Enhanced Control of the Fungus Gnat Bradysia odoriphaga (Diptera: Sciaridae) by Co-Application of Clothianidin and Hexaflumuron
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Field Experiments for Studying Dynamics of Clothianidin, Its Residue in Soil and Chive, and Control Efficacies
2.4. Evluation of Control Efficacy
2.5. Sample Preparation
2.6. HPLC Analysis
2.7. Data Analysis
3. Results
3.1. Method Validation
3.2. Absorption of Clothianidin in Chive
3.3. Dissipation of Clothianidin in Chive
3.4. Dissipation of Clothianidin in Soil
3.5. Terminal Residue of Clothianidin in Chive and Soil
3.6. Control Efficiencies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imahori, Y.; Suzuki, Y.; Uemura, K.; Kishioka, I.; Fujiwara, H.; Ueda, Y.; Chachin, K. Physiological and quality responses of Chinese chive leaves to low oxygen atmosphere. Postharvest Biol. Technol. 2004, 31, 295–303. [Google Scholar] [CrossRef]
- Hu, G.H.; Lu, Y.H.; Wei, D.Z. Chemical characterization of Chinese chive seed (Allium tuberosum Rottl.). Food Chem. 2006, 99, 693–697. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, F.; Mu, W.; Wang, Q.H.; Li, H. Comparison of Bradysia odoriphaga Yang and Zhang reared on artificial diet and different host plants based on an age-stage, two-sex life table. Phytoparasitica 2015, 43, 107–120. [Google Scholar] [CrossRef]
- An, L.; Yang, X.; Lunau, K.; Fan, F.; Li, M.; Wei, G. High innate preference of black substrate in the chive gnat, Bradysia odoriphaga (Diptera: Sciaridae). PLoS ONE 2019, 14, e0210379. [Google Scholar] [CrossRef]
- Shi, C.H.; Hu, J.R.; Wei, Q.W.; Yang, Y.T.; Cheng, J.X.; Han, H.L.; Wu, Q.J.; Wang, S.L.; Xu, B.Y.; Su, Q.; et al. Control of Bradysia odoriphaga (Diptera: Sciaridae) by soil solarization. Crop Protect. 2018, 114, 76–82. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Xie, W.; Wu, Q.; Xu, B.; Wang, S.; Zhu, X.; Wang, S.; Zhang, Y. Effects of temperature on the age-Stage, two-sex life table of Bradysia odoriphaga (Diptera: Sciaridae). J. Econ. Entomol. 2015, 108, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, S.L.; Moens, M.; Han, R.C.; De Clercq, P. Efficacy of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) against the chive gnat, Bradysia odoriphaga. J. Pest Sci. 2013, 86, 551–561. [Google Scholar] [CrossRef]
