Effects of Landscape Patterns and Their Changes to Species Richness, Species Composition, and the Conservation Value of Odonates (Insecta)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
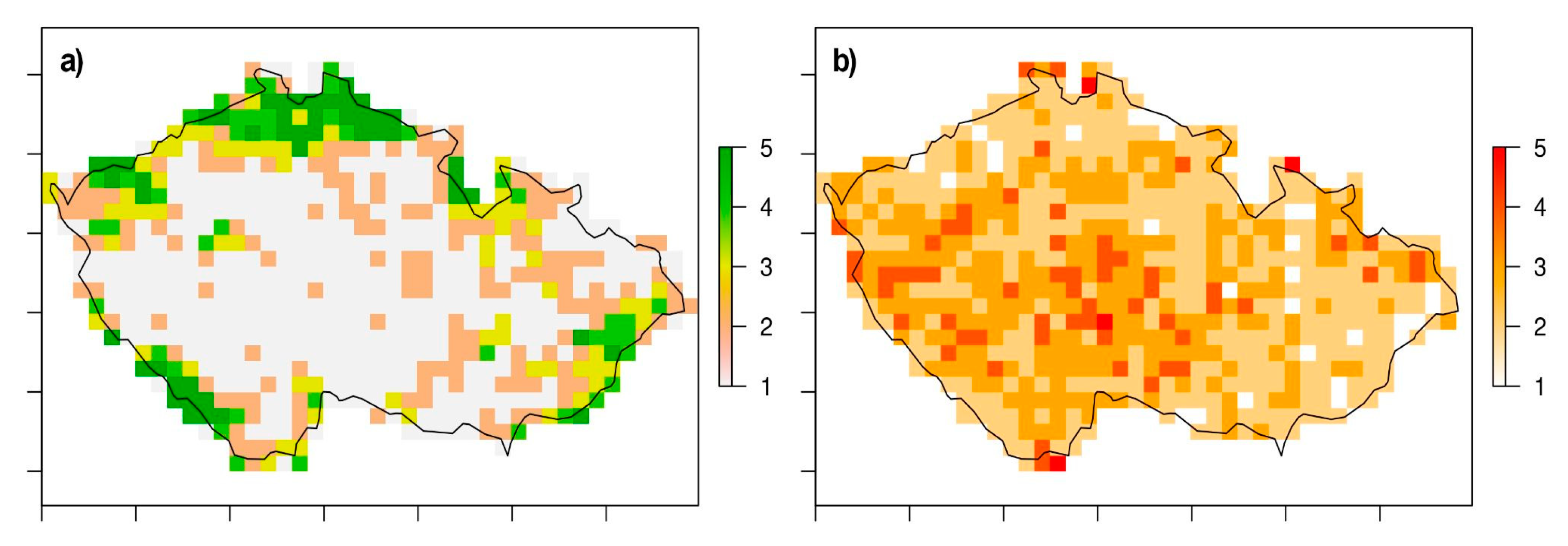
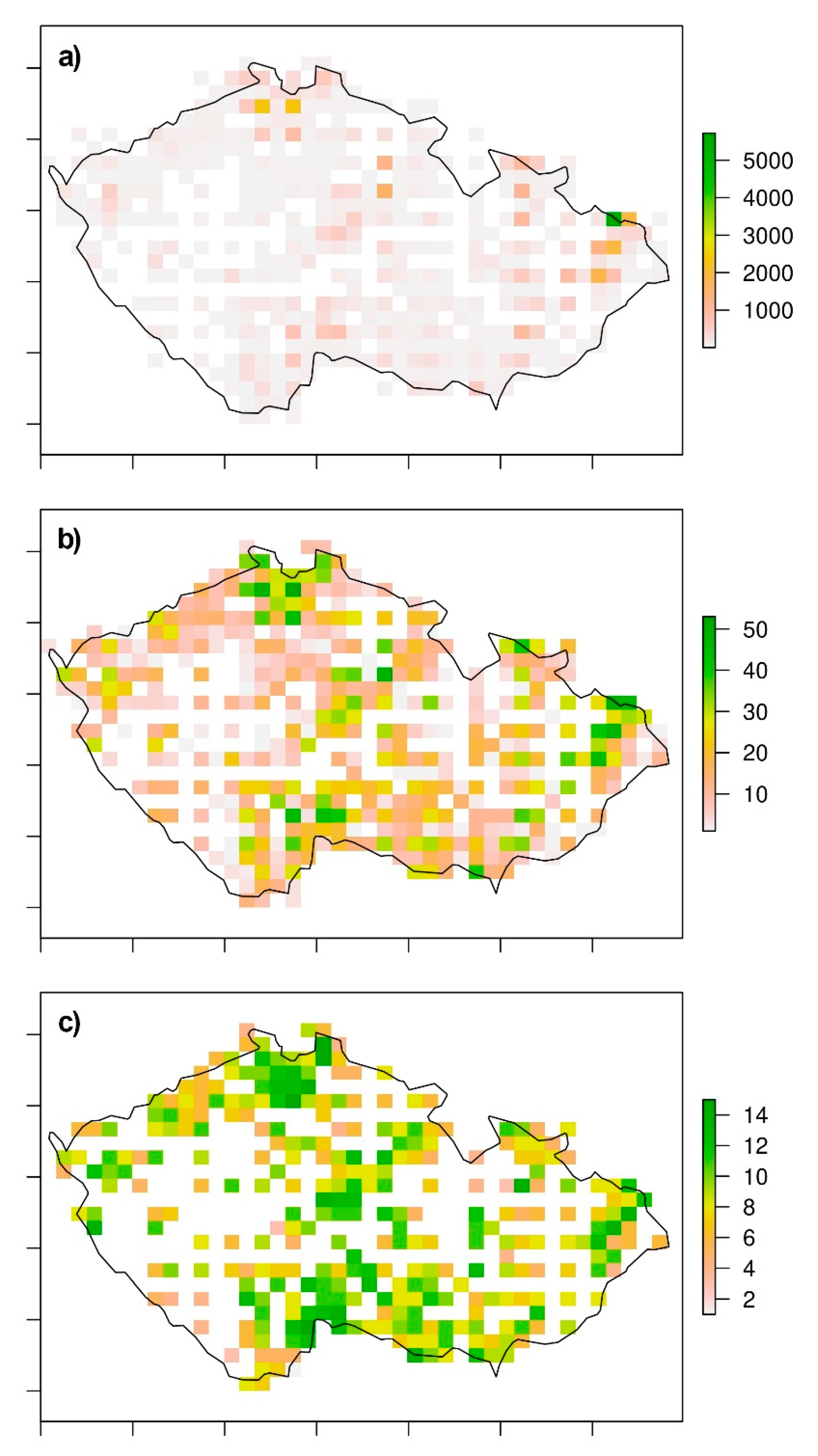
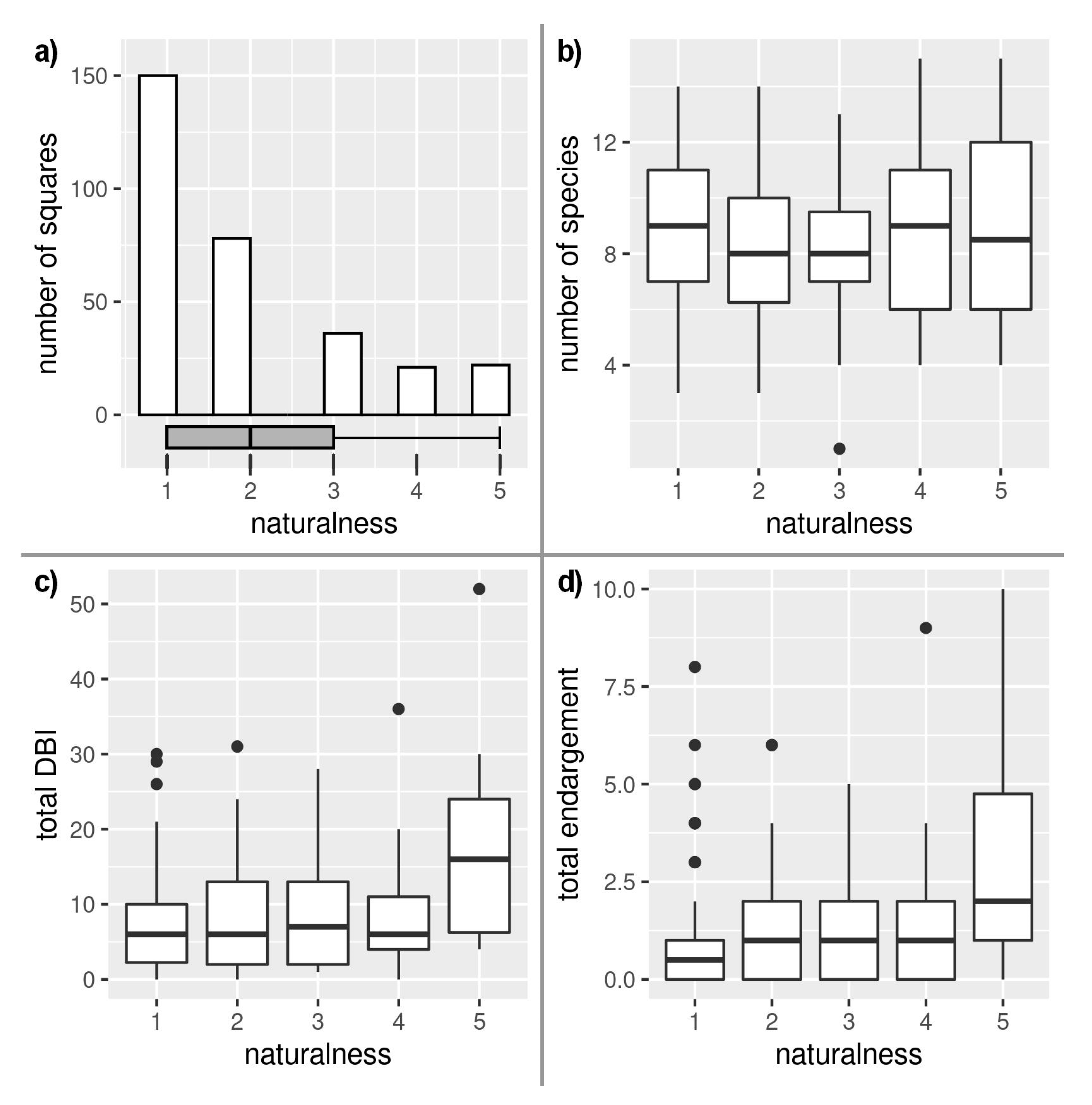
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamer, A.J.; McDonnell, M.J. Amphibian Ecology and Conservation in the Urbanising World: A Review. Biol. Conserv. 2008, 141, 2432–2449. [Google Scholar] [CrossRef]
- Haines-Young, R. Land Use and Biodiversity Relationships. Land Use Policy 2009, 26, S178–S186. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global Effects of Land Use on Local Terrestrial Biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed]
- LaManna, J.A.; Martin, T.E. Logging Impacts on Avian Species Richness and Composition Differ Across Latitudes and Foraging and Breeding Habitat Preferences. Biol. Rev. Camb. Philos. Soc. 2017, 92, 1657–1674. [Google Scholar] [CrossRef]
- Juiling, S.; Leon, S.K.; Jumian, J.; Tsen, S.; Lee, Y.L.; Khoo, E.; Sugau, J.B.; Nilus, R.; Pereira, J.T.; Damit, A.; et al. Conservation assessment and spatial distribution of endemic orchids in Sabah, Borneo. Nat. Conserv. Res. 2020, 5, 136–144. [Google Scholar] [CrossRef]
