Fight or Flight? Alternative Defense of the Pea Aphids, Acyrthosiphon pisum on Different Host Plants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Pea Aphids Rearing
2.2. Infection of Pea Aphids with Bacteria Staphylococcus aureus and Escherichia coli
2.3. Infection of Pea Aphids with Fungus Beauveria bassiana
2.4. Statistical Analysis
3. Results
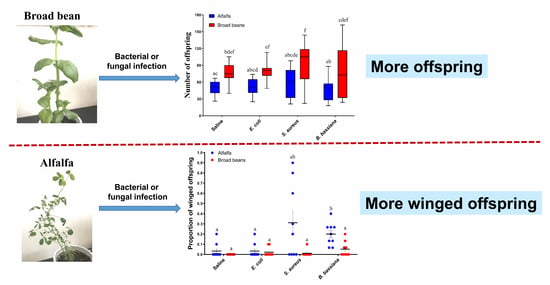
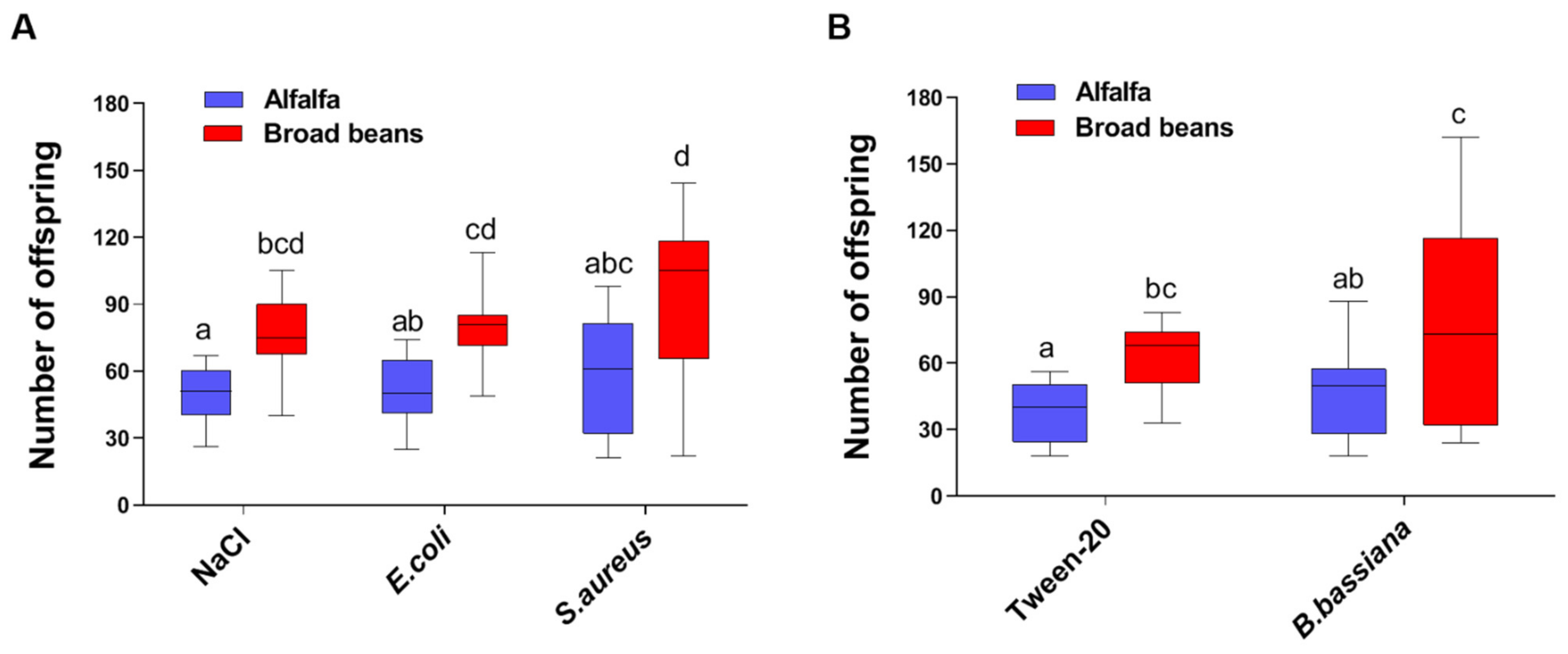
3.1. Host Plants Affect the Fecundity of the Pea Aphids in Both Infected and Uninfected Conditions
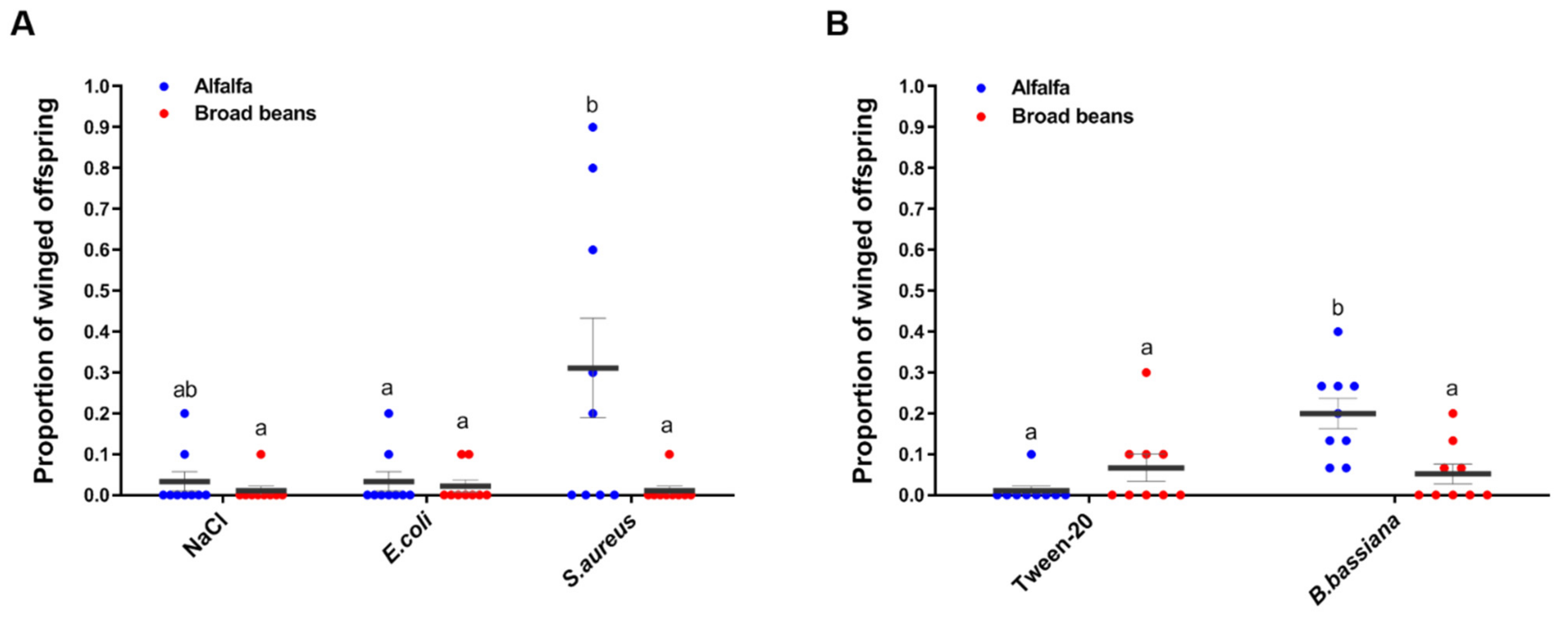
3.2. Pea Aphids Produce Winged Offspring in Response to Pathogens Differently between Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Nappi, A.J.; Ottaviani, E. Cytotoxicity and cytotoxic molecules in invertebrates. BioEssays 2000, 22, 469–480. [Google Scholar] [CrossRef]
- Hanson, M.A.; Lemaitre, B. New insights on Drosophila antimicrobial peptide function in host defense and beyond. Curr. Opin. Immunol. 2020, 62, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Imler, J.L. Overview of Drosophila immunity: A historical perspective. Dev. Comp. Immunol. 2014, 42, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Barribeau, S.M.; Laughton, A.M.; De Roode, J.C.; Gerardo, N.M. Non-immunological defense in an evolutionary framework. Trends Ecol. Evol. 2011, 26, 242–248. [Google Scholar] [CrossRef]
- Elsik, C.G. The pea aphid genome sequence brings theories of insect defense into question. Genome Biol. 2010, 11, 106. [Google Scholar] [CrossRef] [Green Version]
- Hendry, T.A.; Ligon, R.A.; Besler, K.R.; Fay, R.L.; Smee, M.R. Visual Detection and Avoidance of Pathogenic Bacteria by Aphids. Curr. Biol. 2018, 28, 3158–3164.e4. [Google Scholar] [CrossRef] [Green Version]
- Altincicek, B.; Gross, J.; Vilcinskas, A. Wounding-mediated gene expression and accelerated viviparous re-production of the pea aphid Acyrthosiphon pisum. Insect Mol. Biol. 2008, 17, 711–716. [Google Scholar] [CrossRef]
- Barribeau, S.M.; Parker, B.J.; Gerardo, N.M. Exposure to natural pathogens reveals costly aphid response to fungi but not bacteria. Ecol. Evol. 2014, 4, 488–493. [Google Scholar] [CrossRef]
