A Three-Pronged Approach to Studying Sublethal Insecticide Doses: Characterising Mosquito Fitness, Mosquito Biting Behaviour, and Human/Environmental Health Risks
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Effects of Sublethal Prallethrin Doses on Mosquito Fitness
- Cx. pipiens females: Since they came from an autogenous strain, Cx. pipiens females did not need to consume blood to lay eggs. Forty-eight hours after the trial, they were given a tray containing water to allow egg laying. During this period, the number of females that drowned was noted for each treatment group.
- Ae. albopictus females: Forty-eight hours after the trial, Ae. albopictus females were fed calf’s blood using a membrane feeding system (Hemotek, Discovery Workshops, Lancashire, England). Females were given wet paper filters for egg laying, which meant that there was no risk of drowning.
2.2. Effect of Sublethal Prallethrin Doses on Mosquito Biting Behaviour
2.3. Assessments of Human and Environmental Health Risks
2.4. Statistical Analysis
3. Results
3.1. Effects of Sublethal Prallethrin Doses on Mosquito Fitness
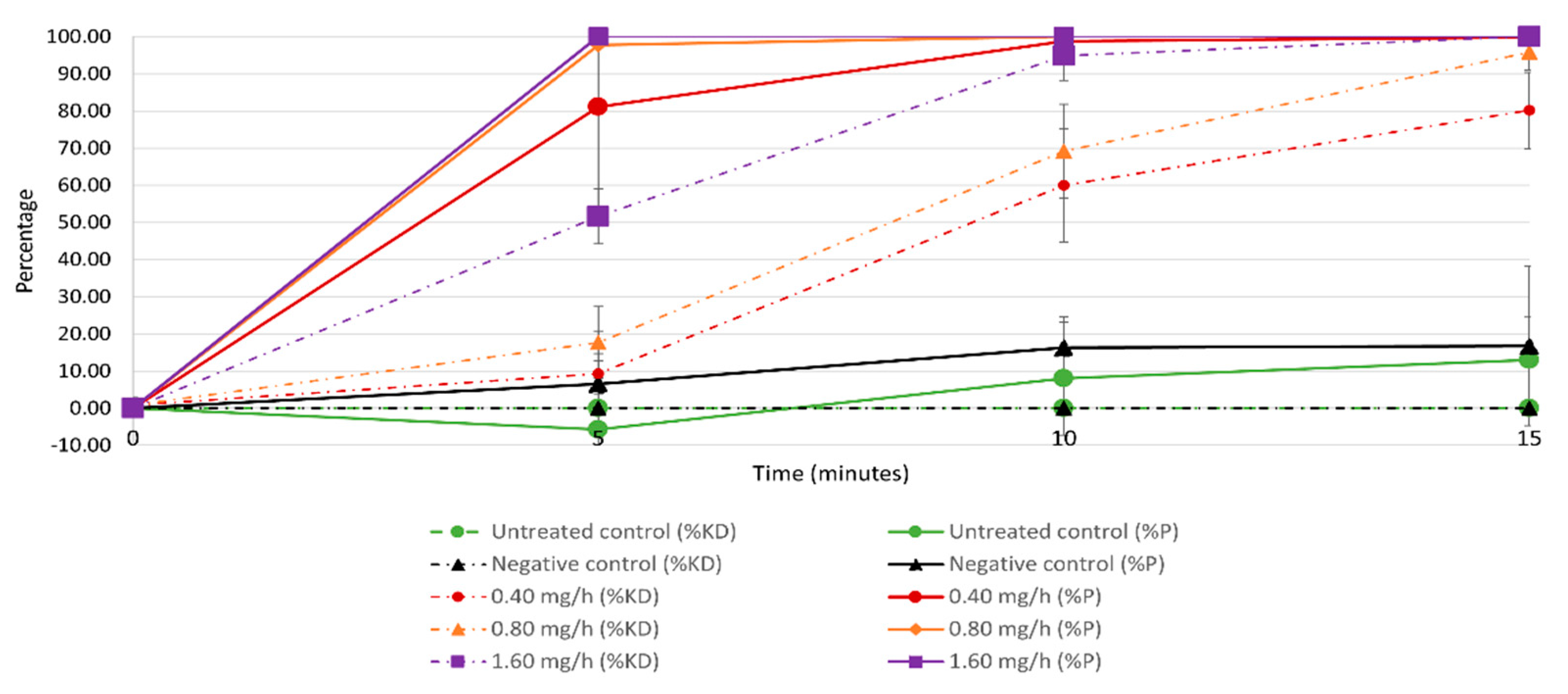
3.1.1. Effects of Species, Sex, and Treatment on KD during the 90-Min Treatment Trial
3.1.2. Percentage of Dead and Affected Mosquitoes 48 h into the Post-Treatment Period
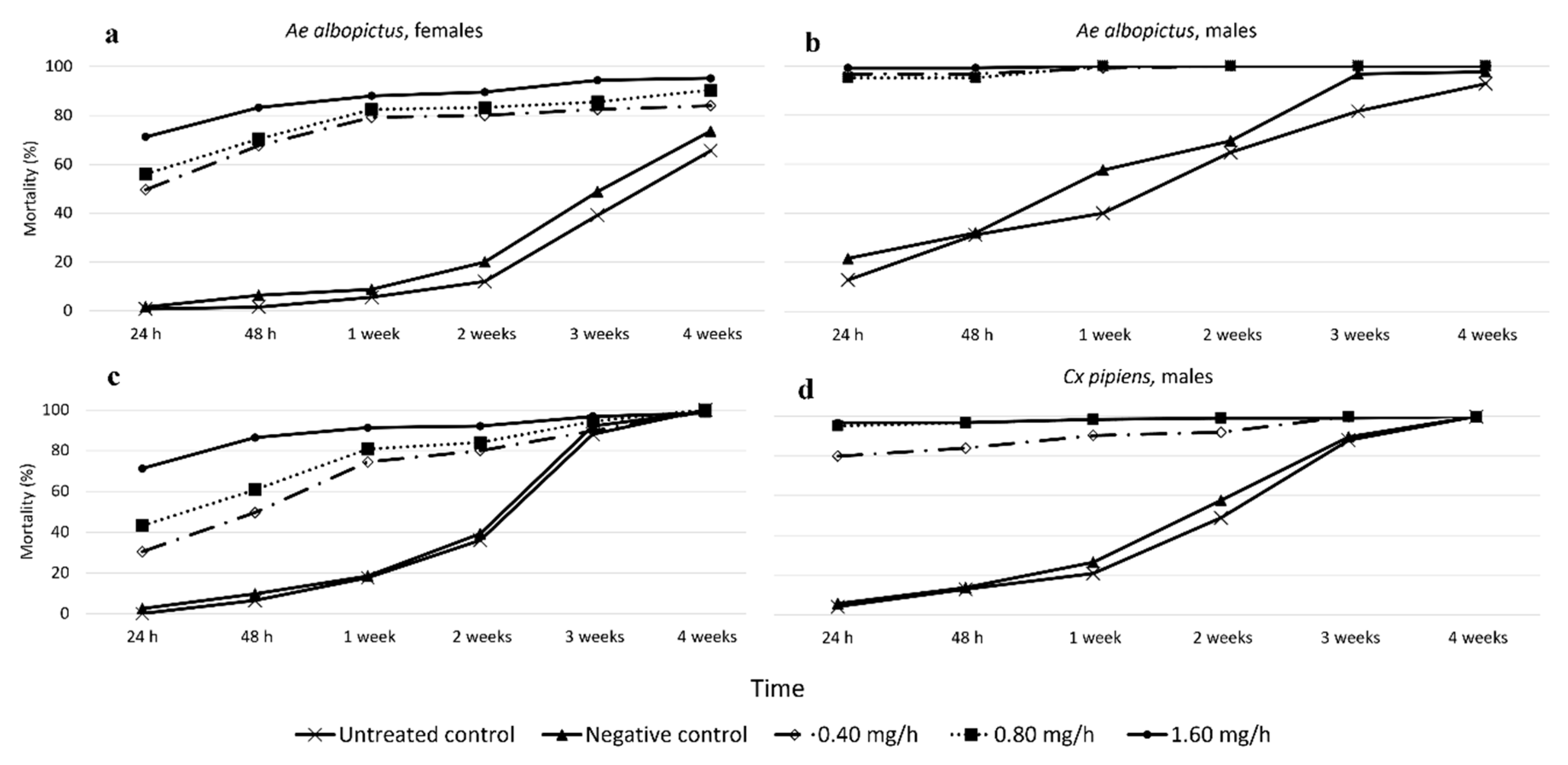
3.1.3. Effects of Species, Sex, and Treatment on Long-Term Mortality
3.1.4. Effects of Species, Sex, and Treatment on Fertility, Egg Laying, and F1 Population Size over the 4-Week Post-Treatment Period
3.2. Effects of Sublethal Prallethrin Doses on Mosquito Biting Behaviour
3.3. Assessments of Human and Environmental Health Risks
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef]