- Thany, S.H. Agonist actions of clothianidin on synaptic and extrasynaptic nicotinic acetylcholine receptors expressed on cockroach sixth abdominal ganglion. Neurotoxicology 2009, 30, 1045–1052. [Google Scholar] [CrossRef][Green Version]
- Zhang, P.; He, M.; Zhao, Y.H.; Ren, Y.P.; Wei, Y.; Mu, W.; Liu, F. Dissipation dynamics of clothianidin and its control efficacy against Bradysia odoriphaga Yang and Zhang in Chinese chive ecosystems. Pest Manag. Sci. 2016, 72, 1396–1404. [Google Scholar] [CrossRef]
- Chen, C.Y.; Shi, X.Y.; Desneux, N.; Han, P.; Gao, X.W. Detection of insecticide resistance in Bradysia odoriphaga Yang and Zhang (Diptera: Sciaridae) in China. Ecotoxicology 2017, 26, 868–875. [Google Scholar] [CrossRef]
- Zhang, P.W.; Wang, S.Y.; Huang, C.L.; Fu, J.T.; Huang, R.L.; Li, Z.H.; Zhang, Z.X. Dissipation and residue of clothianidin in granules and pesticide fertilizers used in cabbage and soil under field conditions. Environ. Sci. Pollut. Res. 2018, 25, 27–33. [Google Scholar] [CrossRef]
- Agossa, F.R.; Padonou, G.G.; Fassinou, A.J.Y.H.; Odjo, E.M.; Akuoko, O.K.; Salako, A.; Koukpo, Z.C.; Nwangwu, U.C.; Akinro, B.; Sezonlin, M.; et al. Small-scale field evaluation of the efficacy and residual effect of Fludora® Fusion (mixture of clothianidin and deltamethrin) against susceptible and resistant Anopheles gambiae populations from Benin, West Africa. Malaria J. 2018, 17, 484. [Google Scholar] [CrossRef]
- Ludwig, S.W.; Oetting, R.D. Evaluation of medium treatments for management of Frankliniella occidentalis (Thripidae: Thysanoptera) and Bradysia coprophila (Diptera: Sciaridae). Pest Manag. Sci. 2001, 57, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.T.; Dong, F.S.; Xu, J.; Liu, X.G.; Li, Y.B.; Kong, Z.Q.; Liang, X.Y.; Liu, N.; Zheng, Y.Q. Residue change of pyridaben in apple samples during apple cider processing. Food Control 2014, 37, 240–244. [Google Scholar] [CrossRef]
- Sheets, J.J.; Karr, L.L.; Dripps, J.E. Kinetics of uptake, clearance, transfer, and metabolism of hexaflumuron by eastern subterranean termites (Isoptera: Rhinotermitidae). J. Econ. Entomol. 2000, 93, 871–877. [Google Scholar] [CrossRef]
- Mirhaghparast, S.K.; Zibaee, A. Effects of hexaflumuron and pyriproxyfen on the purified phenoloxidase of Chilo suppressalis Walker (Lepidoptera: Crambidae). Arch. Phytopathol. Pflanzenschutz. 2013, 46, 1775–1784. [Google Scholar] [CrossRef]