- Rozhnov, V.V.; Lavrinenko, I.A.; Razzhivin, V.Y.; Makarova, O.L.; Lavrinenko, O.V.; Anufriev, V.V.; Babenko, A.B.; Bizin, M.S.; Glazov, P.M.; Goryachkin, S.V.; et al. Biodiversity revision of a large arctic region as a basis for its monitoring and protection under conditions of active economic development (Nenetsky Autonomous Okrug, Russia). Nat. Conserv. Res. 2019, 4, 181–200. [Google Scholar] [CrossRef]
- Leal, C.G.; Lennox, G.D.; Ferraz, S.F.B.; Ferreira, J.; Gardner, T.A.; Thomson, J.R.; Berenguer, E.; Lees, A.C.; Hughes, R.M.; Mac Nally, R.; et al. Integrated terrestrial-freshwater planning doubles conservation of tropical aquatic species. Science 2020, 370, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, M.; Glińska-Lewczuk, K. Principal threats to the conservation of freshwater habitats in the continental biogeographical region of Central Europe. Biodivers. Conserv. 2019, 28, 4065–4097. [Google Scholar] [CrossRef]
- Pereira, H.M.; Navarro, L.M.; Martins, I.S. Global Biodiversity Change: The Bad, the Good, and the Unknown. Annu. Rev. Environ. Resour. 2012, 37, 25–50. [Google Scholar] [CrossRef]
- Kalkman, V.J.; Boudot, J.-P.; Bernard, R.; Conze, K.-J.; De Knijf, G.; Dyatlova, E.; Ferreira, S.; Jović, M.; Ott, J.; Riservato, E.; et al. European Red List of Dragonflies; Publications Office of the European Union: Luxembourg, 2010. [Google Scholar]
- McKinney, M.L.; Lockwood, J.L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evolut. 1999, 14, 450–453. [Google Scholar] [CrossRef]
- Devictor, V.; Julliard, R.; Clavel, J.; Jiguet, F.; Lee, A.; Couvet, D. Functional Biotic Homogenization of Bird Communities in Disturbed Landscapes. Glob. Ecol. Biogeogr. 2008, 17, 252–261. [Google Scholar] [CrossRef]
