Characteristics of the Peritrophic Matrix of the Silkworm, Bombyx mori and Factors Influencing Its Formation
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Thickness of PM
2.3. PM Permeability
2.4. Transmission Electron Microscopy (TEM)
2.5. Scanning Electron Microscopy (SEM)
2.6. Antibiotic Treatment
2.7. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.8. Statistical Analysis
3. Results
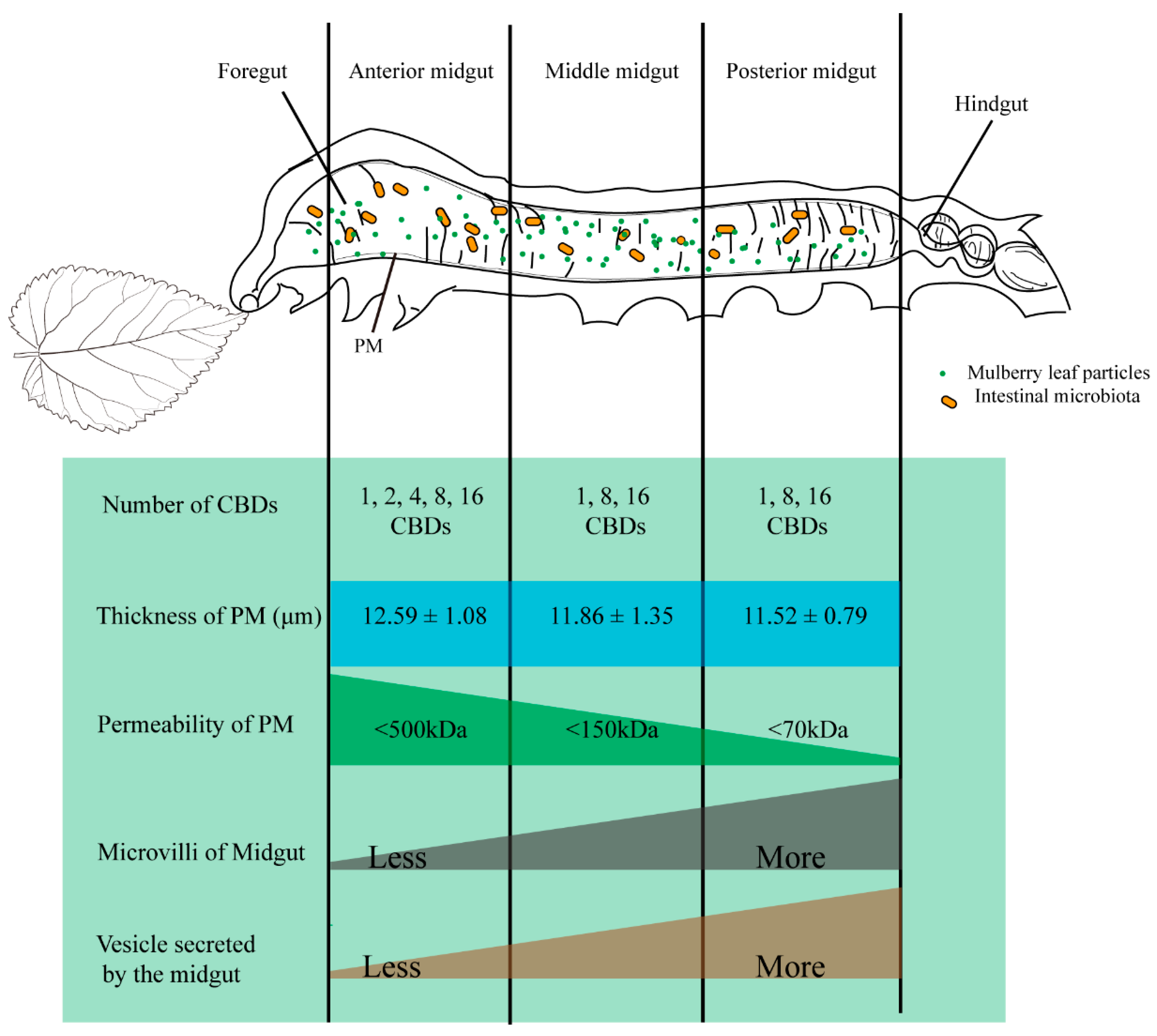
3.1. Measurement of PM Thickness at Different Development Stages of Silkworm
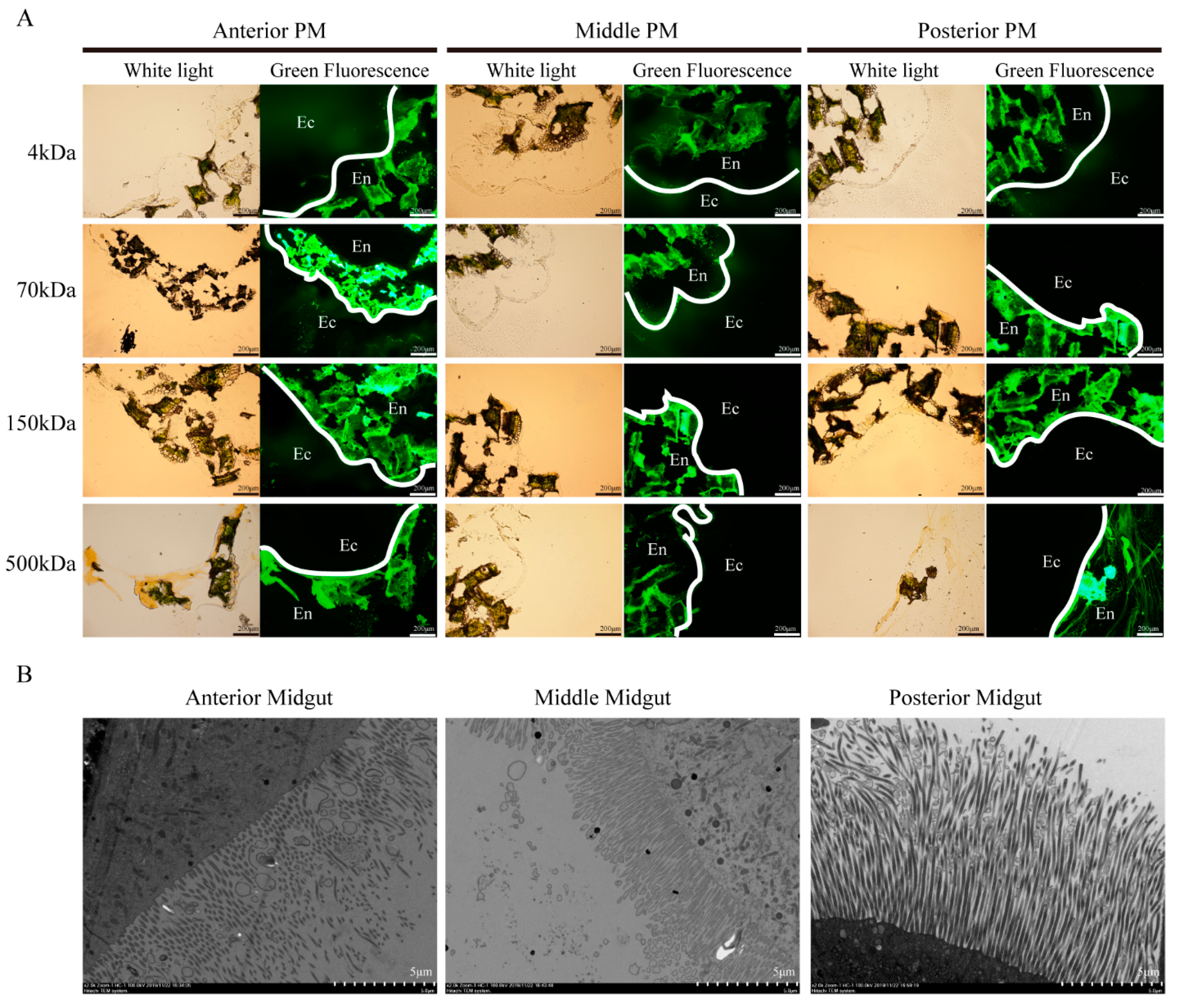
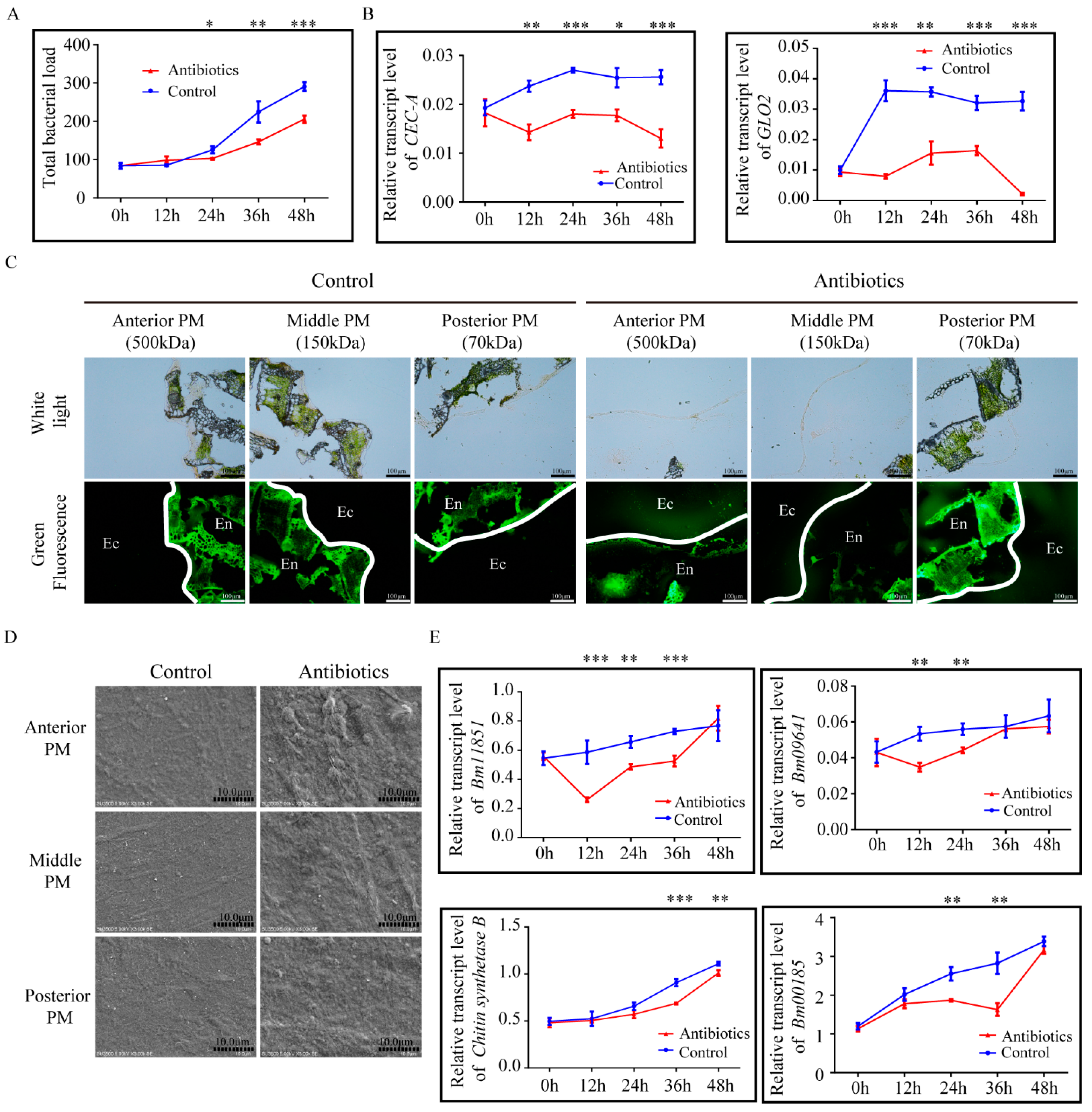
3.2. PM Permeability and Microvilli Morphology of Different Midgut Regions in Silkworm
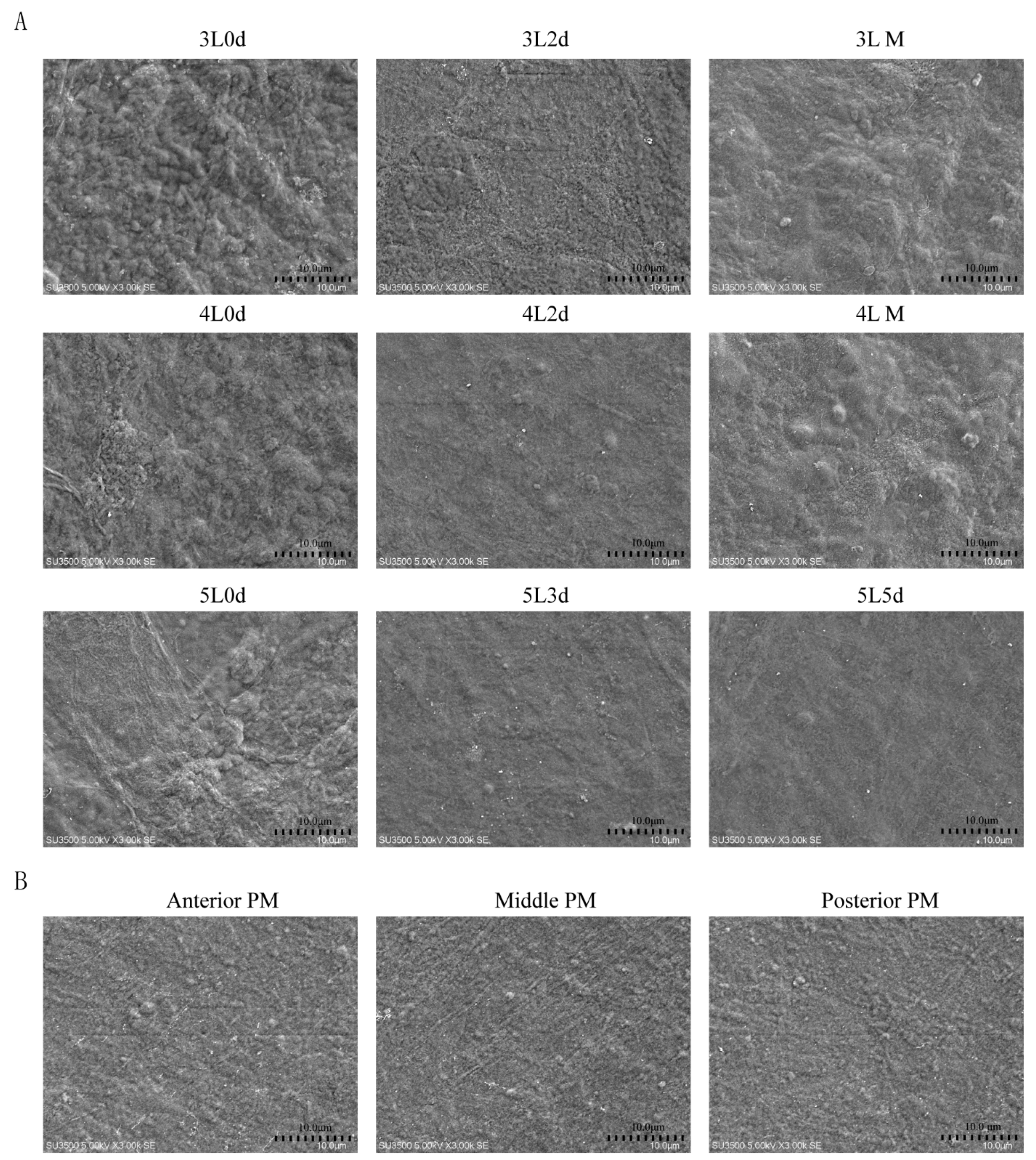
3.3. Silkworm PM Surface Structure at Different Developmental Stages Evaluated by SEM
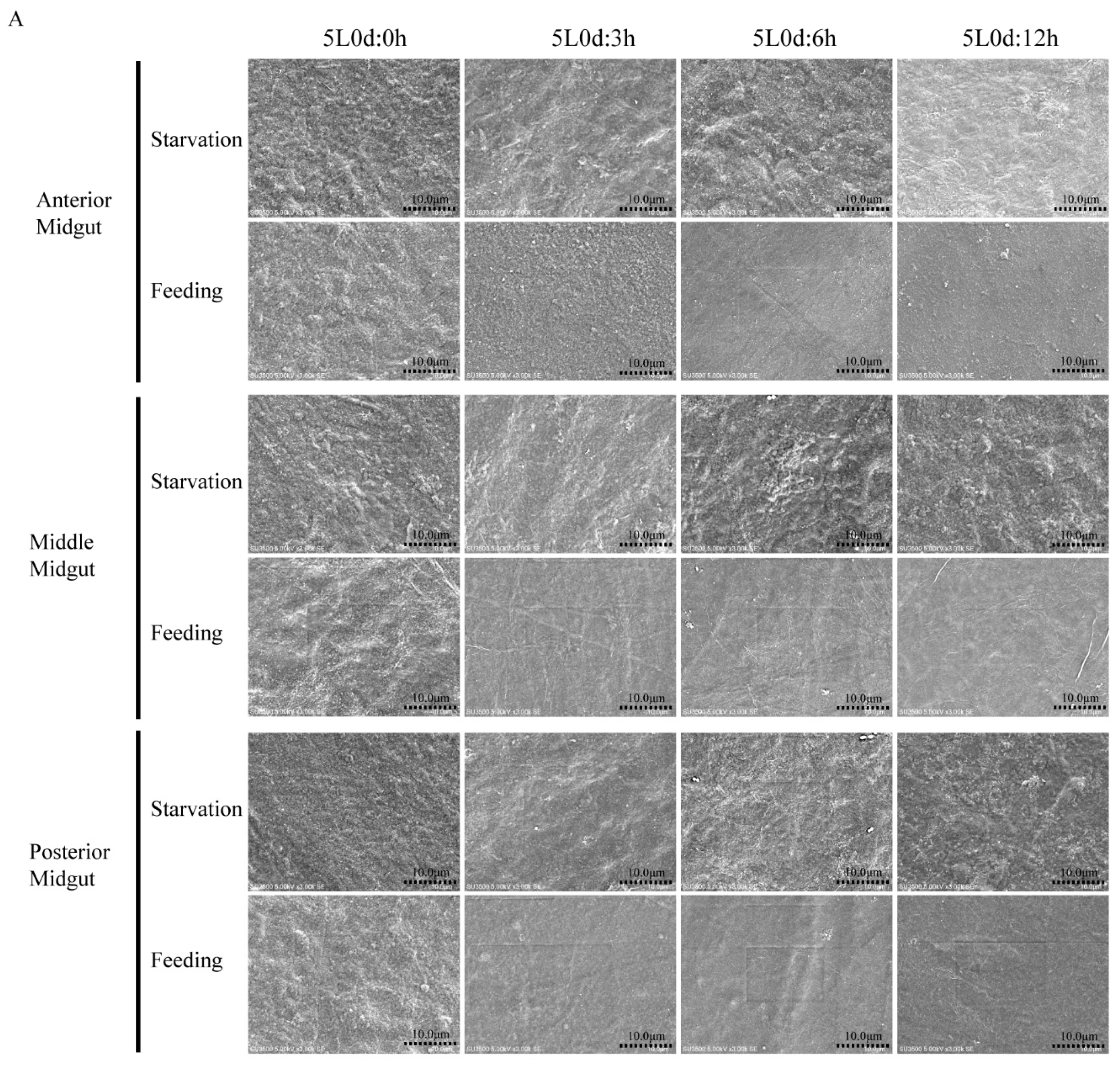
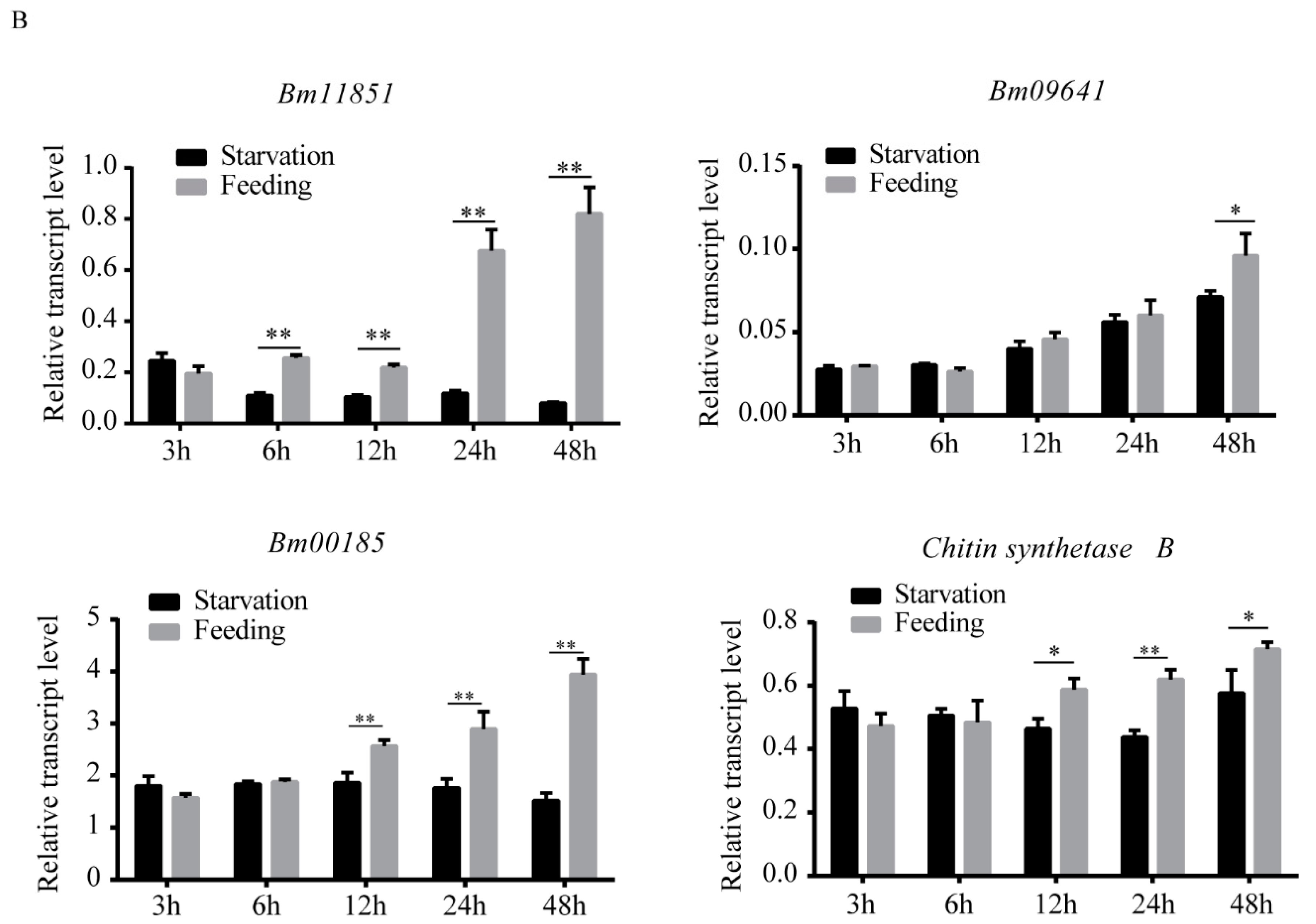
3.4. Silkworm PM Synthesis May Be Induced by Food Ingestion
3.5. Gut Microbiota Play a Role in Maintaining PM Structural Integrity in Silkworm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, N.; Lehane, M.J. Peritrophic membranes, cell surface molecules and parasite tropisms within arthropod vectors. Parasitol. Today 1993, 9, 45–50. [Google Scholar] [CrossRef]
- Elvin, C.M.; Vuocolo, T.; Pearson, R.D.; East, I.J.; Riding, G.A.; Eisemann, C.H.; Tellam, R.L. Characterization of a major peritrophic membrane protein, Peritrophin-44, from the larvae of Lucilia cuprina. J. Biol. Chem. 1996, 271, 8925–8935. [Google Scholar] [CrossRef]
- Tellam, R.L.; Wijffels, G.; Willadsen, P. Peritrophic matrix proteins. Insect Biochem. Mol. Biol. 1998, 29, 87–101. [Google Scholar] [CrossRef]
