Effect of Cold Storage on the Quality of Psyttalia incisi (Hymenoptera: Braconidae), a Larval Parasitoid of Bactrocera dorsalis (Diptera: Tephritidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Colonies
2.2. Effects of Storage Temperature, Storage Duration and Pupal Age Interval on the Emergence Parameters of P. incisi
2.3. Effects of Pupal Cold Storage on Quality of P. incisi Adults
2.3.1. Dry Weight
2.3.2. Flight Capacity
2.3.3. Longevity and Reproductive Parameters
2.4. Statistical Analysis
3. Results
3.1. Effects of PupalCold Storage on the Emergence Parameters of P. incisi
3.1.1. Emergence Rate
3.1.2. Female Proportion
3.2. Effects of Pupal Cold Storage on the Quality of P. incisi Adults
3.2.1. Dry Weight
3.2.2. Flight Capacity
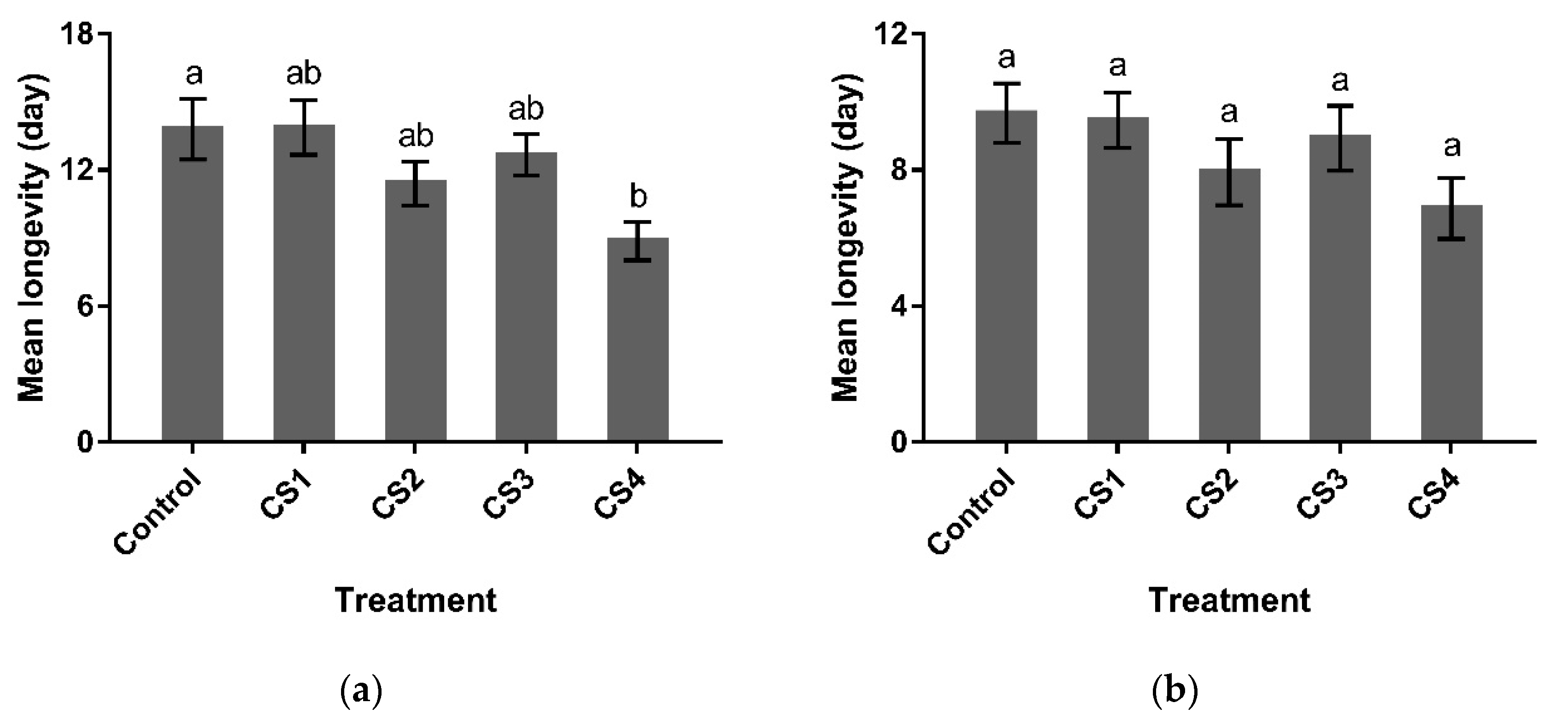
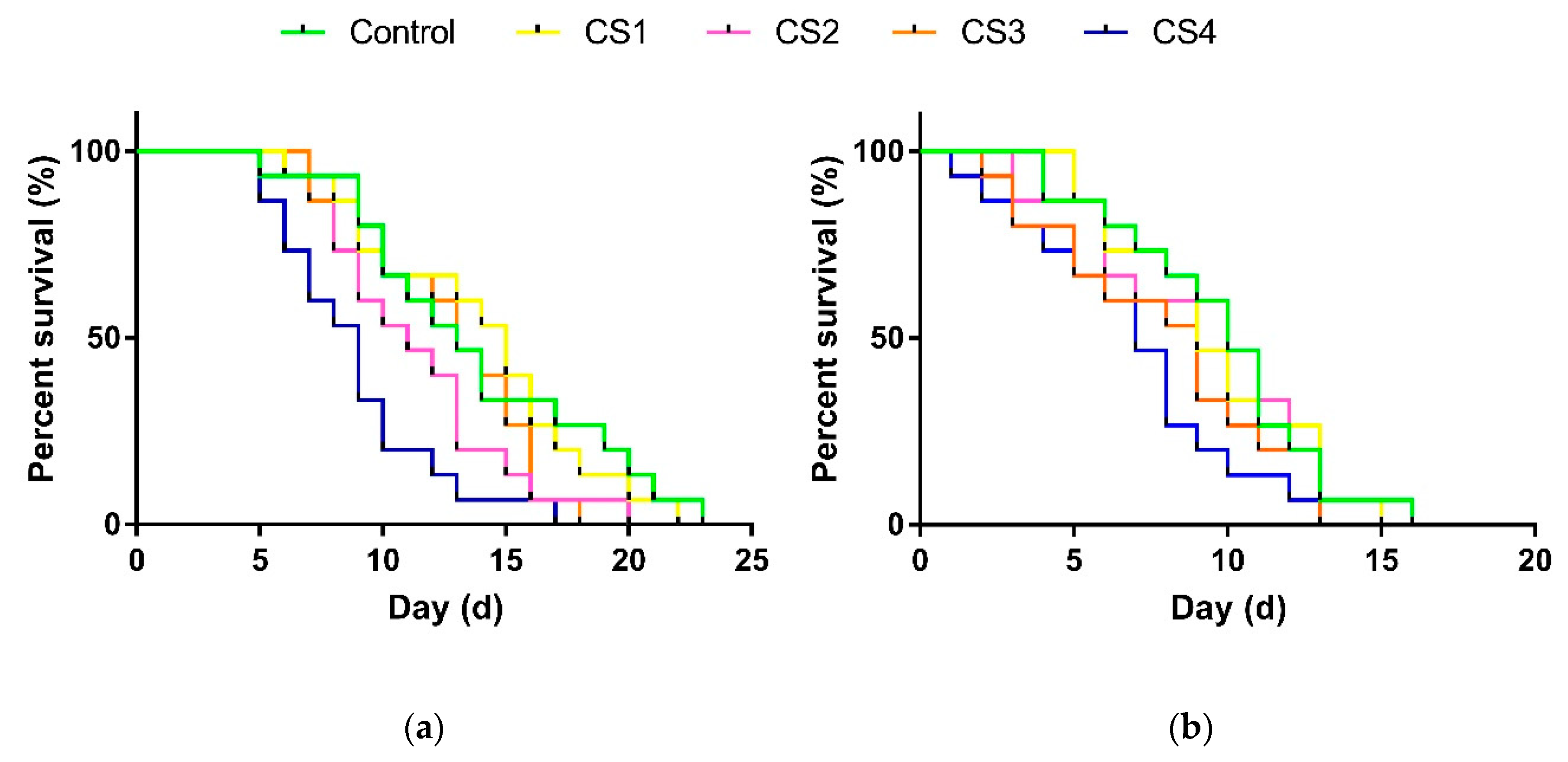
3.2.3. Longevity
3.2.4. G1 Reproductive Performance and G2 Emergence Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Zhang, D.J.; Xu, Y.J.; Wang, L.; Cheng, D.F.; Qi, Y.X.; Zeng, L.; Lu, Y.Y. Invasion, expansion, and control of Bactrocera dorsalis (Hendel) in China. J. Integr. Agric. 2019, 18, 771–787. [Google Scholar] [CrossRef]
- Clarke, A.R.; Li, Z.H.; Qin, Y.J.; Zhao, Z.H.; Liu, L.J.; Schutze, M.K. Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) is not invasive through Asia: It’s been there all along. J. Appl. Entomol. 2019, 143, 797–801. [Google Scholar] [CrossRef]
- Mutamiswa, R.; Nyamukonduwa, C.; Chikowore, G.; Chidawanyika, F. Overview of oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) in Africa: From invasion, bio-ecology to sustainable management. Crop. Prot. 2021, 141, 105402. [Google Scholar] [CrossRef]
- Piñero, J.C.; Souder, S.K.; Smith, T.R.; Vargas, R.I. Attraction of Bactrocera cucurbitae and Bactrocera dorsalis (Diptera: Tephritidae) to beer waste and other protein sources laced with ammonium acetate. Fla. Entomol. 2017, 100, 70–76. [Google Scholar] [CrossRef]
- Jin, T.; Zeng, L.; Lin, Y.Y.; Lu, Y.Y.; Liang, G.W. Insecticide resistance of the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae), in mainland China. Pest. Manag. Sci. 2011, 67, 370–376. [Google Scholar] [CrossRef]