- Gossner, C.M.; Ducheyne, E.; Schaffner, F. Increased risk for autochthonous vector-borne infections transmitted by Aedes albopictus in continental Europe. Eurosurveillance 2018, 23, 1800268. [Google Scholar] [CrossRef]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging arboviruses: Why today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef]
- Flores, H.A.; O’Neill, S.L. Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 2018, 16, 508–518. [Google Scholar] [CrossRef]
- Gratz, N.G.; Jany, W.C. What role for insecticides in vector control programs? Am. J. Trop. Med. Hyg. 1994, 50, 11–20. [Google Scholar] [CrossRef]
- van den Berg, H.; Zaim, M.; Yadav, R.S.; Soares, A.; Ameneshewa, B.; Mnzava, A.; Hii, J.; Dash, A.P.; Ejov, M. Global trends in the use of insecticides to control vector-borne diseases. Environ. Health Perspect. 2012, 120, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Wilks, M.F. Pyrethroids: Mechanisms of toxicity, features and management. J. Toxicol. Clin. Toxicol. 2005, 43, 425. [Google Scholar]
- World Health Organization (WHO). Safety of Pyrethroids for Public Health Use. 2005. Available online: https://apps.who.int/iris/bitstream/handle/10665/69008/?sequence=1 (accessed on 28 April 2021).
- Todd, G.D.; Wohlers, D.; Citra, M.J. Toxicological Profile for Pyrethrins and Pyrethroids; Agency for Toxic Substances and Disease Registry: Atlanta, Georgia, 2003.
- Reglamento (UE) 2018/1472 de la Comisión. De 28 de Septiembre de 2018, por el que se Modifica el Anexo II del Reglamento (CE) n.° 1333/2008 del Parlamento Europeo y del Consejo y el Anexo del Reglamento (UE) n.° 231/2012 de la Comisión en lo que se Refiere al Aditivo E 120 (Cochinilla, Ácido Carmínico, Carmines) (Texto Pertinente a Efectos del EEE). Diario Oficial de la Unión Europa. 2018, pp. 1–4. Available online: https://eur-lex.europa.eu/eli/reg/2018/1472/oj?locale=es (accessed on 27 April 2021).
- European Chemicals Agency (ECHA). Guidance on Biocides Legislation. Available online: https://echa.europa.eu/guidance-documents/guidance-on-biocides-legislation (accessed on 28 April 2021).