- Shibata, T.; Maki, K.; Hadano, J.; Fujikawa, T.; Kitazaki, K.; Koshiba, T.; Kawabata, S. Crosslinking of a peritrophic matrix protein protects gut epithelia from bacterial exotoxins. PLoS Pathog. 2015, 11, e1005244. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Kain, W.; Wang, P. Effects of disruption of the peritrophic membrane on larval susceptibility to Bt toxin Cry1Ac in cabbage loopers. J. Insect Physiol. 2019, 117, 103897. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.M.; Falleiros, Â.M.F.; Moscardi, F.; Gregório, E.A. The role of peritrophic membrane in the resistance of Anticarsia gemmatalis larvae (Lepidoptera: Noctuidae) during the infection by its nucleopolyhedrovirus (AgMNPV). Arthropod. Struct. Dev. 2011, 40, 429–434. [Google Scholar] [CrossRef]
- Weiss, B.L.; Savage, A.F.; Griffith, B.C.; Wu, Y.; Aksoy, S. The peritrophic matrix mediates differential infection outcomes in the tsetse fly gut following challenge with commensal, pathogenic, and parasitic microbes. J. Immunol. 2014, 193, 773–782. [Google Scholar] [CrossRef]
- Sadlova, J.; Homola, M.; Myskova, J.; Jancarova, M.; Volf, P. Refractoriness of Sergentomyia schwetzi to Leishmania spp. is mediated by the peritrophic matrix. PLoS Negl. Trop. Dis. 2018, 12, e0006382. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, S. Tsetse peritrophic matrix influences for trypanosome transmission. J. Insect Physiol. 2019, 118, 103919. [Google Scholar] [CrossRef]
- Erlandson, M.A.; Toprak, U.; Hegedus, D.D. Role of the peritrophic matrix in insect-pathogen interactions. J. Insect Physiol. 2019, 117, 103894. [Google Scholar] [CrossRef]
- Bolognesi, R.; Terra, W.R.; Ferreira, C. Peritrophic membrane role in enhancing digestive efficiency. J. Insect Physiol. 2008, 54, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, D.; Erlandson, M.; Gillott, C.; Toprak, U. New insights into peritrophic matrix synthesis, architecture, and function. Annu. Rev. Entomol. 2009, 54, 285–302. [Google Scholar] [CrossRef]