- Diaz-Fleischer, F.; Perez-Staples, D.; Cabrera-Mireles, H.; Montoya, P.; Liedo, P. Novel insecticides and bait stations for the control of Anastrepha fruit flies in mango orchards. J. Pestic. Sci. 2017, 90, 1–8. [Google Scholar] [CrossRef]
- Yang, J.Q.; Cai, P.M.; Chen, J.; Zhang, H.H.; Wang, C.; Xiang, H.J.; Yang, Y.C.; Chen, J.H.; Ji, Q.E.; Song, D.B. Interspecific competition between Fopius arisanus and Psyttalia incisi (Hymenoptera: Braconidae), parasitoids of Bactrocera dorsalis (Diptera: Tephritidae). Biol. Control 2018, 40, 183–189. [Google Scholar] [CrossRef]
- Liang, G.H.; Chen, J.H.; Huang, J.C. The functional response and disturbance response of larvae of Psyttalia incisi (Silvestri) to oriental fruit flies. Acta Agric. Univ. Jiangxiensis 2006, 28, 200–203. [Google Scholar]
- Liang, G.H.; Wu, Y.; Chen, J.H. Seasonal incidence of Bactrocera dorsalis and its parasitoids in field. J. Southwest For. Coll. 2006, 26, 72–74. [Google Scholar]
- Colinet, H.; Boivin, G. Insect parasitoids cold storage: A comprehensive review of factors of variability and consequences. Biol. Control 2011, 58, 83–95. [Google Scholar] [CrossRef]
- Rathee, M.; Ram, P. Impact of cold storage on the performance of entomophagous insects: An overview. Phytoparasitica 2018, 46, 421–449. [Google Scholar] [CrossRef]
- Rezaei, M.; Talebi, A.A.; Fathipour, Y.; Karimzadeh, J.; Mehrabadi, M.; Reddy, G.V.P. Effects of cold storage on life-history traits of Aphidius matricariae. Entomol. Exp. Appl. 2020, 168, 800–807. [Google Scholar] [CrossRef]
- Ghazy, N.A.; Suzuki, T.; Amano, H.; Ohyama, K. Air temperature optimisation for humidity-controlled cold storage of the predatory mites Neoseiulus californicus and Phytoseiulus persimilis (Acari: Phytoseiidae). Pest. Manag. Sci. 2014, 70, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.; Han, S.C.; Li, J.; Liu, J.S.; Li, Z.G. Effects of cold storage on the quality of Trichogramma dendrolimi Matsumura (Hymenoptera: Trichogrammatidae) reared on artificial medium. Pest. Manag. Sci. 2019, 75, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Daane, K.M.; Wang, X.G.; Johnson, M.W.; Cooper, M.L. Low temperature storage effects on two olive fruit fly parasitoids. BioControl 2013, 58, 175–185. [Google Scholar] [CrossRef]
