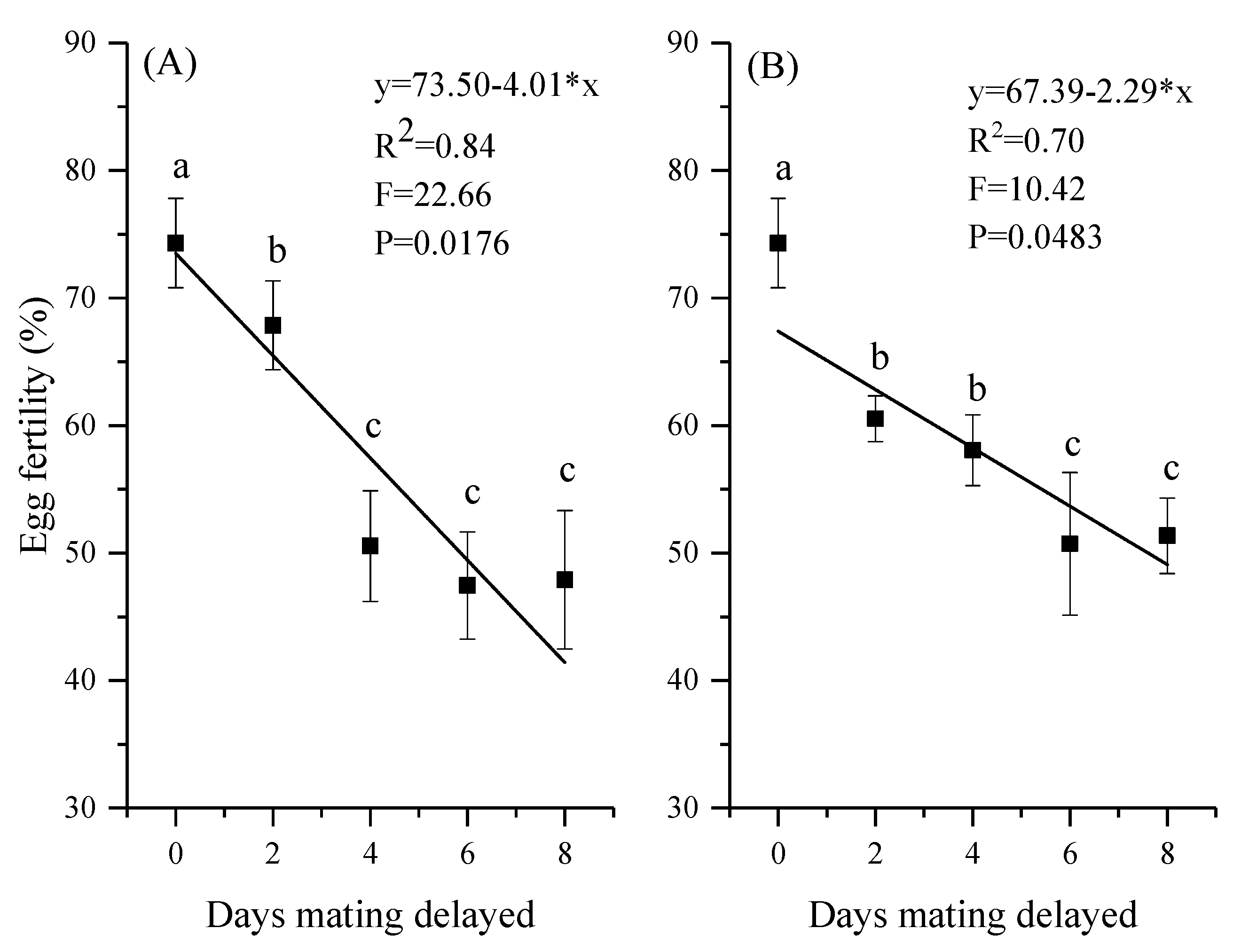
| Days Mating Delayed | Mating Success Ratio (%) | Pre-Oviposition Period (Days) | Oviposition Period (Days) | |||
|---|---|---|---|---|---|---|
| Female-Delayed Mating | Male-Delayed Mating | Female-Delayed Mating | Male-Delayed Mating | Female-Delayed Mating | Male-Delayed Mating | |
| 0 | 75.00 | 75.00 | 13.17 ± 1.17 a | 13.17 ± 1.17 a | 94.33 ± 13.82 a | 94.33 ± 13.82 a |
| 2 | 66.67 | 60.00 | 5.83 ± 1.94 b | 7.33 ± 0.82 b | 75.83 ± 6.74 b | 81.67 ± 8.17 b |
| 4 | 54.55 | 66.67 | 5.83 ± 1.47 b | 5.83 ± 0.75 c | 66.00 ± 7.46 b | 72.00 ± 6.69 bc |
| 6 | 60.00 | 46.15 | 6.00 ± 0.89 b | 5.50 ± 0.84 c | 64.33 ± 6.98 b | 67.50 ± 6.66 c |
| 8 | 50.00 | 42.86 | 4.50 ± 2.74 b | 5.33 ± 0.52 c | 45.33 ± 23.43 c | 65.33 ± 7.06 c |
| Variable | Correlation Coefficient | |
|---|---|---|
| Female-Delayed Mating Days | Male-Delayed Mating Days | |
| Mating success | −0.9058 * | −0.9096 * |
| Pre-oviposition period | −0.7834 * | −0.8381 * |
| Oviposition period | −0.9682 * | −0.9558 * |
| Fecundity | −0.9810 * | −0.9403 * |
| Hatch rate Female longevity Male longevity | −0.9217 * 0.6023 0.8118 | −0.9228 * 0.1848 0.8142 |
| Days Mating Delayed | Net Reproductive Rate (R0) | Mean Generation Time (T) | Intrinsic Rate of Increase (rm) | Doubling Time (D) | Finite Rate of Increase (λ) |
|---|---|---|---|---|---|
| 0 | 463.097 ± 25.704 a | 156.167 ± 7.012 ab | 0.0397 ± 0.0020 a | 17.686 ± 0.8983 c | 1.0405 ± 0.0021 a |
| 2 | 331.696 ± 21.236 b | 146.833 ± 3.877 b | 0.0396 ± 0.0014 a | 17.589 ± 0.5643 c | 1.0404 ± 0.0014 a |
| 4 | 149.382 ± 8.917 c | 153.167 ± 6.887 ab | 0.0331 ± 0.0022 b | 21.299 ± 1.1324 b | 1.0337 ± 0.0023 b |
| 6 | 131.615 ± 7.116 cd | 154.00 ± 2.745 ab | 0.0317 ± 0.0003 bc | 21.905 ± 0.2342 b | 1.0322 ± 0.0003 bc |
| 8 | 95.620 ± 5.914 d | 165.333 ± 5.426 a | 0.0277 ± 0.0010 c | 25.200 ± 0.8348 a | 1.0281 ± 0.0010 c |