- Rodríguez-de la Noval, C.; Rodríguez-Cabrera, L.; Izquierdo, L.; Espinosa, L.A.; Hernandez, D.; Ponce, M.; Moran-Bertot, I.; Tellez-Rodríguez, P.; Borras-Hidalgo, O.; Huang, S. Functional expression of a peritrophin A-like SfPER protein is required for larval development in Spodoptera frugiperda (Lepidoptera: Noctuidae). Sci. Rep. 2019, 9, 2630. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Yang, J.; Niu, N.; Zhang, J.; Yang, Q. Mucin family genes are essential for the growth and development of the migratory locust, Locusta migratoria. Insect Biochem. Mol. Biol. 2020, 123, 103404. [Google Scholar] [CrossRef]
- Agrawal, S.; Kelkenberg, M.; Begum, K.; Steinfeld, L.; Williams, C.E.; Kramer, K.J.; Beeman, R.W.; Park, Y.; Muthukrishnan, S.; Merzendorfer, H. Two essential peritrophic matrix proteins mediate matrix barrier functions in the insect midgut. Insect Biochem. Mol. Biol. 2014, 49, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Casu, R.; Eisemann, C.; Pearson, R.; Riding, G.; East, I.; Donaldson, A.; Cadogan, L.; Tellam, R. Antibody-mediated inhibition of the growth of larvae from an insect causing cutaneous myiasis in a mammalian host. Proc. Natl. Acad. Sci. USA 1997, 94, 8939–8944. [Google Scholar] [CrossRef]
- Malta, J.; Martins, G.F.; Weng, J.L.; Fernandes, K.M.; Munford, M.L.; Ramalho-Ortigão, M. Effects of specific antisera targeting peritrophic matrix-associated proteins in the sand fly vector Phlebotomus papatasi. Acta Tropica 2016, 159, 161–169. [Google Scholar] [CrossRef]
- Oliveira, A.H.; Fernandes, K.M.; Goncalves, W.G.; Zanuncio, J.C.; Serrao, J.E. A peritrophin mediates the peritrophic matrix permeability in the workers of the bees Melipona quadrifasciata and Apis mellifera. Arthropod. Struct. Dev. 2019, 53, 100885. [Google Scholar] [CrossRef]
- Khajuria, C.; Buschman, L.L.; Chen, M.S.; Muthukrishnan, S.; Zhu, K.Y. A gut-specific chitinase gene essential for regulation of chitin content of peritrophic matrix and growth of Ostrinia nubilalis larvae. Insect Biochem. Mol. Biol. 2010, 40, 621–629. [Google Scholar] [CrossRef]