- Long, X.Z.; Chen, K.W.; Xian, J.D.; Lu, Y.Y.; Zeng, L. Cold storage technique of Diachasmimorpha longicaudata (Ashmead). J. Environ. Entomol. 2014, 36, 115–121. [Google Scholar]
- Wang, H.L. Effects of Spinosad and Temperature and Humidity on the Parasitoid Fopius arisanus (Sonan). Master’s Thesis, Fujian Agricultural and Forestry University, Fuzhou, China, 2011. [Google Scholar]
- Ji, Q.E.; Dong, C.Z.; Chen, J.H. A new record species—Opius incisi Silvestri (Hymenoptera: Braconidae) parasitizing on Dacus dorsalis (Hendal) in China. Entomotaxonomia 2004, 26, 144–145. [Google Scholar]
- Chang, C.L.; Vargas, R.I.; Caceres, C.; Jang, E.; Cho, I.K. Development and assessment of a liquid larval diet for Bactrocera dorsalis (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2006, 99, 1191–1198. [Google Scholar] [CrossRef]
- Wang, X.G.; Messing, R.H. Potential interactions between pupal and egg-or larval-pupal parasitoids of Tephritid fruit flies. Environ. Entomol. 2004, 33, 1313–1320. [Google Scholar] [CrossRef][Green Version]
- Vargas, R.I.; Leblanc, L.; Harris, E.; Manoukis, N.C. Regional suppression of Bactrocera fruit flies (Diptera: Tephritidae) in the Pacific through biological control and prospects for future introductions into other areas of the world. Insects 2012, 3, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.J.; Xie, C.H.; He, Y.B.; Qiu, B.; Chen, H.Y.; Xu, Z.F. Investigation on hylmenopterous parasitoids of Bactrocera dorsalis (Hendel) in Guangdong. J. Environ. Entomol. 2008, 30, 350–356. [Google Scholar]
- Benelli, M.; Ponton, F.; Lallu, U.; Mitchell, K.A.; Taylor, P.W. Cool storage of Queensland fruit fly pupae for improved management of mass production schedules. Pest. Manag. Sci. 2019, 75, 3184–3192. [Google Scholar] [CrossRef]
- Sakaki, S.; Jalali, M.A.; Kamali, H.; Nedved, O. Effect of low-temperature storage on the life history parameters and voracity of Hippodamia variegata (Coleoptera: Coccinellidae). Eur. J. Entomol. 2019, 116, 10–15. [Google Scholar] [CrossRef]