- Bibbs, C.S.; Xue, R.-D. OFF! Clip-on repellent device with metofluthrin tested on Aedes aegypti (Diptera: Culicidae) for mortality at different time intervals and distances. J. Med. Entomol. 2016, 53, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Bibbs, C.S.; Fulcher, A.; Xue, R.-D. Allethrin-Based mosquito control device causing knockdown, morbidity, and mortality in four species of field-caught mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2015, 52, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, S.A.; Devine, G.J. Confusion, knock-down and kill of Aedes aegypti using metofluthrin in domestic settings: A powerful tool to prevent dengue transmission? Parasit. Vectors 2013, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.-D.; Qualls, W.A.; Smith, M.L.; Gaines, M.K.; Weaver, J.H.; Debboun, M. Field evaluation of the Off! Clip-on mosquito repellent (Metofluthrin) against Aedes albopictus and Aedes taeniorhynchus (Diptera: Culicidae) in Northeastern Florida. J. Med. Entomol. 2012, 49, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Achee, N.L.; Bangs, M.J.; Farlow, R.; Killeen, G.F.; Lindsay, S.; Logan, J.G.; Moore, S.J.; Rowland, M.; Sweeney, K.; Torr, S.J.; et al. Spatial repellents: From discovery and development to evidence-based validation. Malar. J. 2012, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Bibbs, C.S.; Kaufman, P.E. Volatile pyrethroids as a potential mosquito abatement tool: A review of pyrethroid-containing spatial repellents. J. Integr. Pest Manag. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Guidance on Biocidal Products Regulation Volume II Parts B+C: Assessment and Evaluation, vol. 2.1 (March 2017). Available online: https://echa.europa.eu/guidance-documents/guidance-on-biocides-legislation (accessed on 28 April 2021).