- Ma, X.D.; Xue, M.; Li, C.X.; Zhao, H.P.; Ji, G.X. Toxic effects of five insect growth regulators on chive gnat Bradysia odoriphaga. J. Plant Protect. 2015, 42, 271–277. [Google Scholar] [CrossRef]
- Kim, M.; Shim, C.; Kim, Y.; Ko, B.; Park, J.; Hwang, S.; Kim, B. Effect of biostimulator Chlorella fusca on improving growth and qualities of Chinese chives and Spinach in organic farm. Plant Pathol. J. 2018, 34, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.Z.; Luo, J.H.; Wang, M.Y.; Wang, X.L.; Qin, A.L. Determination of nicotine pesticide residues in vegetable with sulfur matrix by ultra-performance liquid chromatography-rlectrospray tandem mass spectrometry. Agrochemicals 2011, 50, 359–361. [Google Scholar] [CrossRef]
- Li, L.; Jiang, G.Q.; Liu, C.Y.; Liang, H.W.; Sun, D.L.; Li, W. Clothianidin dissipation in tomato and soil, and distribution in tomato peel and flesh. Food Control 2012, 25, 265–269. [Google Scholar] [CrossRef]
- Meng, B.; Yu, Y.; Zhang, Q.; Wang, S.; Hu, D.; Zhang, K. Simultaneous determination of residues of thiamethoxam and its metabolite clothianidin in tobacco leaf and soil using liquid chromatography-tandem mass spectrometry. Biomed. Chromatogr. 2018, 32, e4225. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Ma, X.D.; Xue, M.; Zhao, H.P.; Li, C.X. Toxic effects of clothianidin and other five kinds of insecticides to Bradysia odoriphaga. Acta Phytophylacica Sin. 2014, 41, 225–229. [Google Scholar] [CrossRef]
- Zhou, F.J.; Qun, Y.; Wei, Y.; Wang, Y.L. Effect of LED source on growth and nutritional quality of Chinese chive in different cutting stages. J. Northeast. Agri. Univ. 2017, 48, 52–62. [Google Scholar] [CrossRef]
- Chen, H.; Fan, Y.J.; Fan, X.J.; Zhuang, Q.Y.; Zhou, X.H.; Yu, Y. Effects of plastic film mulching on Bradysia odoriphaga in leek field and high temperature treatment on leek growth. Shandong Agri. Sci. 2017, 49, 105–109. [Google Scholar] [CrossRef]
- Li, Z.G.; Zhang, G.S.; Liu, Y.; Wan, K.Y.; Zhang, R.H.; Chen, F. Soil nutrient assessment for urban ecosystems in Hubei, China. PLoS ONE 2013, 9, e75856. [Google Scholar] [CrossRef]
- Zeng, Z.; Tang, J.; Liu, Y.; Zhang, M.; Lin, B. Changes and Driving Forces of Farmland Organic Matter in Guangdong Province, China. Soils 2013, 43, 84–90. [Google Scholar] [CrossRef]