- Devictor, V.; Robert, A. Measuring Community Responses to Large-Scale Disturbance in Conservation Biogeography. Divers. Distrib. 2009, 15, 122–130. [Google Scholar] [CrossRef]
- Worthen, W.B.; Fravel, R.K.; Horne, C.P. Downstream Changes in Odonate (Insecta: Odonata) Communities along a Suburban to Urban Gradient: Untangling Natural and Anthropogenic Effects. Insects 2021, 12, 201. [Google Scholar] [CrossRef]
- Rocha-Ortega, M.; Rodríguez, P.; Córdoba-Aguilar, A. Can Dragonfly and Damselfly Communities Be Used as Bioindicators of Land Use Intensification? Ecol. Indic. 2019, 107, 105553. [Google Scholar] [CrossRef]
- Honkanen, M.; Sorjanen, A.M.; Mönkkönen, M. Deconstructing Responses of Dragonfly Species Richness to Area, Nutrients, Water Plant Diversity and Forestry. Oecologia 2011, 166, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Goertzen, D.; Suhling, F. Urbanization Versus Other Land Use: Diverging Effects on Dragonfly Communities in Germany. Divers. Distrib. 2019, 25, 38–47. [Google Scholar] [CrossRef]
- Simaika, J.P.; Samways, M.J. Large-Scale Estimators of Threatened Freshwater Catchment Species Relative to Practical Conservation Management. Biol. Conserv. 2010, 143, 311–320. [Google Scholar] [CrossRef]
- Simaika, J.P.; Samways, M.J. Comparative Assessment of Indices of Freshwater Habitat Conditions Using Different Invertebrate Taxon Sets. Ecol. Indic. 2011, 11, 370–378. [Google Scholar] [CrossRef]