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Stark, J.D.; Banks, J.E. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 2003, 48, 505–519. [Google Scholar] [CrossRef]
- Bibbs, C.S.; Hahn, D.A.; Kaufman, P.E.; Xue, R.-D. Sublethal effects of a vapour-active pyrethroid, transfluthrin, on Aedes aegypti and Ae. albopictus (Diptera: Culicidae) fecundity and oviposition behaviour. Parasit. Vectors 2018, 11, 486. [Google Scholar] [CrossRef]
- Darbro, J.M.; Muzari, M.O.; Giblin, A.; Adamczyk, R.M.; Ritchie, S.A.; Devine, G.J. Reducing biting rates of Aedes aegypti with metofluthrin: Investigations in time and space. Parasit. Vectors 2017, 10, 69. [Google Scholar] [CrossRef]
- Bibbs, C.S.; Bloomquist, J.R.; Hahn, D.A.; Kaufman, P.E.; Xue, R.-D. Gone in 60 seconds: Sub-lethal effects of metofluthrin vapors on behavior and fitness of resistant and field strains of Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2019, 56, 1087–1094. [Google Scholar] [CrossRef]
- Yap, H.H.; Lim, M.P.; Chong, N.L.; Lee, C.Y. Efficacy and sublethal effects of mosquito coils on Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). In Proceedings of the the Second International Conference on Urban Pests, Edinburgh, Scotland, 7–10 July 1996; pp. 177–184. Available online: http://www.chowyang.com/uploads/2/4/3/5/24359966/011.pdf (accessed on 27 April 2021).
- Chareonviriyaphap, T. Behavioral responses of mosquitoes to insecticides. In Insecticides—Pest Engineering; Perveen, F.K., Ed.; InTech: Rijeka, Croatia, 2012; pp. 201–220. [Google Scholar]
- Matsunaga, T.; Makita, M.; Higo, A.; Nishibe, I.; Dohara, K.; Shinjo, G. Studies on prallethrin, a new synthetic pyrethroid, for indoor applications: I. The insecticidal activities of prallethrin isomers. Med. Entomol. Zool. 1987, 38, 219–223. [Google Scholar] [CrossRef][Green Version]
- Moreno-Gómez, M.; Bueno-Marí, R.; Drago, A.; Miranda, M.A. From the field to the laboratory: Quantifying outdoor mosquito landing rate to better evaluate topical repellents. J. Med. Entomol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gómez, M.; Bueno-Marí, R.; Carr, B.T.; Bowman, G.R.; Faherty, G.W.; Gobbi, C.; Palm, J.M.; Van Sloun, P.; Miranda, M.Á. Two new alternatives to the conventional arm-in-cage test for assessing topical repellents. J. Med. Entomol. 2021. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency (ECHA). Product Type 18—Insecticides, Acaricides and Products to Control other Arthropods and Product Type 19—Repellents and Attractants (only Concerning Arthropods). 2011. Available online: https://echa.europa.eu/documents/10162/16960215/bpd_guid_tnsg_efficacy_pt18-19_final_en.pdf/9c72241e-0eea-4f23-8e5f-f52d00a83382 (accessed on 27 April 2021).
- Debboun, M.; Frances, S.P.; Strickman, D. Insect Repellents Handbook, 2nd ed.; Debboun, M., Frances, S.P., Strickman, D., Eds.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD). Guidance Document on Assays for Testing the Efficacy of Baits against Cockroaches; Series on Testing and Assessment No. 183; OECD Publishing: París, France, 2013; Volume 3, p. JT03333177. Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2013)3&doclanguage=en (accessed on 28 April 2021).
- National Institute for Public Health and the Environment. ConsExpo. Available online: https://www.rivm.nl/en/consexpo (accessed on 28 April 2021).
- European Chemicals Agency (ECHA). Default human factor values for use in exposure assessments for biocidal products. In Recommendation No. 14 of the BPC Ad Hoc Working Group on Human Exposure; ECHA Publishing: Helsinki, Finland, 2017; Available online: https://echa.europa.eu/documents/10162/21664016/recom_14+_default+human_factor_values_biocidal+products_en.pdf/88354d31-8a3a-475a-9c7d-d8ef8088d004 (accessed on 28 April 2021).
- European Chemicals Agency (ECHA). Technical Agreements for Biocides Human Health (TOX). 2018. Available online: https://webgate.ec.europa.eu/s-circabc/sd/d/0428d181-3849-4fe7-806e-936ceb32f693/TOX-TAB_version_2_0.pdf (accessed on 28 April 2021).
- Organisation for Economic Co-Operation and Development (OECD). Emission Scenario Document for Insecticides, Acaricides and Products to Control Other Arthropods for Household and Professional Uses; Series on Emission Scenario No. 18; OECD Publishing: Paris, France, 2008; Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?doclanguage=en&cote=env/jm/mono(2008)14 (accessed on 28 April 2021).