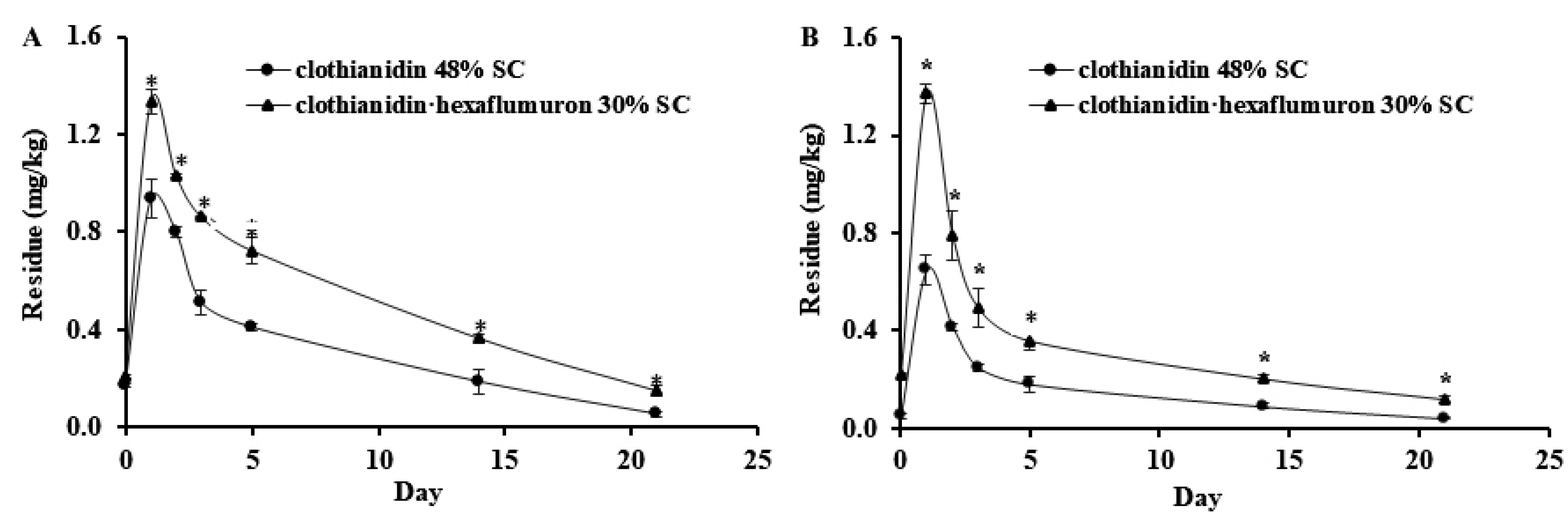
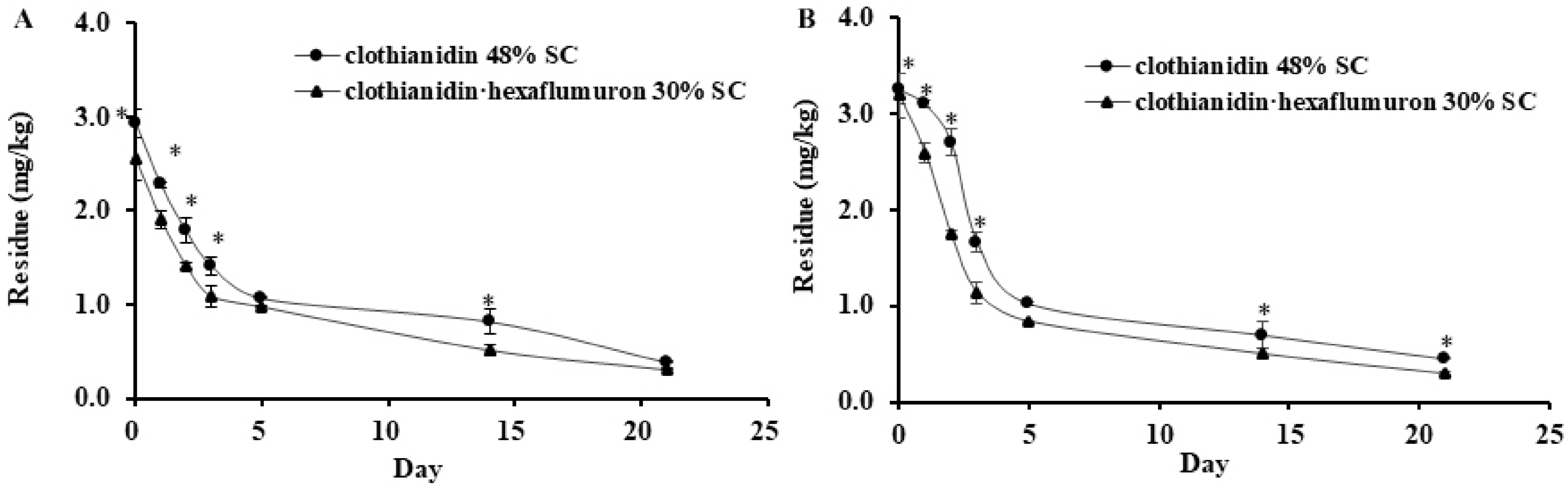
| Sample | Insecticide z | Sample Location | Dissipation Equations | Correlation Coefficient (R2) | Half-Lives (Day) |
|---|---|---|---|---|---|
| Chive | Clo + Hex | Guangdong | C = 1.2923 × 10−0.100T | 0.9829 | 6.93 |
| Clo + Hex | Hubei | C = 0.8949 × 10−0.103T | 0.8593 | 6.73 | |
| Clo | Guangdong | C = 0.9371 × 10−0.133T | 0.9718 | 5.21 | |
| Clo | Hubei | C = 0.4748 × 10−0.121T | 0.9181 | 5.73 | |
| Soil | Clo + Hex | Guangdong | C = 1.8386 × 10−0.089T | 0.8758 | 7.87 |
| Clo + Hex | Hubei | C = 2.1715 × 10−0.103T | 0.9753 | 6.73 | |
| Clo | Guangdong | C = 2.2034 × 10−0.082T | 0.9065 | 8.45 | |
| Clo | Hubei | C = 2.6938 × 10−0.093T | 0.8776 | 7.45 |
| Sample | Dosage (g.a.i.ha−1) | Application Times | Time (d) | Residues (mg/kg) | |||
|---|---|---|---|---|---|---|---|
| Guangdong | Hubei | ||||||
| Clo + Hex | Clo | Clo + Hex | Clo | ||||
| Chive | 675 | 2 | 7 | 0.205 a | 0.150 c | 0.191 a,b | 0.173 b |
| 14 | 0.185 a | 0.107 b | <LOD | 0.024 c | |||
| 30 | <LOD | <LOD | <LOD | <LOD | |||
| 675 | 3 | 7 | 1.764 a | 1.647 a | 0.288 b | 0.213 b | |
| 14 | 0.236 a | 0.254 a | 0.020 b | 0.019 b | |||
| 30 | 0.211 a | 0.136 b | <LOD | <LOD | |||
| 1350 | 2 | 7 | 0.568 a | 0.205 c | 0.279 b | 0.117 d | |
| 14 | 0.303 a | 0.085 c | 0.172 b | 0.023 d | |||
| 30 | 0.066 a | <LOD | <LOD | <LOD | |||
| 1350 | 3 | 7 | 2.227 a | 1.864 b | 0.568 c | 0.460 c | |
| 14 | 0.719 a | 0.569 b | 0.200 c | 0.021 d | |||
| 30 | 0.459 a | 0.259 b | <LOD | <LOD | |||
| Soil | 675 | 2 | 7 | 0.192 c | 0.214 c | 0.510 b | 0.624 a |
| 14 | 0.096 d | 0.130 c | 0.219 b | 0.327 a | |||
| 30 | 0.023 b | 0.027 b | 0.080 a | 0.074 a | |||
| 675 | 3 | 7 | 0.318 b | 0.324 b | 0.693 a | 0.718 a | |
| 14 | 0.041 c | 0.062 b | 0.682 a | 0.678 a | |||
| 30 | 0.027 c | 0.043 c | 0.481 b | 0.539 a | |||
| 1350 | 2 | 7 | 1.254 c | 1.437 b | 1.540 b | 1.863 a | |
| 14 | 0.120 c | 0.716 a | 0.490 b | 0.514 b | |||
| 30 | 0.044 b | 0.035 b | 0.327 a | 0.331 a | |||
| 1350 | 3 | 7 | 2.103 b | 2.534 a | 1.605 d | 1.792 c | |
| 14 | 0.185 c | 0.140 d | 1.526 a | 1.439 a | |||
| 30 | 0.044 d | 0.054 c | 0.304 b | 0.432 a | |||