- Briggs, A.J.; Pryke, J.S.; Samways, M.J.; Conlong, D.E. Complementarity Among Dragonflies Across a Pondscape in a Rural Landscape Mosaic. Insect Conserv. Divers. 2019, 12, 241–250. [Google Scholar] [CrossRef]
- Dolný, A.; Harabiš, F. Underground Mining Can Contribute to Freshwater Biodiversity Conservation: Allogenic Succession Forms Suitable Habitats for Dragonflies. Biol. Conserv. 2012, 145, 109–117. [Google Scholar] [CrossRef]
- Harabiš, F.; Dolný, A. Military Training Areas as Refuges for Threatened Dragonfly Species: Effect of Spatial Isolation and Military Activity. Biol. Conserv. 2018, 217, 28–35. [Google Scholar] [CrossRef]
- Vorster, C.; Samways, M.J.; Simaika, J.P.; Kipping, J.; Clausnitzer, V.; Suhling, F.; Dijkstra, K.-D.B. Development of a New Continental-Scale Index for Freshwater Assessment Based on Dragonfly Assemblages. Ecol. Indic. 2020, 109, 105819. [Google Scholar] [CrossRef]
- Watts, P.C.; Rouquette, J.R.; Saccheri, I.J.; Kemp, S.J.; Thompson, D.J. Molecular and Ecological Evidence for Small-Scale Isolation by Distance in an Endangered Damselfly, Coenagrion mercuriale. Mol. Ecol. 2004, 13, 2931–2945. [Google Scholar] [CrossRef]
- Harabiš, F.; Dolný, A. Necessity for the Conservation of Drainage Systems as Last Refugia for Threatened Damselfly Species, Coenagrion ornatum. Insect Conserv. Divers. 2015, 8, 143–151. [Google Scholar] [CrossRef]