- Beament, J.; Corbet, S.A. Surface properties of Culex pipiens pipiens eggs and the behaviour of the female during egg-raft assembly. Physiol. Entomol. 1981, 6, 135–148. [Google Scholar] [CrossRef]
- Isaacs, A.; Lynd, A.; Donnelly, M. Insecticide-induced leg loss does not eliminate biting and reproduction in Anopheles gambiae mosquitoes. Sci. Rep. 2017, 7, 46674. [Google Scholar] [CrossRef]
- Khoo, B.K.; Sutherland, D.J. Leg Fracture in Adult Mosquitos Induced by Bioresmethrin. Mosq. News. 1981, 41, 802–804. [Google Scholar]
- Wu, C.W.; Kong, X.; Wu, D. Micronanostructures of the scales on a mosquito’s legs and their role in weight support. Phys. Rev. E 2007, 76, 017301. [Google Scholar] [CrossRef]
- Balabanidou, V.; Kefi, M.; Aivaliotis, M.; Koidou, V.; Girotti, J.R.; Mijailovsky, S.J.; Juárez, M.P.; Papadogiorgaki, E.; Chalepakis, G.; Kampouraki, A.; et al. Mosquitoes cloak their legs to resist insecticides. Proc. R. Soc. B 2019, 286, 20191091. [Google Scholar] [CrossRef]
- Soderlund, D.M.; Bloomquist, J.R. Neurotoxic actions of pyrethroid insecticides. Annu. Rev. Entomol. 1989, 34, 77–96. [Google Scholar] [CrossRef]
- Tooming, E.; Merivee, E.; Must, A.; Merivee, M.-I.; Sibul, I.; Nurme, K.; Williams, I.H. Behavioural effects of the neonicotinoid insecticide thiamethoxam on the predatory insect Platynus assimilis. Ecotoxicology 2017, 26, 902–913. [Google Scholar] [CrossRef]
- Kong, X.Q.; Liu, J.L.; Zhang, W.J.; Qu, Y.D. Load-bearing ability of the mosquito tarsus on water surfaces arising from its flexibility. AIP Adv. 2015, 5, 037101. [Google Scholar] [CrossRef]
- Davidson, G. Studies on insecticide resistance in anopheline mosquitos. Bull. World Health Organ. 1958, 18, 579–621. [Google Scholar]
- Unlu, I.; Farajollahi, A.; Rochlin, I.; Crepeau, T.N.; Strickman, D.; Gaugler, R. Differences in male–female ratios of Aedes albopictus (Diptera: Culicidae) following ultra-low volume adulticide applications. Acta Trop. 2014, 137, 201–205. [Google Scholar] [CrossRef]
- Boubidi, S.C.; Rossignol, M.; Chandre, F.; Tounsi, R.; Lagneau, C.; Fontenille, D.; Reiter, P. Gender bias in insecticide susceptibility of Aedes albopictusis solely attributable to size. J. Am. Mosq. Control. Assoc. 2016, 32, 251–253. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes, 2nd ed.; Updated June 2018; WHO: Geneva, Switzerland, 2018; Available online: https://apps.who.int/iris/bitstream/handle/10665/250677/9789241511575-eng.pdf (accessed on 28 April 2021).