- Benelli, M.; Ponton, F.; Taylor, P.W. Cool sotrage of Queensland fruit fly eggs for increased flexibility in rearing programs. Pest. Manag. Sci. 2019, 75, 1056–1064. [Google Scholar] [CrossRef]
- Luczynski, A.; Nyrop, J.P.; Shi, A. Influence of cold storage on pupal development and mortality during storage and on post-storage performance of Encarsia formosa and Eretmocerus eremicus (Hymenoptera: Aphelinidae). Biol. Control 2006, 40, 107–117. [Google Scholar] [CrossRef]
- Neven, L.G.; Hansen, L.D. Effects of temperature and controlled atmospheres on codling moth metabolism. Ann. Entomol. Soc. Am. 2010, 103, 418–423. [Google Scholar] [CrossRef]
- Lins, J.C.; Bueno, V.H.P.; Sidney, L.A.; Silva, D.B.; Sampaio, M.V.; Pereira, J.M.; Nomelini, Q.S.S.; van Lenteren, J.C. Cold storage affects mortality, body mass, lifespan, reproduction and flight capacity of Praon volucre (Hymenoptera: Braconidae). Eur. J. Entomol. 2013, 110, 263–270. [Google Scholar] [CrossRef]
- Jervis, M.A.; Ferns, P.N.; Heimpel, G.E. Body size and the timing of egg production in parasitoid wasps: A comparative analysis. Funct. Ecol. 2003, 17, 375–383. [Google Scholar] [CrossRef]
- Colinet, H.; Boivin, G.; Hance, T. Manipulation of parasitoid size using the temperature-size rule: Fitness consequences. Oecologia 2007, 152, 425–433. [Google Scholar] [CrossRef]
- Visser, B.; le Lann, C.; den Blanken, F.J.; Harvey, J.A.; van Alphen, J.M.; Ellers, J. Loss of lipid synthesis as an evolutionary consequence of a parasitic lifestyle. Proc. Natl. Acad. Sci. USA 2010, 107, 8677–8682. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; van Baaren, J.; Hance, T.; Perre, J.S.; Vernon, P. Stress intensity and fitness in the parasitoid Aphidius ervi (Hymenoptera: Braconidae): Temperature below the development threshold combined with a fluctuating thermal regime is a must. Ecol. Entomol. 2013, 38, 355–363. [Google Scholar] [CrossRef]
- Alam, M.S.; Alam, M.Z.; Alam, S.N.; Miah, M.R.U.; Mian, M.I.H. Effect of storage duration on the stored pupae of parasitoid Bracon hebetor (Say) and its impact on parasitoid quality. Bangladesh J. Agric. Res. 2016, 41, 297–310. [Google Scholar] [CrossRef]