| Sites | Insecticide z | Dosage (g.a.i.ha−1) | Corrected Mortality (%) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 d | 3 d | 5 d | 7 d | 14 d | 30 d | |||
| Guangdong | Clo + Hex | 675 | 48.14 ± 1.32 b | 57.85 ± 1.25 abc | 70.83 ± 5.61 ab | 85.61 ± 3.30 ab | 90.84 ± 4.79 ab | 94.03 ± 2.98 ab |
| 1350 | 58.50 ± 3.20 a | 63.58 ± 1.94 a | 75.37 ± 2.07 a | 88.60 ± 3.25 a | 94.64 ± 2.21 a | 96.82 ± 1.58 a | ||
| Clo | 675 | 34.97 ± 2.12 cd | 45.95 ± 2.60 de | 49.39 ± 2.08 d | 58.13 ± 1.48 d | 64.18 ± 2.22 de | 69.60 ± 2.13 d | |
| 1350 | 40.68 ± 4.36 bc | 58.91 ± 1.97 ab | 62.94 ± 2.61 bc | 68.62 ± 2.57 c | 73.29 ± 2.40 cd | 77.76 ± 1.87 c | ||
| Hubei | Clo + Hex | 675 | 41.72 ± 0.69 bc | 51.41 ± 1.60 cd | 70.83 ± 1.38 ab | 78.24 ± 1.94 b | 82.45 ± 1.26 bc | 87.56 ± 3.27 b |
| 1350 | 48.06 ± 3.04 b | 58.12 ± 2.92 ab | 69.06 ± 1.30 ab | 82.20 ± 4.43 ab | 90.02 ± 4.71 ab | 92.71 ± 1.45 ab | ||
| Clo | 675 | 29.23 ± 4.60 d | 43.49 ± 2.15 e | 47.39 ± 5.61 d | 52.00 ± 1.28 d | 59.63 ± 2.46 e | 64.80 ± 3.51 d | |
| 1350 | 36.74 ± 4.36 cd | 55.08 ± 1.35 bc | 57.41 ± 4.56 cd | 59.20 ± 2.39 d | 68.36 ± 5.24d e | 71.42 ± 2.12 cd | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wan, K.; Wang, R.; Wu, J.; Hou, R.; Zhao, K.; Zhang, Z.; Chen, J.; Cheng, D. Enhanced Control of the Fungus Gnat Bradysia odoriphaga (Diptera: Sciaridae) by Co-Application of Clothianidin and Hexaflumuron. Insects 2021, 12, 571. https://doi.org/10.3390/insects12070571
Wang Y, Wan K, Wang R, Wu J, Hou R, Zhao K, Zhang Z, Chen J, Cheng D. Enhanced Control of the Fungus Gnat Bradysia odoriphaga (Diptera: Sciaridae) by Co-Application of Clothianidin and Hexaflumuron. Insects. 2021; 12(7):571. https://doi.org/10.3390/insects12070571
Chicago/Turabian StyleWang, Yongqing, Kai Wan, Ruifei Wang, Jiyingzi Wu, Ruiquan Hou, Kunyu Zhao, Zhixiang Zhang, Jianjun Chen, and Dongmei Cheng. 2021. "Enhanced Control of the Fungus Gnat Bradysia odoriphaga (Diptera: Sciaridae) by Co-Application of Clothianidin and Hexaflumuron" Insects 12, no. 7: 571. https://doi.org/10.3390/insects12070571
APA StyleWang, Y., Wan, K., Wang, R., Wu, J., Hou, R., Zhao, K., Zhang, Z., Chen, J., & Cheng, D. (2021). Enhanced Control of the Fungus Gnat Bradysia odoriphaga (Diptera: Sciaridae) by Co-Application of Clothianidin and Hexaflumuron. Insects, 12(7), 571. https://doi.org/10.3390/insects12070571