- Buhagiar, T.S.; Devine, G.J.; Ritchie, S.A. Effects of sublethal exposure to metofluthrin on the fitness of Aedes aegypti in a domestic setting in Cairns, Queensland. Parasit. Vectors 2017, 10, 274. [Google Scholar] [CrossRef]
- Kawada, H.; Iwasaki, T.; Le Loan, L.; Tien, T.K.; Mai, N.T.; Shono, Y.; Katayama, Y.; Takagi, M. Field evaluation of spatial repellency of metofluthrin-impregnated latticework plastic strips against Aedes aegypti (L.) and analysis of environmental factors affecting its efficacy in My Tho City, Tien Giang, Vietnam. Am. J. Trop. Med. Hyg. 2006, 75, 1153–1157. [Google Scholar] [CrossRef]
- Maciver, D.R. Mosquito coils-Part II. Studies on the action of mosquito coil smoke on mosquitoes. Pyrethrum Post 1964, 7, 7–14. [Google Scholar]
- Enayati, A.; Hemingway, J. Malaria management: Past, present, and future. Annu. Rev. Entomol. 2010, 55, 569–591. [Google Scholar] [CrossRef] [PubMed]
- Boonyuan, W.; Kongmee, M.; Bangs, M.J.; Prabaripai, A.; Chareonviriyaphap, T. Host feeding responses of Aedes aegypti (L.) exposed to deltamethrin. J. Vector Ecol. 2011, 36, 361–372. [Google Scholar] [CrossRef]
- Dye-Braumuller, K.C.; Haynes, K.F.; Brown, G.C. Quantitative analysis of Aedes albopictus movement behavior following sublethal exposure to prallethrin. J. Am. Mosq. Control. Assoc. 2017, 33, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Wagman, J.M.; Achee, N.L.; Grieco, J.P. Insensitivity to the spatial repellent action of transfluthrin in Aedes aegypti: A heritable trait associated with decreased insecticide susceptibility. PLoS Negl. Trop. Dis. 2015, 9, e0003726. [Google Scholar] [CrossRef]
- Valerio, L.; Marini, F.; Bongiorno, G.; Facchinelli, L.; Pombi, M.; Caputo, B.; Maroli, M.; della Torre, A. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in urban and rural contexts within Rome province, Italy. Vector Borne Zoonotic Dis. 2010, 10, 291–294. [Google Scholar] [CrossRef]
- Dusfour, I.; Achee, N.L.; Roberts, D.R.; Grieco, J.P. Contact irritancy and spatial repellency behaviors in Anopheles albimanus Wiedemann (Diptera: Culicidae) collected in Orange Walk, Belize, CA. J. Vector Ecol. 2009, 34, 232–237. [Google Scholar] [CrossRef]
- Lengeler, C. Insecticide-treated bednets and curtains for preventing malaria. In The Cochrane Database of Systematic Reviews (Complete Reviews); John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1998. [Google Scholar]
- Noor, A.M.; Mutheu, J.J.; Tatem, A.J.; Hay, S.I.; Snow, R.W. Insecticide-treated net coverage in Africa: Mapping progress in 2000-07. Lancet 2009, 373, 58–67. [Google Scholar] [CrossRef]
- Lee, C.-Y. Sublethal effects of insecticides on longevity, fecundity and behaviour of insect pests: A review. J. Biosci. 2000, 11, 107–112. [Google Scholar]
- Haynes, K.F. Sublethal effects of neurotoxic insecticides on insect behavior. Annu. Rev. Entomol. 1988, 33, 149–168. [Google Scholar] [CrossRef]
- Collins, E.; Vaselli, N.M.; Sylla, M.; Beavogui, A.H.; Orsborne, J.; Lawrence, G.; Wiegand, R.E.; Irish, S.R.; Walker, T.; Messenger, L.A. The relationship between insecticide resistance, mosquito age and malaria prevalence in Anopheles gambiae s.l. from Guinea. Sci. Rep. 2019, 9, 8846. [Google Scholar] [CrossRef]
- Protopopoff, N.; Matowo, J.; Malima, R.; Kavishe, R.; Kaaya, R.; Wright, A.; West, P.A.; Kleinschmidt, I.; Kisinza, W.; Mosha, F.W.; et al. High level of resistance in the mosquito Anopheles gambiae to pyrethroid insecticides and reduced susceptibility to bendiocarb in north-western Tanzania. Malar. J. 2013, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Kleinschmidt, I.; Bradley, J.; Knox, T.B.; Mnzava, A.P.; Kafy, H.T.; Mbogo, C.; Ismail, B.A.; Bigoga, J.D.; Adechoubou, A.; Raghavendra, K.; et al. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: A WHO-coordinated, prospective, international, observational cohort study. Lancet Infect. Dis. 2018, 18, 640–649. [Google Scholar] [CrossRef]
- European Commission. Report on the Fees Payable to Members States Competent Authorities Pursuant to Article 80(2) of the Biocidal Product Regulation. 2014. Available online: https://circabc.europa.eu/sd/a/896cf317-7b62-4604-a736-c18e02fc3ead/CA-Nov14-Doc.7.2%20-%20Report%20on%20fees.doc (accessed on 28 April 2021).