- Shao, L.; Devenport, M.; Jacobs-Lorena, M. The peritrophic matrix of hematophagous insects. Arch. Insect Biochem. Physiol. 2001, 47, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Toprak, U.; Erlandson, M.; Hegedus, D.D. Peritrophic matrix proteins. Trends Entomol. 2010, 6, 23–51. [Google Scholar]
- Jacobs-Lorena, M.; Shen, Z. A type I peritrophic matrix protein from the malaria vector anopheles gambiae binds to chitin. J. Biol. Chem. 1998, 273, 17665–17670. [Google Scholar]
- Tellam, R.L.; Eisemann, C.; Casu, R.; Pearson, R. The intrinsic peritrophic matrix protein peritrophin-95 from larvae of Lucilia cuprina is synthesised in the cardia and regurgitated or excreted as a highly immunogenic protein. Insect Biochem. Mol. Biol. 2000, 30, 9–17. [Google Scholar] [CrossRef]
- Kawakita, H.; Miyamoto, K.; Wada, S.; Mitsuhashi, W. Analysis of the ultrastructure and formation pattern of the peritrophic membrane in the cupreous chafer, Anomala cuprea (Coleoptera: Scarabaeidae). Appl. Entomol. Zool. 2015, 51, 133–142. [Google Scholar] [CrossRef]
- Teixeira, A.D.D.; Marques-Araujo, S.; Zanuncio, J.C.; Serrao, J.E. Peritrophic membrane origin in adult bees (Hymenoptera): Immunolocalization. Micron 2015, 68, 91–97. [Google Scholar] [CrossRef]
- Harper, M.S.; Granados, R.R. Peritrophic membrane structure and formation of larval Trichoplusia ni with an investigation on the secretion patterns of a PM mucin. Tissue Cell 1999, 31, 202–211. [Google Scholar] [CrossRef]
- Bolognesi, R.; Ribeiro, A.F.; Terra, W.R.; Ferreira, C. The peritrophic membrane of Spodoptera frugiperda: Secretion of peritrophins and role in immobilization and recycling digestive enzymes. Arch. Insect Biochem. Physiol. 2001, 47, 62–75. [Google Scholar] [CrossRef]
- Terra, W.R. The origin and functions of the insect peritrophic membrane and peritrophic gel. Arch. Insect Biochem. Physiol. 2001, 47, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, D.D.; Toprak, U.; Erlandson, M. Peritrophic matrix formation. J. Insect Physiol. 2019, 117, 103898. [Google Scholar] [CrossRef] [PubMed]