- Metge, T.; Goertzen, D.; Suhling, F. Libellen der Flora-Fauna-Habitat Richtlinie in Braunschweig (Niedersachsen). Braunschw. Nat. Schr. 2020, 16, 1–20. [Google Scholar] [CrossRef]
- Harabiš, F.; Dolný, A. Human Altered Ecosystems: Suitable Habitats as Well as Ecological Traps for Dragonflies (Odonata): The Matter of Scale. J. Insect Conserv. 2012, 16, 121–130. [Google Scholar] [CrossRef]
- Capmourteres, V.; Anand, M. ‘Conservation value’: A Review of the Concept and Its Quantification. Ecosphere 2016, 7, e1476. [Google Scholar] [CrossRef]
- Boučníková, E.; Kučera, T. How Natural and Cultural Aspects Influence Land Cover Changes in the Czech Republic. Ekológia 2005, 24, 1–24. [Google Scholar]
- Dolný, A.; Bárta, D.; Waldhauser, M.; Holuša, O.; Hanel, L. The Dragonflies of the Czech Republic: Ecology, Conservation and Distribution; Český Svaz Ochránců Přírody Vlašim: Vlašim, Czech Republic, 2007. [Google Scholar]
- Nature Conservation Agency CR. Species Occurrence Database. Available online: http://portal.nature.cz (accessed on 7 January 2016).
- Farkač, J.; Král, D.; Škorpík, M. List of Threatened Species in the Czech Republic. Invertebrates. In Agentura Ochrany Přírody a Krajiny ČR; Česká Republika: Praha, Czech Republic, 2005. [Google Scholar]
- Simaika, J.P.; Samways, M.J. An Easy-to-Use Index of Ecological Integrity for Prioritizing Freshwater Sites and for Assessing Habitat Quality. Biodivers. Conserv. 2009, 18, 1171–1185. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 18 May 2021).