- Townson, H.; Nathan, M.B.; Zaim, M.; Guillet, P.; Manga, L.; Bos, R.; Kindhauser, M. Exploiting the potential of vector control for disease prevention. Bull. World Health Organ. 2005, 83, 942–947. [Google Scholar] [PubMed]






| Parameter | Value |
|---|---|
| Spray duration | 24 h (worst-case scenario) |
| Exposure duration | To be determined (max. number of hours that exposure remained safe for adults and children) |
| Weight fraction compound | 100% (the prallethrin release rate is considered in the mass generation rate) |
| Room volume | 16 m3 (fixed value) |
| Room height | 2.5 m (fixed value) |
| Ventilation rate | 1/h (fixed value) |
| Inhalation rate | 16 m3/d (adult) |
| 10.1 m3/d (child of 2–3 years old) | |
| Mass generation rate | 4.03 × 10−5 g/s (=1.6 mg/h) |
| 2.27 × 10−5 g/s (=0.8 mg/h) | |
| 1.02 × 10−5 g/s (=0.4 mg/h) | |
| Airborne fraction | 1 (fixed value) |
| Density, non-volatile | 0.85 g/cm3 (density corrected to formulation) |
| Inhalation cut-off diameter | 15 µm (fixed value) |
| Aerosol diameter distribution | log normal (fixed value) |
| Median diameter | 8 µm (fixed value) |
| Coefficient of variation | 0.3 (fixed value) |
| Maximum diameter | 50 µm (fixed value) |
| Body weight | 60 kg (adult), 15.6 kg (child 2–3 years old) |
| Absorption | 100% (fixed value) |
| Parameter | Value |
|---|---|
| Weight fraction compound | 100% (the prallethrin release rate is considered in the mass generation rate) |
| Transfer coefficient 1 | 0.24 m2/h (fixed value) |
| Dislodgeable amount | 2.93 mg/m2 |
| Contact time | 60 min (fixed value) |
| Rubbed surface | 7 m2 (fixed value) |
| Absorption model | Fixed fraction |
| Absorption | 6% (based on experimental results provided by the AS supplier) |
| Species | Sex | Treatment Comparisons | χ2 | p-Value |
|---|---|---|---|---|
| Ae. albopictus | Females | Untreated vs. negative control 1 | - | - |
| Controls vs. prallethrin groups 2 | - | p < 0.0001 in all cases | ||
| 0.4 mg/h vs. 0.8 mg/h | 34.59 | p < 0.0001 | ||
| 0.4 mg/h vs. 1.6 mg/h | 63.02 | p < 0.0001 | ||
| 0.8 mg/h vs. 1.6 mg/h | 6.18 | p < 0.05 | ||
| Males | Untreated vs. negative control | 17.03 | p < 0.0001 | |
| Controls vs. prallethrin groups 2 | - | p < 0.0001 in all cases | ||
| 0.4 mg/h vs. 0.8 mg/h | 61.76 | p < 0.0001 | ||
| 0.4 mg/h vs. 1.6 mg/h | 65.21 | p < 0.0001 | ||
| 0.8 mg/h vs. 1.6 mg/h | 0.15 | p = 0.698 | ||
| Cx. pipiens | Females | Untreated vs. negative control 1 | - | - |
| Controls vs. prallethrin groups 2 | - | p < 0.0001 in all cases | ||
| 0.4 mg/h vs. 0.8 mg/h | 39.88 | p < 0.0001 | ||
| 0.4 mg/h vs. 1.6 mg/h | 67.29 | p < 0.0001 | ||
| 0.8 mg/h vs. 1.6 mg/h | 5.49 | p < 0.05 | ||
| Males | Untreated vs. negative control | - | - | |
| Controls vs. prallethrin groups 2 | - | p < 0.0001 in all cases | ||
| 0.4 mg/h vs. 0.8 mg/h | 25.28 | p < 0.0001 | ||
| 0.4 mg/h vs. 1.6 mg/h | 102.49 | p < 0.0001 | ||
| 0.8 mg/h vs. 1.6 mg/h | 22.23 | p < 0.0001 |
| Species | Sex | Treatment Comparisons | χ2 | p-Value |
|---|---|---|---|---|
| Ae. albopictus | Females | Untreated vs. negative control | 3.15 | p = 0.07 |
| Controls vs. prallethrin groups 1 | - | p < 0.0001 in all cases | ||
| 0.4 mg/h vs. 0.8 mg/h | 0.15 | p = 0.69 | ||
| 0.4 mg/h vs. 1.6 mg/h | 6.40 | p < 0.05 | ||
| 0.8 mg/h vs. 1.6 mg/h | 5.72 | p < 0.05 | ||
| Males | Untreated vs. negative control | 6.32 | p < 0.05 | |