- Hendry, T.A.; Clark, K.J.; Baltrus, D.A. A highly infective plant-associated bacterium influences reproductive rates in pea aphids. R. Soc. Open Sci. 2016, 3, 150478. [Google Scholar] [CrossRef] [Green Version]
- Weisser, W.W.; Braendle, C.; Minoretti, N. Predator-induced morphological shift in the pea aphid. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1999, 266, 1175–1181. [Google Scholar] [CrossRef] [Green Version]
- Hatano, E.; Baverstock, J.; Kunert, G.; Pell, J.K.; Weisser, W.W. Entomopathogenic fungi stimulate transgen-erational wing induction in pea aphids, Acyrthosiphon pisum (Hemiptera: Aphididae). Ecol. Entomol. 2012, 37, 75–82. [Google Scholar] [CrossRef]
- Ter Braak, B.; Laughton, A.M.; Altincicek, B.; Parker, B.J.; Gerardo, N.M. Exposure to Bacterial Signals Does Not Alter Pea Aphids’ Survival upon a Second Challenge or Investment in Production of Winged Offspring. PLoS ONE 2013, 8, e73600. [Google Scholar] [CrossRef]
- Parker, B.J.; Barribeau, S.M.; Laughton, A.M.; Griffin, L.H.; Gerardo, N.M. Life-history strategy determines constraints on immune function. J. Anim. Ecol. 2017, 86, 473–483. [Google Scholar] [CrossRef] [Green Version]
- Braendle, C.; Miura, T.; Bickel, R.; Shingleton, A.W.; Kambhampati, S.; Stern, D.L. Developmental Origin and Evolution of Bacteriocytes in the Aphid-Buchnera Symbiosis. PLoS Biol. 2003, 1, e21. [Google Scholar] [CrossRef]
- Martínez, A.; Costamagna, A. Effects of crowding and host plant quality on morph determination in the soybean aphid, Aphis glycines. Èntomol. Exp. Appl. 2018, 166, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.; Williams, I.; Hardie, J. The role of nutrition, crowding and interspecific interactions in the development of winged aphids. Ecol. Entomol. 2001, 26, 330–340. [Google Scholar] [CrossRef]
- Schaefers, G.A.; Judge, F.D. Effects of temperature, photoperiod, and host plant on alary polymorphism in the aphid, Chaetosiphon fragaefolii. J. Insect Physiol. 1971, 17, 365–379. [Google Scholar] [CrossRef]