- Devenport, M.; Fujioka, H.; Jacobs-Lorena, M. Storage and secretion of the peritrophic matrix protein Ag-Aper1 and trypsin in the midgut of Anopheles gambiae. Insect Mol. Biol. 2004, 13, 349–358. [Google Scholar] [CrossRef]
- Devenport, M.; Fujioka, H.; Donnelly-Doman, M.; Shen, Z.; Jacobs-Lorena, M. Storage and secretion of Ag-Aper14, a novel peritrophic matrix protein, and Ag-Muc1 from the mosquito Anopheles gambiae. Cell Tissue Res. 2005, 320, 175–185. [Google Scholar] [CrossRef]
- Toprak, U.; Baldwin, D.; Erlandson, M.; Gillott, C.; Hegedus, D.D. Insect intestinal mucins and serine proteases associated with the peritrophic matrix from feeding, starved and moulting Mamestra configurata larvae. Insect Mol. Biol. 2010, 19, 163–175. [Google Scholar] [CrossRef]
- Toprak, U.; Hegedus, D.D.; Baldwin, D.; Coutu, C.; Erlandson, M. Spatial and temporal synthesis of Mamestra configurata peritrophic matrix through a larval stadium. Insect Biochem. Mol. Biol. 2014, 54, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.J.; Huang, L.X.; Hu, D.; Liu, L.Y.; Gu, J.; Huang, L.H.; Feng, Q.L. Cloning, expression and chitin-binding activity of two peritrophin-like protein genes in the common cutworm, Spodoptera litura. Insect Sci. 2013, 21, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.R. Composition and functional roles of the gut microbiota in mosquitoes. Curr. Opin. Insect Sci. 2018, 28, 59–65. [Google Scholar] [CrossRef]
- Li, F.; Li, M.; Mao, T.; Wang, H.; Chen, J.; Lu, Z.; Qu, J.; Fang, Y.; Gu, Z.; Li, B. Effects of phoxim exposure on gut microbial composition in the silkworm, Bombyx mori. Ecotox. Environ. Saf. 2020, 189, 110011. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, Z.; Yu, J.; Li, Z.; Liu, X.; Xu, H. Comparison of gut bacterial communities and their associations with host diets in four fruit borers. Pest Manag. Sci. 2020, 76, 1353–1362. [Google Scholar] [CrossRef]
