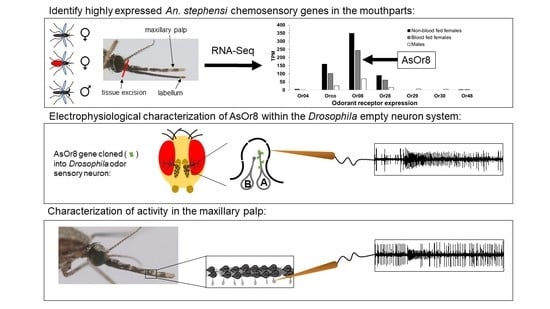
Characterization of Anopheles stephensi Odorant Receptor 8, an Abundant Component of the Mouthpart Chemosensory Transcriptome
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Rearing
2.2. An. stephensi Mouthpart Transcriptome
2.2.1. RNA Sample Collection and Sequencing
2.2.2. Quality Control of Raw Reads
2.3. Electrophysiological AsOR8 Characterization in the Drosophila Empty Neuron System
2.3.1. Cloning
2.3.2. Drosophila Stocks and Transgenes
2.3.3. Electrophysiology
2.4. Odorant Panel Selection
3. Results
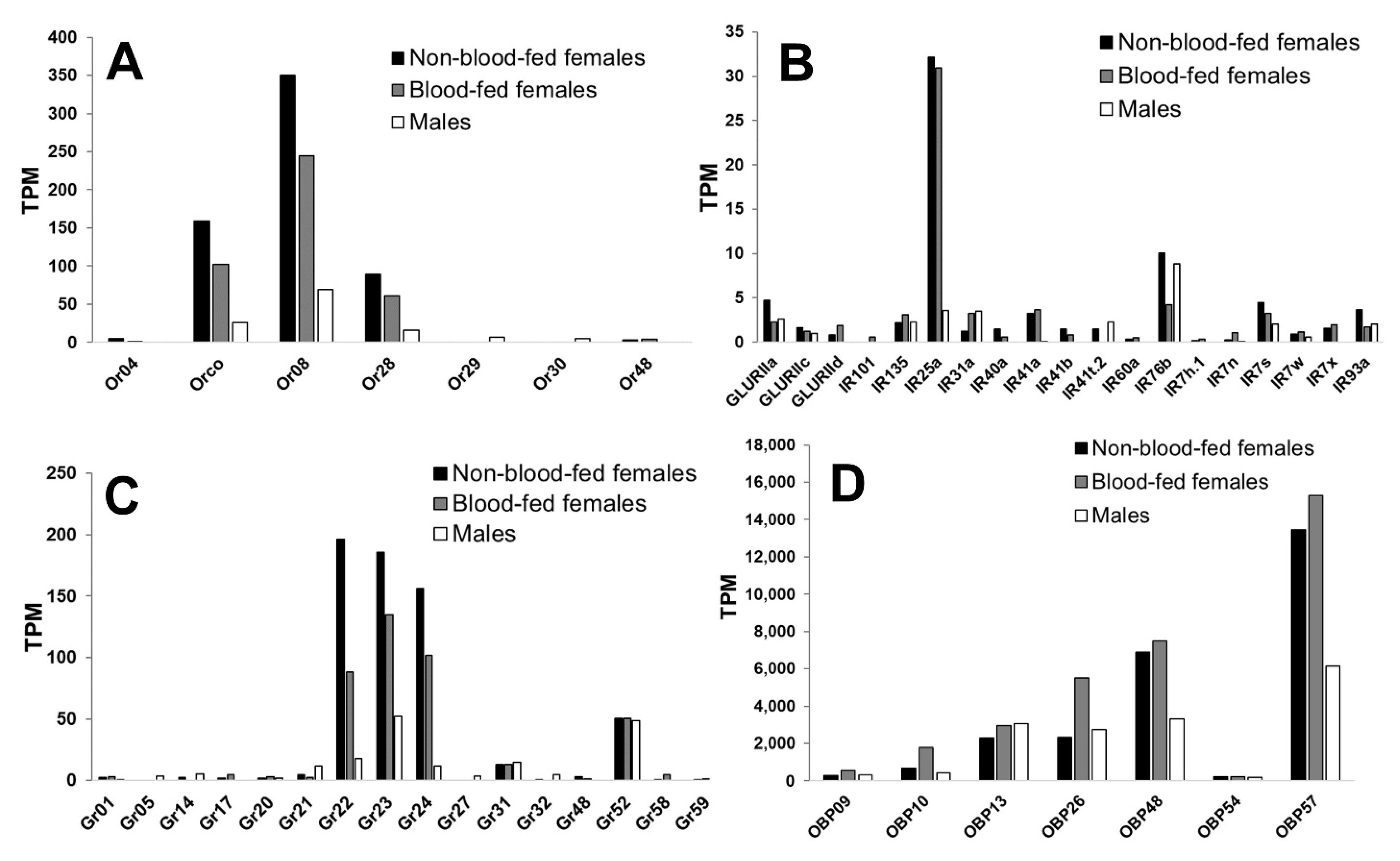
3.1. An. stephensi Mouthpart Transcriptome Reveals High Expression of Or8
3.2. AsOr8 Is Receptive to Alcohols and Ketones in the Human Volatile Spectrum
3.3. Maxillary Palp Capitate Peg Recordings Mirror AsOr8 Recordings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2020. 2020. Available online: https://www.who.int/publications/i/item/9789240015791 (accessed on 17 May 2021).