- Sentis, A.; Bertram, R.; Dardenne, N.; Ramon-Portugal, F.; Louit, I.; Le Trionnaire, G.; Simon, J.-C.; Magro, A.; Pujol, B.; Hemptinne, J.-L.; et al. Different phenotypic plastic responses to predators observed among aphid lineages specialized on different host plants. Sci. Rep. 2019, 9, 9017. [Google Scholar] [CrossRef]
- Xu, L.; Ma, L.; Wang, W.; Li, L.; Lu, Z. Phenoloxidases are required for the pea aphid’s defence against bacterial and fungal infection. Insect Mol. Biol. 2019, 28, 176–186. [Google Scholar] [CrossRef]
- Reyes, M.L.; Laughton, A.M.; Parker, B.J.; Wichmann, H.; Fan, M.; Sok, D.; Hrček, J.; Acevedo, T.; Gerardo, N.M. The influence of symbiotic bacteria on reproductive strategies and wing polyphenism in pea aphids responding to stress. J. Anim. Ecol. 2019, 88, 601–611. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, R.; Kotze, J. Do not log-transform count data. Methods Ecol. Evol. 2010, 1, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Heilbron, D.C. Zero-Altered and other Regression Models for Count Data with Added Zeros. Biom. J. 1994, 36, 531–547. [Google Scholar] [CrossRef]
- Tu, W. Zero-Inflated Data. In Encyclopedia of Environmetrics; Wiley: Hoboken, NJ, USA, 2006; Volume 4, pp. 2387–2391. [Google Scholar]
- Zeileis, A.; Kleiber, C.; Jackman, S. Regression Models for Count Data in R. J. Stat. Softw. 2008, 1, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; SAGE Publications: Thousand Oaks, CA, USA, 2018; p. 608. [Google Scholar]
- Ferrari, J.; West, J.A.; Via, S.; Godfray, H.C. Population genetic structure and secondary symbionts in host associated populations of the pea aphid complex. Evolution 2012, 66, 375–390. [Google Scholar] [CrossRef]
- Fukatsu, T.; Nikoh, N. Two intracellular symbiotic bacteria from the mulberry psyllid Anomoneura mori (Insecta, Homoptera). Appl. Environ. Microbiol. 1998, 64, 3599–3606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuchida, T.; Koga, R.; Horikawa, M.; Tsunoda, T.; Maoka, T.; Matsumoto, S.; Simon, J.-C.; Fukatsu, T. Symbiotic Bacterium Modifies Aphid Body Color. Science 2010, 330, 1102. [Google Scholar] [CrossRef]
- Tsuchida, T.; Koga, R.; Shibao, H.; Matsumoto, T.; Fukatsu, T. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 2002, 11, 2123–2135. [Google Scholar] [CrossRef]