- Amice, G.; Vernon, P.; Outreman, Y.; van Alphen, J.; van Baaren, J. Variability in responses to thermal stress in parasitoids. Ecol. Entomol. 2008, 33, 701–708. [Google Scholar] [CrossRef]
- Yocum, G.D.; Zdárek, J.; Joplin, K.H.; Lee, R.E.; Smith, D.C.; Manter, K.D.; Denlinger, D.L. Alteration of the eclosion rhythm and eclosion behavior in the flesh fly, Sarcophaga crassipalpis, by low and high temperature stress. J. Insect. Physiol. 1994, 40, 13–21. [Google Scholar] [CrossRef]
- Yan, Z.; Yue, J.J.; Bai, C.; Peng, Z.Q.; Zhang, C.H. Effects of cold storage on the biological characteristics of Microplitis prodeniae (Hymenoptera: Braconidae). Bull. Entomol. Res. 2017, 107, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.I.; Ramadan, M.; Hussain, T.; Mochizuki, N.; Bautista, R.C.; Stark, J.D. Comparative demography of six fruit fly (Diptera: Tephritidae) parasitoids (Hymenoptera: Braconidae). Biol. Control 2002, 25, 30–40. [Google Scholar] [CrossRef]
- Liang, G.H.; Huang, J.C.; Chen, J.H. Comparative analysis on RAPD of two geographic populations of Psyttalia incisi. J. Fujian Coll. For. 2007, 27, 16–19. [Google Scholar]
- Leopold, R. Colony maintenance and mass-rearing: Using cold storage technology for extending the shelf-life of insects. In Area-Wide Control of Insect Pests: From Research to Field Implementation, 2nd ed.; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 149–162. [Google Scholar]
| Emergence Rate (%) | ||||
|---|---|---|---|---|
| Storage Temperature (℃) | Storage Time (d) | Pupal Age Interval | ||
| Prepupae | Middle-Age | Late-Age | ||
| 4 | 10 | 7.57 ± 1.66 c | 16.84 ± 1.96 b | 4.55 ± 1.13 cd |
| 15 | 1.77 ± 0.89 ef | 6.64 ± 1.50 c | 0 f | |
| 20 | 0 f | 1.14 ± 0.76 f | 0 f | |
| 25 | 0 f | 0 f | 0 f | |
| 25 (control) | 83.27 ± 2.36 a | |||
| 7 | 10 | 13.71 ± 2.17 c | 25.22 ± 2.74 b | 11.47 ± 1.28 c |
| 15 | 3.43 ± 1.2 de | 11.08 ± 0.87 c | 1.53 ± 0.84 de | |
| 20 | 0.51 ± 0.51 e | 5.44 ± 1.63 d | 0 e | |
| 25 | 0 e | 0 e | 0 e | |
| 25 (control) | 83.27 ± 2.36 a | |||