- Bowler, D.E.; Eichenberg, D.; Conze, K.J.; Suhling, F.; Baumann, K.; Bönsel, A.; Bittner, T.; Drews, A.; Günther, A.; Isaac, N.J.B.; et al. Winners and Losers Over 35 Years of Dragonfly and Damselfly Distributional Change in Germany. bioRxiv 2020. [Google Scholar] [CrossRef]
- Šigutová, H.; Šipoš, J.; Dolný, A. A novel approach involving the use of Odonata as indicators of tropical forest degradation: When family matters. Ecol. Indic. 2019, 104, 229–236. [Google Scholar] [CrossRef]
- Harabiš, F.; Dolný, A. Odonates Need Natural Disturbances: How Human-Induced Dynamics Affect the Diversity of Dragonfly Assemblages. Freshw. Sci. 2015, 34, 1050–1057. [Google Scholar] [CrossRef]
- Iversen, L.L.; Rannap, R.; Briggs, L.; Sand-Jensen, K. Variable History of Land Use Reduces the Relationship to Specific Habitat Requirements of a Threatened Aquatic Insect. Popul. Ecol. 2016, 58, 155–164. [Google Scholar] [CrossRef]
- Tang, D.H.Y.; Visconti, P. Biases of Odonata in Habitats Directive: Trends, Trend Drivers, and Conservation Status of European Threatened Odonata. Insect Conserv. Divers. 2021, 14, 1–14. [Google Scholar] [CrossRef]
- Hochkirch, A.; Schmitt, T.; Beninde, J.; Hiery, M.; Kinitz, T.; Kirschey, J.; Matenaar, D.; Rohde, K.; Stoefen, A.; Wagner, N.; et al. Europe Needs a New Vision for a Natura 2020 Network. Conserv. Lett. 2013, 6, 462–467. [Google Scholar] [CrossRef]
- Cardoso, P. Habitats Directive Species Lists: Urgent Need of Revision. Insect Conserv. Divers. 2012, 5, 169–174. [Google Scholar] [CrossRef]
- Hermoso, V.; Morán-Ordóñez, A.; Canessa, S.; Brotons, L. Realising the Potential of Natura 2000 to Achieve EU Conservation Goals as 2020 Approaches. Sci. Rep. 2019, 9, 16087. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging Threats and Persistent Conservation Challenges for Freshwater Biodiversity. Biol. Rev. Camb. Philos. Soc. 2019, 94, 849–873. [Google Scholar] [CrossRef]
- Raebel, E.M.; Merckx, T.; Feber, R.E.; Riordan, P.; Thompson, D.J.; MacDonald, D.W. Multi-Scale Effects of Farmland Management on Dragonfly and Damselfly Assemblages of Farmland Ponds. Agric. Ecosyst. Environ. 2012, 161, 80–87. [Google Scholar] [CrossRef]
- Dolný, A.; Mižičová, H.; Harabiš, F. Natal Philopatry in Four European Species of Dragonflies (Odonata: Sympetrinae) and Possible Implications for Conservation Management. J. Insect Conserv. 2013, 17, 821–829. [Google Scholar] [CrossRef]
- Dolný, A.; Harabiš, F.; Mižičová, H. Home Range, Movement, and Distribution Patterns of the Threatened Dragonfly Sympetrum depressiusculum (Odonata: Libellulidae): A Thousand Times Greater Territory to Protect? PLoS ONE 2014, 9, e100408. [Google Scholar] [CrossRef] [PubMed]
- Termaat, T.; Van Grunsven, R.H.A.; Plate, C.L.; Van Strien, A.J. Strong Recovery of Dragonflies in Recent Decades in the Netherlands. Freshw. Sci. 2015, 34, 1094–1104. [Google Scholar] [CrossRef]
- Cardoso, P.; Erwin, T.L.; Borges, P.A.V.; New, T.R. The Seven Impediments in Invertebrate Conservation and How to Overcome Them. Biol. Conserv. 2011, 144, 2647–2655. [Google Scholar] [CrossRef]