- Narasimhan, S.; Rajeevan, N.; Liu, L.; Zhao, Y.O.; Heisig, J.; Pan, J.; Eppler-Epstein, R.; Deponte, K.; Fish, D.; Fikrig, E. Gut microbiota of the tick vector Ixodes scapularis modulate colonization of the Lyme disease spirochete. Cell Host Microbe 2014, 15, 58–71. [Google Scholar] [CrossRef]
- Gao, L.; Song, X.; Wang, J. Gut microbiota is essential in PGRP-LA regulated immune protection against Plasmodium berghei infection. Parasit Vectors 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, M.; Dong, L.; Zhu, H.; Wang, J. PGRP-LD mediates A. stephensi vector competency by regulating homeostasis of microbiota-induced peritrophic matrix synthesis. PLoS Pathog. 2018, 14, e1006899. [Google Scholar] [CrossRef]
- Rodgers, F.H.; Gendrin, M.; Wyer, C.A.S.; Christophides, G.K. Microbiota-induced peritrophic matrix regulates midgut homeostasis and prevents systemic infection of malaria vector mosquitoes. PLoS Pathog. 2017, 13, e1006391. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Wan, Q.X.; Zhao, Q.; Wang, K.X.; Zha, X.F. Spliceosomal protein gene bmspx regulates reproductive organ development in Bombyx mori. Int. J. Mol. Sci. 2020, 21, 2579. [Google Scholar] [CrossRef]
- Zha, X.L.; Yu, X.B.; Zhang, H.Y.; Wang, H.; Huang, X.Z.; Shen, Y.H.; Lu, C. Identification of peritrophins and antiviral effect of Bm01504 against BmNPV in the silkworm, Bombyx mori. Int. J. Mol. Sci. 2020, 21, 7973. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, E.; Genersch, E. Honey bee larval peritrophic matrix degradation during infection with Paenibacillus larvae, the aetiological agent of American foulbrood of honey bees, is a key step in pathogenesis. Environ. Microbiol. 2013, 15, 2894–2901. [Google Scholar] [PubMed]
- Sandoval-Mojica, A.F.; Scharf, M.E. Silencing gut genes associated with the peritrophic matrix of Reticulitermes flavipes (Blattodea: Rhinotermitidae) increases susceptibility to termiticides. Insect Mol. Biol. 2016, 25, 734–744. [Google Scholar] [CrossRef]