- Jiang, X.; Peery, A.; Hall, A.B.; Sharma, A.; Chen, X.G.; Waterhouse, R.M.; Komissarov, A.; Riehle, M.M.; Shouche, Y.; Sharakhova, M.V.; et al. Genome analysis of a major urban malaria vector mosquito, Anopheles stephensi. Genome Biol. 2014, 15, 459. [Google Scholar] [CrossRef] [PubMed]
- Seyfarth, M.; Khaireh, B.A.; Abdi, A.A.; Bouh, S.M.; Faulde, M.K. Five years following first detection of Anopheles stephensi (Diptera:Culicidae) in Djibouti, Horn of Africa: Populations established—Malaria emerging. Parasitol. Res. 2019, 118, 725–732. [Google Scholar] [CrossRef]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Chareonviriyaphap, T.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Elyazar, I.R.; Kabaria, C.W.; Harbach, R.E.; et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: Occurrence data, distribution maps and bionomic precis. Parasites Vectors 4 2011, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBride, C.S. Genes and odors underlying the recent evolution of mosquito preference for humans. Curr. Biol. 2016, 26, R41–R46. [Google Scholar] [CrossRef] [Green Version]
- Carey, A.F.; Wang, G.; Su, C.-Y.; Zwiebel, L.J.; Carlson, J.R. Odorant reception in the malaria mosquito Anopheles gambiae. Nature 2010, 464, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Carey, A.F.; Carlson, J.R.; Zwiebel, L.J. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2010, 107, 4418–4423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitts, R.J.; Rinker, D.C.; Jones, P.L.; Rokas, A.; Zwiebel, L.J. Transcriptome profiling of chemosensory appendages in the malaria vector Anopheles gambiae reveals tissue-and sex-specific signatures of odor coding. BMC Genom. 2011, 12, 271. Available online: http://www.biomedcentral.com/1471-2164/12/271 (accessed on 7 August 2017). [CrossRef] [Green Version]
- Rinker, D.C.; Zhou, X.; Pitts, R.J.; Rokas, A.; Zwiebel, L.J. Antennal transcriptome profiles of anopheline mosquitoes reveal human host olfactory specialization in Anopheles gambiae. BMC Genom. 2013, 14, 749. [Google Scholar] [CrossRef] [Green Version]
- Rinker, D.C.; Pitts, R.J.; Zhou, X.; Suh, E.; Rokas, A.; Zwiebel, L.J. Blood meal-induced changes to antennal transcriptome profiles reveal shifts in odor sensitivities in Anopheles gambiae. Proc. Natl. Acad. Sci. USA 2013, 110, 8260–8265. [Google Scholar] [CrossRef] [Green Version]
- Athrey, G.; Cosme, L.V.; Popkin-Hall, Z.; Pathikonda, S.; Takken, W.; Slotman, M.A. Chemosensory gene expression in olfactory organs of the anthropophilic Anopheles coluzzii and zoophilic Anopheles quadriannulatus. BMC Genom. 2017, 18, 751. [Google Scholar] [CrossRef]
- Raji, J.I.; Melo, N.; Castillo, J.S.; Gonzalez, S.; Saldana, V.; Stensmyr, M.C.; DeGennaro, M. Aedes aegypti Mosquitoes Detect Acidic Volatiles Found in Human Odor Using the IR8a Pathway. Curr. Biol. 2019, 29, 1253–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neafsey, D.E.; Waterhouse, R.M.; Abai, M.R.; Aganezov, S.S.; Alekseyev, M.A.; Allen, J.E.; Amon, J.; Arcà, B.; Arensburger, P.; Artemov, G.; et al. Highly evolvable malaria vectors: The genomes of 16 Anopheles mosquitoes. Science 2015, 347. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, E.; Aonuma, H.; Nelson, B.; Yoshimura, A.; Tokunaga, F.; Fukkumoto, S.; Kanuka, H. The role of proboscis of the malaria vector mosquito Anopheles stephensi in host-seeking behavior. Parasites Vectors 4 2011, 10. [Google Scholar] [CrossRef] [Green Version]