- Suhonen, J.; Hilli-Lukkarinen, M.; Korkeamäki, E.; Kuitunen, M.; Kullas, J.; Penttinen, J.; Salmela, J. Local Extinction of Dragonfly and Damselfly Populations in Low- and High-Quality Habitat Patches. Conserv. Biol. 2010, 24, 1148–1153. [Google Scholar] [CrossRef]

| Species | DBI | Red List CR (2005) | Degradation (p-Value) | Naturalness (p-Value) |
|---|---|---|---|---|
| Aeshna juncea (Linnaeus, 1758) | 4 | VU | 0.9338 | <0.0001 |
| Leucorrhinia dubia (Vander Linden, 1825) | 6 | VU | 0.9167 | <0.0001 |
| Somatochlora alpestris (Selys, 1840) | 7 | EN | 0.0867 | <0.0001 |
| Somatochlora arctica (Zetterstedt, 1840) | 7 | EN | 0.5130 | <0.0001 |
| Cordulegaster bidentata (Selys, 1843) | 5 | VU | 0.0728 | 0.0001 |
| Sympetrum danae (Sulzer, 1776) | 1 | 0.4139 | 0.0004 | |
| Aeshna caerulea (Ström, 1783) | 9 | CR | 0.3008 | 0.0006 |
| Aeshna cyanea (Müller, 1764) | 0 | 0.9445 | 0.0017 | |
| Platycnemis pennipes (Pallas, 1771) | 0 | 0.7900 | 0.0036 | |
| Cordulegaster boltonii (Donovan, 1807) | 3 | VU | 0.2165 | 0.0066 |
| Aeshna subarctica (Walker, 1908) | 9 | CR | 0.6880 | 0.0083 |
| Leucorrhinia rubicunda (Linnaeus, 1758) | 7 | EN | 0.4556 | 0.0106 |
| Pyrrhosoma nymphula (Sulzer, 1776) | 0 | 0.4084 | 0.0109 | |
| Ischnura elegans (Vander Linden, 1820) | 0 | 0.5523 | 0.0147 | |
| Sympecma paedisca (Brauer, 1877) | 6 | CR | 0.8281 | 0.0215 |
| Coenagrion hastulatum (Charpentier, 1825) | 4 | NT | 0.0050 | 0.0243 |
| Orthetrum coerulescens (Fabricius, 1798) | 5 | EN | 0.8589 | 0.0299 |
| Enallagma cyathigerum (Charpentier, 1840) | 0 | 0.0211 | 0.0302 | |
| Crocothemis erythraea (Brullé, 1832) | 1 | 0.4823 | 0.0330 | |
| Sympetrum pedemontanum (Müller in Allioni, 1766) | 7 | EN | 0.7181 | 0.0471 |
| Orthetrum albistylum (Selys, 1848) | 1 | 0.3890 | 0.0486 | |
| Anax parthenope (Selys, 1839) | 1 | VU | 0.2992 | 0.0505 |
| Lestes barbarus (Fabricius, 1798) | 5 | VU | 0.5384 | 0.1180 |
| Cordulia aenea (Linnaeus, 1758) | 0 | 0.4309 | 0.1276 | |
| Coenagrion ornatum (Selys, 1850) | 7 | CR | 0.6649 | 0.1474 |
| Somatochlora metallica (Vander Linden, 1825) | 0 | 0.0315 | 0.1700 | |
| Anax imperator (Leach in Brewster, 1815) | 0 | 0.1206 | 0.1828 | |
| Somatochlora flavomaculata (Vander Linden, 1825) | 6 | EN | 0.6152 | 0.2008 |
| Libellula quadrimaculata (Linnaeus, 1758) | 0 | 0.2112 | 0.2205 | |
| Lestes sponsa (Hansemann, 1823) | 0 | 0.0040 | 0.2346 | |
| Leucorrhinia pectoralis (Charpentier, 1825) | 5 | VU | 0.6519 | 0.2407 |
| Erythromma lindenii (Selys, 1840) | 6 | 1.0000 | 0.2573 | |
| Calopteryx virgo (Linnaeus, 1758) | 1 | 0.3937 | 0.2908 | |
| Erythromma najas (Hansemann, 1823) | 1 | 0.0942 | 0.3338 | |
| Leucorrhinia albifrons (Burmeister, 1839) | 8 | CR | 0.7565 | 0.3802 |
| Brachytron pratense (Müller, 1764) | 5 | EN | 0.1447 | 0.3869 |
| Coenagrion puella (Linnaeus, 1758) | 0 | 0.3854 | 0.3994 | |
| Aeshna grandis (Linnaeus, 1758) | 1 | 0.0219 | 0.4091 | |
| Ophiogomphus cecilia (Geoffroy in Fourcroy, 1785) | 4 | EN | 0.1385 | 0.4137 |