| 10 | 10 | 19.03 ± 2.02 c | 45.81 ± 2.50 b | 39.07 ± 3.23 b |
| 15 | 15.23 ± 2.14 cd | 36.19 ± 2.34 b | 20.05 ± 2.04 c | |
| 20 | 9.34 ± 1.56 de | 16.29 ± 2.86 cd | 3.36 ± 1.11e fg | |
| 25 | 2.64 ± 0.84 fg | 5.46 ± 1.42 ef | 0 g | |
| 25 (control) | 83.27 ± 2.36 a | |||
| 13 | 10 | 37.89 ± 3.16 c | 81.29 ± 2.50 a | 80.62 ± 1.92 a |
| 15 | 33.52 ± 4.32 cd | 73.22 ± 2.34 a | 75.71 ± 3.26 a | |
| 20 | 20.06 ± 3.06 d | 54.80 ± 2.73 b | 59.33 ± 2.44 bE | |
| 25 | 6.01± 1.48 e | 38.49 ± 2.95 cE | 46.67± 2.64 bcE | |
| 25 (control) | 83.27 ± 2.36 a | |||
| Female Proportion (%) | ||||
|---|---|---|---|---|
| Storage Temperature (℃) | Storage Time (d) | Pupal Age Interval | ||
| Prepupae | Middle-Age | Late-Age | ||
| 4 | 10 | 80.21 ± 7.56 a | 69.51 ± 5.06 a | - |
| 15 | - | 72.92 ± 11.86 a | - | |
| 20 | - | - | - | |
| 25 | - | - | - | |
| 25 (control) | - | 71.53 ± 2.02 a | ||
| 7 | 10 | 71.48 ± 6.78 a | 71.42 ± 4.92 a | 74.07 ± 5.97 a |
| 15 | - | 64.82 ± 10.09 a | - | |
| 20 | - | 80.56 ± 7.38 a | - | |
| 25 | - | - | - | |
| 25 (control) | 71.53 ± 2.02 a | |||
| 10 | 10 | 74.23 ± 6.01 a | 68.21 ± 2.96 a | 69.81 ± 2.85 a |
| 15 | 71.09 ± 6.17 a | 71.65 ± 3.06 a | 72.67 ± 4.30 a | |
| 20 | 71.88 ± 8.78 a | 70.95 ± 4.15 a | - | |
| 25 | - | 80.95 ± 8.13 a | - | |
| 25 (control) | 71.53 ± 2.02 a | |||
| 13 | 10 | 70.65 ± 5.34 a | 69.20 ± 3.08 a | 66.79 ± 1.99 a |
| 15 | 69.58 ± 4.01 a | 70.12 ± 2.62 a | 68.43 ± 1.98 a | |
| 20 | 73.70 ± 5.78 a | 69.23 ± 4.06 a | 71.56 ± 3.21 aE | |
| 25 | 68.75 ± 15.27 a | 70.56 ± 4.26 aE | 69.42 ± 2.43 aE | |
| 25 (control) | 71.53 ± 2.02 a | |||
| Treatment | Dry Weight (mg) | Flight Ability (%) | ||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Control (25 °C) | 59.67 ± 0.76 a | 40.30 ± 0.59 a | 69.33 ± 3.65 a | 62.67 ± 2.75 a |
| CS1 | 59.05 ± 0.88 ab | 40.55 ± 0.38 a | 67.56 ± 4.15 ab | 60.22 ± 2.99 ab |
| CS2 | 59.15 ± 0.59 ab | 40.10 ± 0.50 ab | 60.22 ± 1.61 ab | 54.44 ± 2.97 ab |
| CS3 | 57.72 ± 0.60 ab | 39.24 ± 0.34 ab | 61.33 ± 1.91 ab | 55.11 ± 2.21 ab |
| CS4 | 56.46 ± 0.97 b | 38.19 ± 0.64 b | 56.67 ± 1.86 b | 52.00 ± 1.70 b |