- Konno, K.; Mitsuhashi, W. The peritrophic membrane as a target of proteins that play important roles in plant defense and microbial attack. J. Insect Physiol. 2019, 117, 103912. [Google Scholar] [CrossRef]
- Yang, X.; Koci, J.; Smith, A.A.; Zhuang, X.; Sharma, K.; Dutta, S.; Rana, V.S.; Kitsou, C.; Yas, O.B.; Mongodin, E.F. A novel tick protein supports integrity of gut peritrophic matrix impacting existence of gut microbiome and Lyme disease pathogens. Cell. Microbiol. 2020, 23, e13275. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.S.; Hopkins, T.L. Peritrophic membrane structure and secretion in European corn borer larvae (Ostrinia nubilalis). Tissue Cell 1997, 29, 463–475. [Google Scholar] [CrossRef]
- Hopkins, T.L.; Harper, M.S. Lepidopteran peritrophic membranes and effects of dietary wheat germ agglutinin on their formation and structure. Arch. Insect Biochem. Physiol. 2001, 47, 100–109. [Google Scholar] [CrossRef]
- Kenmoku, H.; Ishikawa, H.; Ote, M.; Kuraishi, T.; Kurata, S. A subset of neurons controls the permeability of the peritrophic matrix and midgut structure in Drosophila adults. J. Exp. Biol. 2016, 219, 2331–2339. [Google Scholar]
- Levy, S.M.; Falleiros, Â.M.F.; Moscardi, F.; Gregório, E.A. Susceptibility/resistance of Anticarsia gemmatalis larvae to its nucleopolyhedrovirus (AgMNPV): Structural study of the peritrophic membrane. J. Invertebr. Pathol. 2007, 96, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.; Cardoso, C.; Ribeiro, A.F.; Terra, W.R.; Ferreira, C. Midgut proteins released by microapocrine secretion in Spodoptera frugiperda. J. Insect Physiol. 2013, 59, 70–80. [Google Scholar] [CrossRef] [PubMed][Green Version]
| Name | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| Bm09641 | CTGAAGGTTCGGGCTTGGGT | TGTGCCTGCTGAGTCTGCTGTG |