- Nuss, A.B.; Brown, M.R. Isolation of an insulin-like peptide from the Asian malaria mosquito, Anopheles stephensi, that acts as a steroidogenic gonadotropin across diverse mosquito taxa. Gen. Comp. Endocrinol. 2018, 258, 140–148. [Google Scholar] [CrossRef]
- Sharma, A.; Reyes, J.; Borgmeyer, D.; Ayala-Chavez, C.; Snow, K.; Arshad, F.; Nuss, A.B.; Gulia-Nuss, M. The sugar substitute erythritol shortens the lifespan of Aedes aegypti potentially by N-linked protein glycosylation. Sci. Rep. 2020, 10, 6195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 22 January 2021).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Wagner, G.P.; Kin, K.; Lynch, V.J. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci. 2012, 131, 281–285. [Google Scholar] [CrossRef]
- Giraldo-Calderón, G.I.; Emrich, S.J.; MacCallum, R.M.; Maslen, G.; Dialynas, E.; Topalis, P.; Ho, N.; Gesing, S. VectorBase: An updated bioinformatics resource for invertebrate vectors and other organisms related with human diseases. Nucleic Acids Res. 2015, 43, D707–D713. [Google Scholar] [CrossRef]
- Lu, T.; Qiu, Y.T.; Wang, G.; Kwon, J.Y.; Rutzler, M.; Kwon, H.-W.; Pitts, R.J.; van Loon, J.J.A.; Takken, W.; Carlson, J.R. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr. Biol. 2007, 17, 1533–1544. [Google Scholar] [CrossRef] [Green Version]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef]
- Dobritsa, A.A.; van Naters, W.V.D.G.; Warr, C.G.; Steinbrecht, R.A.; Carlson, J.R. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron 2003, 37, 827–841. [Google Scholar] [CrossRef] [Green Version]
- Hallem, E.A.; Ho, M.G.; Carlson, J.R. The molecular basis of odor coding in the Drosophila antenna. Cell 2004, 117, 965–979. [Google Scholar] [CrossRef] [Green Version]
- Mathew, D.; Martelli, C.; Kelley-Swift, E.; Brusalis, C.; Gershow, M.; Samuel, A.D.; Emonet, T.; Carlson, J.R. Functional diversity among sensory receptors in a Drosophila olfactory circuit. Proc. Natl. Acad. Sci. USA 2013, 110, E2134–E2143. [Google Scholar] [CrossRef] [Green Version]
- Ashburner, M. Drosophila: A Laboratory Handbook; Cold Spring Harbor Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Pitts, R.; Derryberry, S.; Zhang, Z.; Zwiebel, L. Variant Ionotropic Receptors in the Malaria Vector Mosquito Anopheles gambiae Tuned to Amines and Carboxylic Acids. Sci. Rep. 2017, 7, 40297. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.; Potter, S.C.; Finn, R.D. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohbot, J.D.; Dickens, J.C. Characterization of an enantioselevtive odorant receptor in the yellow fever mosquito Aedes aegypti. PLoS ONE 2009, 4, e7032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, A.J.; Dickens, J.C. Functional characterization of the octenol receptor neuron on the maxillary palps of the yellow fever mosquito, Aedes aegypti. PLoS ONE 2011, 6, e21785. [Google Scholar] [CrossRef] [Green Version]
- Dekel, A.; Pitts, R.J.; Yakir, E.; Bohbot, J.D. Evolutionarily conserved odorant receptor function questions ecological context of octenol role in mosquitoes. Sci. Rep. 2016, 6, 37330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBride, C.S.; Baier, F.; Omondi, A.B.; Spitzer, S.A.; Lutomiah, J.; Sang, R.; Ignell, R.; Vosshall, L.B. Evolution of mosquito preference for humans linked to an odorant receptor. Nature 2014, 515, 222–227. [Google Scholar] [CrossRef]