| Correlation coefficient | −0.9406 * | 0.6025 | −0.9663 * | 0.9575 * | −0.9666 * |
| Days Mating Delayed | Net Reproductive Rate (R0) | Mean Generation Time (T) | Intrinsic Rate of Increase (rm) | Doubling Time (D) | Finite Rate of Increase (λ) |
|---|---|---|---|---|---|
| 0 | 463.097 ± 25.704 a | 156.167 ± 7.012 a | 0.0397 ± 0.0020 a | 17.685 ± 0.8981 b | 1.0405 ± 0.0021 a |
| 2 | 282.662 ± 15.758 b | 156.000 ± 8.266 a | 0.0367 ± 0.0020 ab | 19.188 ± 1.039 b | 1.0374 ± 0.0021 ab |
| 4 | 247.203 ± 8.569 b | 152.667 ± 6.682 a | 0.0365 ± 0.0018 ab | 19.233 ± 0.907 b | 1.0372 ± 0.0019 ab |
| 6 | 148.633 ± 9.897 c | 151.667 ± 6.800 a | 0.0333 ± 0.0019 b | 21.122 ± 1.104 b | 1.0339 ± 0.0019 b |
| 8 | 67.606 ± 4.235 d | 160.333 ± 9.649 a | 0.0269 ± 0.0022 c | 26.527 ± 1.811 a | 1.0272 ± 0.0022 c |
| Correlation coefficient | −0.9767 * | 0.1851 | −0.9408 * | 0.8987 * | −0.9397 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-L.; Jin, Q.-N.; Wang, X.-P. Effects of Delayed Mating on the Reproductive Performance of Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae). Insects 2021, 12, 629. https://doi.org/10.3390/insects12070629
Wang Y-L, Jin Q-N, Wang X-P. Effects of Delayed Mating on the Reproductive Performance of Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae). Insects. 2021; 12(7):629. https://doi.org/10.3390/insects12070629
Chicago/Turabian StyleWang, Ya-Ling, Qi-Nian Jin, and Xiang-Ping Wang. 2021. "Effects of Delayed Mating on the Reproductive Performance of Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae)" Insects 12, no. 7: 629. https://doi.org/10.3390/insects12070629
APA StyleWang, Y.-L., Jin, Q.-N., & Wang, X.-P. (2021). Effects of Delayed Mating on the Reproductive Performance of Henosepilachna vigintioctopunctata (F.) (Coleoptera: Coccinellidae). Insects, 12(7), 629. https://doi.org/10.3390/insects12070629