| Bm01504 | TGGCCTCAGAATGTCGACT | CAATAATCTAAAATCCATAATGCTAC |
| Bm00185 | CATCCTCCCCTGGGCTCAC | CGTAATCAAGGTCATTTGTTCGC |
| Bm11851 | GCAGAACAGGTTTGCGACTG | GCTCAGGCTCTTGTTCTGGT |
| Bm01491 | AAAGCTCCAGGGAGACAACG | TCCTCACCTGGAACGACTCT |
| sw22934 | TTCGTACTGGCTCTTCTCGT | CAAAGTTGATAGCAATTCCCT |
| GLO2 | CTAAATAGACAAATCGGTGGC | GCGGATCTCTGCTTGAAGAC |
| CecA | CTTCGTCTTCGCGTTGGT | AAGGATTTCGCTTGCCCTAT |
| 16sRNA | TACGGGAGGCAGCAG | ATTACCGCGGCTGCTGG |
| PM Thickness [μm] (Mean ± SEM) | ||||
|---|---|---|---|---|
| Instar | Number of PMs | Anterior PM | Middle PM | Posterior PM |
| Third instar | 20 | 9.13 ± 1.55 b | 9.42 ± 1.35 b | 9.85 ± 0.06 b |
| Fourth instar | 20 | 9.33 ± 0.85 b | 9.67 ± 0.54 b | 9.89 ± 1.23 b |
| Fifth instar | 20 | 12.59 ± 1.08 a | 11.86 ± 1.35 a | 11.52 ± 0.79 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zha, X.-L.; Wang, H.; Sun, W.; Zhang, H.-Y.; Wen, J.; Huang, X.-Z.; Lu, C.; Shen, Y.-H. Characteristics of the Peritrophic Matrix of the Silkworm, Bombyx mori and Factors Influencing Its Formation. Insects 2021, 12, 516. https://doi.org/10.3390/insects12060516
Zha X-L, Wang H, Sun W, Zhang H-Y, Wen J, Huang X-Z, Lu C, Shen Y-H. Characteristics of the Peritrophic Matrix of the Silkworm, Bombyx mori and Factors Influencing Its Formation. Insects. 2021; 12(6):516. https://doi.org/10.3390/insects12060516
Chicago/Turabian StyleZha, Xu-Le, Han Wang, Wei Sun, Hong-Yan Zhang, Jin Wen, Xian-Zhi Huang, Cheng Lu, and Yi-Hong Shen. 2021. "Characteristics of the Peritrophic Matrix of the Silkworm, Bombyx mori and Factors Influencing Its Formation" Insects 12, no. 6: 516. https://doi.org/10.3390/insects12060516
APA StyleZha, X.-L., Wang, H., Sun, W., Zhang, H.-Y., Wen, J., Huang, X.-Z., Lu, C., & Shen, Y.-H. (2021). Characteristics of the Peritrophic Matrix of the Silkworm, Bombyx mori and Factors Influencing Its Formation. Insects, 12(6), 516. https://doi.org/10.3390/insects12060516