- Hughes, D.T.; Wang, G.; Zwiebel, L.J.; Luetje, C.W. A determinant of odorant specificity is located at the extracellular loop 2-transmembrane domain 4 interface of an Anopheles gambiae odorant receptor subunit. Chem. Senses 2014, 39, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Omondi, A.B.; Ghaninia, M.; Dawit, M.; Svensson, T.; Ignell, R. Age-dependent regulation of host seeking in Anopheles coluzzii. Sci. Rep. 2019, 9, 9699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernier, U.R.; Kline, D.L.; Barnard, D.R.; Schreck, C.E.; Yost, R.A. Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Anal. Chem. 2000, 72, 747–756. [Google Scholar] [CrossRef] [Green Version]
- Omondi, B.A.; Majeed, S.; Ignell, R. Functional development of carbon dioxide detection in the maxillary palp of Anopheles gambiae. J. Exp. Biol. 2015, 218, 2482–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saveer, A.M.; Pitts, R.J.; Ferguson, S.T.; Zwiebel, L.J. Characterization of chemosensory responses on the labellum of the malaria vector mosquito, Anopheles coluzzii. Sci. Rep. 2018, 8, 5656. [Google Scholar] [CrossRef]
- Chida, A.R.; Ravi, S.; Jayaprasad, S.; Paul, K.; Saha, J.; Suresh, C.; Whadgar, S.; Kumar, N.; Rao, K.R.; Ghosh, S.; et al. A near-chromosome level genome assembly of Anopheles stephensi. Front. Genet. 2020, 11, 565626. [Google Scholar] [CrossRef]



| Sample | Total Read Pairs | Mapped Reads | Overall Alignment Rate | Proper Pairs | Fragment Length | Total Feature Counts | Total FPKMs |
|---|---|---|---|---|---|---|---|
| Blood-fed female | 54,257,482 | 45,434,941 | 83.7% | 94.9% | 171 | 29,106,087 | 1,493,597 |
| Non-fed female | 47,808,166 | 38,786,401 | 81.1% | 95.1% | 179 | 25,379,805 | 1,382,585 |
| Male | 36,364,670 | 30,038,819 | 82.6% | 95.2% | 152 | 20,034,497 | 1,279,250 |
| Chemosensory Genes | Or | Ir | Gr | OBP |
|---|---|---|---|---|
| Total An. stephensi chemosensory genes (number less than An. gambiae) | 59 (17) | 37 (12) | 60 (0) | 42 (24) |
| Unnumbered or unannotated | 17 | 14 | 8 | 35 |
| Expression detected | 18 | 22 | 35 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speth, Z.; Kaur, G.; Mazolewski, D.; Sisomphou, R.; Siao, D.D.C.; Pooraiiouby, R.; Vasquez-Gross, H.; Petereit, J.; Gulia-Nuss, M.; Mathew, D.; et al. Characterization of Anopheles stephensi Odorant Receptor 8, an Abundant Component of the Mouthpart Chemosensory Transcriptome. Insects 2021, 12, 593. https://doi.org/10.3390/insects12070593
Speth Z, Kaur G, Mazolewski D, Sisomphou R, Siao DDC, Pooraiiouby R, Vasquez-Gross H, Petereit J, Gulia-Nuss M, Mathew D, et al. Characterization of Anopheles stephensi Odorant Receptor 8, an Abundant Component of the Mouthpart Chemosensory Transcriptome. Insects. 2021; 12(7):593. https://doi.org/10.3390/insects12070593
Chicago/Turabian StyleSpeth, Zachary, Gurlaz Kaur, Devin Mazolewski, Rayden Sisomphou, Danielle Denise C. Siao, Rana Pooraiiouby, Hans Vasquez-Gross, Juli Petereit, Monika Gulia-Nuss, Dennis Mathew, and et al. 2021. "Characterization of Anopheles stephensi Odorant Receptor 8, an Abundant Component of the Mouthpart Chemosensory Transcriptome" Insects 12, no. 7: 593. https://doi.org/10.3390/insects12070593
APA StyleSpeth, Z., Kaur, G., Mazolewski, D., Sisomphou, R., Siao, D. D. C., Pooraiiouby, R., Vasquez-Gross, H., Petereit, J., Gulia-Nuss, M., Mathew, D., & Nuss, A. B. (2021). Characterization of Anopheles stephensi Odorant Receptor 8, an Abundant Component of the Mouthpart Chemosensory Transcriptome. Insects, 12(7), 593. https://doi.org/10.3390/insects12070593