- Baverstock, J.; Roy, H.E.; Clark, S.J.; Alderson, P.G.; Pell, J.K. Effect of fungal infection on the reproductive potential of aphids and their progeny. J. Invertebr. Pathol. 2006, 91, 136–139. [Google Scholar] [CrossRef]
- Tan, W.H.; Reyes, M.L.; Hoang, K.L.; Acevedo, T.; Leon, F.; Barbosa, J.D.; Gerardo, N.M. How symbiosis and ecological context influence the variable expression of transgenerational wing induction upon fungal infection of aphids. PLoS ONE 2018, 13, e0201865. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Xu, Y.; Jiang, J.; Lavine, M.; Lavine, L.C. Host quality induces phenotypic plasticity in a wing poly-phenic insect. Proc. Natl. Acad. Sci. USA 2018, 115, 7563. [Google Scholar] [CrossRef] [Green Version]
- Shang, F.; Niu, J.; Ding, B.-Y.; Zhang, W.; Wei, D.-D.; Wei, D.; Jiang, H.-B.; Wang, J.-J. The miR-9b microRNA mediates dimorphism and development of wing in aphids. Proc. Natl. Acad. Sci. USA 2020, 117, 8404–8409. [Google Scholar] [CrossRef]
- Tabadkani, S.M.; Ahsaei, S.M.; Hosseininaveh, V.; Nozari, J. Food stress prompts dispersal behavior in apterous pea aphids: Do activated aphids incur energy loss? Physiol. Behav. 2013, 110, 221–225. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, M.J.; Li, Y.; Ma, L.; Feng, Y.; Lu, Z. Fight or Flight? Alternative Defense of the Pea Aphids, Acyrthosiphon pisum on Different Host Plants. Insects 2021, 12, 614. https://doi.org/10.3390/insects12070614
Martin MJ, Li Y, Ma L, Feng Y, Lu Z. Fight or Flight? Alternative Defense of the Pea Aphids, Acyrthosiphon pisum on Different Host Plants. Insects. 2021; 12(7):614. https://doi.org/10.3390/insects12070614
Chicago/Turabian StyleMartin, Martin John, Yueming Li, Li Ma, Yi Feng, and Zhiqiang Lu. 2021. "Fight or Flight? Alternative Defense of the Pea Aphids, Acyrthosiphon pisum on Different Host Plants" Insects 12, no. 7: 614. https://doi.org/10.3390/insects12070614
APA StyleMartin, M. J., Li, Y., Ma, L., Feng, Y., & Lu, Z. (2021). Fight or Flight? Alternative Defense of the Pea Aphids, Acyrthosiphon pisum on Different Host Plants. Insects, 12(7), 614. https://doi.org/10.3390/insects12070614