| Erythromma viridulum (Charpentier, 1840) | 1 | NT | 0.7449 | 0.4604 |
| Sympetrum striolatum (Charpentier, 1840) | 2 | NT | 0.8467 | 0.4790 |
| Calopteryx splendens (Harris, 1780) | 0 | 0.3071 | 0.4797 | |
| Orthetrum cancellatum (Linnaeus, 1758) | 0 | 0.5501 | 0.4910 | |
| Nehalennia speciosa (Charpentier, 1840) | 9 | CR | 0.4593 | 0.5114 |
| Chalcolestes viridis (Vander Linden, 1820) | 1 | 0.4308 | 0.5149 | |
| Gomphus vulgatissimus (Linnaeus, 1758) | 2 | VU | 0.1256 | 0.5161 |
| Coenagrion lunulatum (Charpentier, 1840) | 9 | CR | 0.0340 | 0.5713 |
| Epitheca bimaculata (Charpentier, 1825) | 8 | CR | 0.7103 | 0.6077 |
| Libellula depressa (Linnaeus, 1758) | 0 | 0.2256 | 0.6144 | |
| Sympetrum meridionale (Selys, 1841) | 5 | EN | 1.0000 | 0.6435 |
| Sympetrum fonscolombii (Selys, 1840) | 3 | EN | 0.6530 | 0.6462 |
| Aeshna affinis (Vander Linden, 1820) | 3 | VU | 1.0000 | 0.6575 |
| Stylurus flavipes (Charpentier, 1825) | 6 | EN | 0.8271 | 0.6630 |
| Aeshna isoceles (Müller, 1767), | 4 | VU | 0.6342 | 0.7061 |
| Aeshna mixta (Latreille, 1805) | 1 | 0.4712 | 0.7132 | |
| Lestes virens (Charpentier, 1825) | 2 | VU | 0.8623 | 0.7134 |
| Orthetrum brunneum (Fonscolombe, 1837) | 4 | EN | 0.6302 | 0.7219 |
| Onychogomphus forcipatus (Linnaeus, 1758) | 5 | EN | 0.4745 | 0.7235 |
| Sympetrum sanguineum (Müller, 1764) | 0 | 0.5376 | 0.7354 | |
| Coenagrion pulchellum (Vander Linden, 1825) | 3 | 0.8519 | 0.7732 | |
| Sympecma fusca (Vander Linden, 1820) | 1 | NT | 0.2797 | 0.7989 |
| Sympetrum depressiusculum (Selys, 1841) | 9 | CR | 0.7883 | 0.8177 |
| Sympetrum flaveolum (Linnaeus, 1758) | 4 | 0.3339 | 0.8472 | |
| Lestes dryas (Kirby, 1890) | 5 | VU | 0.3553 | 0.8598 |
| Ischnura pumilio (Charpentier, 1825) | 3 | NT | 0.8598 | 0.8622 |
| Sympetrum vulgatum (Linnaeus, 1758) | 0 | 0.2521 | 0.8780 | |
| Libellula fulva (Müller, 1764) | 6 | CR | 0.4937 | 0.9696 |
| Coenagrion scitulum (Rambur, 1842) | 5 | CR | 0.9077 | 1.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolný, A.; Ožana, S.; Burda, M.; Harabiš, F. Effects of Landscape Patterns and Their Changes to Species Richness, Species Composition, and the Conservation Value of Odonates (Insecta). Insects 2021, 12, 478. https://doi.org/10.3390/insects12060478
Dolný A, Ožana S, Burda M, Harabiš F. Effects of Landscape Patterns and Their Changes to Species Richness, Species Composition, and the Conservation Value of Odonates (Insecta). Insects. 2021; 12(6):478. https://doi.org/10.3390/insects12060478
Chicago/Turabian StyleDolný, Aleš, Stanislav Ožana, Michal Burda, and Filip Harabiš. 2021. "Effects of Landscape Patterns and Their Changes to Species Richness, Species Composition, and the Conservation Value of Odonates (Insecta)" Insects 12, no. 6: 478. https://doi.org/10.3390/insects12060478
APA StyleDolný, A., Ožana, S., Burda, M., & Harabiš, F. (2021). Effects of Landscape Patterns and Their Changes to Species Richness, Species Composition, and the Conservation Value of Odonates (Insecta). Insects, 12(6), 478. https://doi.org/10.3390/insects12060478