| Treatment | Pre-Oviposition Period (d) | Oviposition Period (d) | Post-Oviposition Period (d) | Total No. of Offspring | No. of Daily Offspring |
|---|---|---|---|---|---|
| 25 °C (control) | 0.33 ± 0.13 a | 10.73 ± 0.73 a | 2.73 ± 0.85 a | 69.13 ± 4.44 a | 6.72 ± 0.41 a |
| CS1 | 0.27 ± 0.15 a | 11.07 ± 0.72 a | 2.53 ± 0.56 a | 68.27 ± 3.75 ab | 6.28 ± 0.45 ab |
| CS2 | 0.20 ± 0.11 a | 9.60 ± 0.66 ab | 1.60 ± 0.42 a | 54.80 ± 5.51 b | 5.53 ± 0.37 ab |
| CS3 | 0.27 ± 0.12 a | 10.40 ± 0.58 ab | 2.00 ± 0.48 a | 65.60 ± 3.84 ab | 6.46 ± 0.31 a |
| CS4 | 0.40 ± 0.13 a | 7.73 ± 0.67 b | 0.73 ± 0.25 a | 37.07 ± 4.59 c | 4.92 ± 0.40 b |
| Treatment | G2 Emergence Rate (%) | G2 Female Proportion (%) |
|---|---|---|
| Control (25 °C) | 85.51 ± 1.31 a | 71.36 ±1.44 a |
| CS1 | 83.75 ± 1.20 a | 69.43 ±1.13 a |
| CS2 | 83.45 ± 1.52 a | 70.73 ± 0.95 a |
| CS3 | 83.96 ± 1.11 a | 71.02 ± 0.73 a |
| CS4 | 84.23 ± 1.21 a | 67.26 ± 1.58 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Yang, D.; Hao, X.; Cai, P.; Guo, Y.; Shi, S.; Liu, C.; Ji, Q. Effect of Cold Storage on the Quality of Psyttalia incisi (Hymenoptera: Braconidae), a Larval Parasitoid of Bactrocera dorsalis (Diptera: Tephritidae). Insects 2021, 12, 558. https://doi.org/10.3390/insects12060558
Lin J, Yang D, Hao X, Cai P, Guo Y, Shi S, Liu C, Ji Q. Effect of Cold Storage on the Quality of Psyttalia incisi (Hymenoptera: Braconidae), a Larval Parasitoid of Bactrocera dorsalis (Diptera: Tephritidae). Insects. 2021; 12(6):558. https://doi.org/10.3390/insects12060558
Chicago/Turabian StyleLin, Jia, Deqing Yang, Xuxing Hao, Pumo Cai, Yaqing Guo, Shuang Shi, Changming Liu, and Qinge Ji. 2021. "Effect of Cold Storage on the Quality of Psyttalia incisi (Hymenoptera: Braconidae), a Larval Parasitoid of Bactrocera dorsalis (Diptera: Tephritidae)" Insects 12, no. 6: 558. https://doi.org/10.3390/insects12060558
APA StyleLin, J., Yang, D., Hao, X., Cai, P., Guo, Y., Shi, S., Liu, C., & Ji, Q. (2021). Effect of Cold Storage on the Quality of Psyttalia incisi (Hymenoptera: Braconidae), a Larval Parasitoid of Bactrocera dorsalis (Diptera: Tephritidae). Insects, 12(6), 558. https://doi.org/10.3390/insects12060558