| Controls vs. prallethrin groups 1 | - | p < 0.0001 in all cases | ||
| 0.4 mg/h vs. 0.8 mg/h | 0.06 | p = 0.80 | ||
| 0.4 mg/h vs. 1.6 mg/h | 2.07 | p = 0.14 | ||
| 0.8 mg/h vs. 1.6 mg/h | 3.66 | p = 0.056 | ||
| Cx. pipiens | Females | Untreated vs. negative control | 3.15 | p = 0.07 |
| Controls vs. prallethrin groups 1 | - | p < 0.0001 in all cases | ||
| 0.4 mg/h vs. 0.8 mg/h | 0.15 | p = 0.69 | ||
| 0.4 mg/h vs. 1.6 mg/h | 6.40 | p < 0.05 | ||
| 0.8 mg/h vs. 1.6 mg/h | 5.72 | p < 0.05 | ||
| Males | Untreated vs. negative control | 1.48 | p = 0.22 | |
| Controls vs. prallethrin groups 1 | - | p < 0.0001 in all cases | ||
| 0.4 mg/h vs. 0.8 mg/h | 0.93 | p = 0.33 | ||
| 0.4 mg/h vs. 1.6 mg/h | 4.69 | p < 0.05 | ||
| 0.8 mg/h vs. 1.6 mg/h | 2.14 | p = 0.14 |
| Variables Measured | Untreated Control | Negative Control | 0.4 mg/h | 0.8 mg/h | 1.6 mg/h | |
|---|---|---|---|---|---|---|
| No. of females alive after 48 h | 117 | 113 | 63 | 49 | 17 | |
| % of females found dead in the egg laying tray | 8.55 | 9.73 | 23.81 | 38.78 | 41.18 | |
| No. third/fourth instar larvae | 4137 | 3985 | 2595 | 2066 | 637 | |
| Ratio of larvae/females | 35.36 | 35.27 | 41.19 | 42.16 | 37.47 | |
| % larvae reaching adulthood | Males | 36 | ND | 35.8 | 39.8 | 39.7 |
| Females | 32.1 | ND | 38.8 | 24.1 | 30.5 | |
| Total no. of adults in F1 population | 2816 | ND | 1936 | 1320 | 447 | |
| % reduction in F1 population size 1 | - | ND | 31.25 | 53.13 | 84.13 | |
| Variables Measured | Untreated Control | Negative Control | 0.4 mg/h | 0.8 mg/h | 1.6 mg/h | |
|---|---|---|---|---|---|---|
| No. of females alive after 48 h | 123 | 117 | 41 | 37 | 21 | |
| % of females found dead in the egg laying tray | 0.81 | 0 | 0 | 5.41 | 0 | |
| No. eggs laid | 3434 | 2525 | 1187 | 508 | 356 | |
| No. third/fourth instar larvae | 1624 | 1104 | 639 | 143 | 173 | |
| Ratio of larvae/females | 13.20 | 9.44 | 15.59 | 3.86 | 8.24 | |
| % larvae reaching adulthood | Males | 37.32 | 37.77 | 33.80 | 41.26 | 37.57 |
| Females | 42.86 | 41.30 | 46.32 | 58.04 | 38.15 | |
| Total no. of adults in F1 population | 1.302 | 873 | 512 | 110 | 131 | |
| % reduction in F1 population size 1 | - | 32.95 | 60.68 | 91.55 | 89.94 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Gómez, M.; Bueno-Marí, R.; Miranda, M.A. A Three-Pronged Approach to Studying Sublethal Insecticide Doses: Characterising Mosquito Fitness, Mosquito Biting Behaviour, and Human/Environmental Health Risks. Insects 2021, 12, 546. https://doi.org/10.3390/insects12060546
Moreno-Gómez M, Bueno-Marí R, Miranda MA. A Three-Pronged Approach to Studying Sublethal Insecticide Doses: Characterising Mosquito Fitness, Mosquito Biting Behaviour, and Human/Environmental Health Risks. Insects. 2021; 12(6):546. https://doi.org/10.3390/insects12060546
Chicago/Turabian StyleMoreno-Gómez, Mara, Rubén Bueno-Marí, and Miguel. A. Miranda. 2021. "A Three-Pronged Approach to Studying Sublethal Insecticide Doses: Characterising Mosquito Fitness, Mosquito Biting Behaviour, and Human/Environmental Health Risks" Insects 12, no. 6: 546. https://doi.org/10.3390/insects12060546
APA StyleMoreno-Gómez, M., Bueno-Marí, R., & Miranda, M. A. (2021). A Three-Pronged Approach to Studying Sublethal Insecticide Doses: Characterising Mosquito Fitness, Mosquito Biting Behaviour, and Human/Environmental Health Risks. Insects, 12(6), 546. https://doi.org/10.3390/insects12060546








