Effect of Processed Beverage By-Product-Based Diets on Biological Parameters, Conversion Efficiency and Body Composition of Hermetia illucens (L) (Diptera: Stratiomyidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin and Preparation of the Insect Diets
2.2. Experimental Procedure
2.3. Chemical Analysis
2.4. Calculations and Statistics
3. Results
3.1. Effect of Diet on Development Time, Survival Rate, Dry Mater, Yield (DM), Mean Wet and Dry Mass of the Larvae
3.2. Conversion Parameters and Substrate Mass Reduction
3.3. Body Composition
3.4. Correlation and Linear Regression
- Mean larval dry mass (y) = −0.028 + 0.265 (C. Protein) + 0.41 (ash)
- PrCR (y) = 0.025 + 0.83 (CP) + 0.54 (Fat) − 0.44 (NFC) + 3.75 (Ash)
- BCR (y) = −1.11 + 2.71 (Fat) + 1.93 (CP) + 1.28 (NDF) + 6.74 (Ash)
- Substrate mass reduction (y) = 0.897 − 2.811 (C.P) + 2.178 (NFC) − 5.12 (Fat) − 8.80 (Ash)
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, K. A review on upcycling: Current body of literature, knowledge gaps and a way forward. In Proceedings of the 17th International Conference on Environment, Cultural, Economic and Social Sustainability, Venice, Italy, 13–14 April 2015; WASET: Venice, Italy, 2015. [Google Scholar]
- FAO. Available online: http://www.fao.org/3/i2373e/i2373e00.pdf (accessed on 11 November 2020).
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef]
- FAO. Available online: http://www.fao.org/3/i2697e/i2697e.pdf (accessed on 11 November 2020).
- LRI. Options for the Livestock Sector in Developing and Emerging Economies to 2030 and Beyond. Meat: The Future Series; World Economic Forum: Geneva, Switzerland, 2019. [Google Scholar]
- FAO. Available online: http://www.fao.org/family-farming/detail/en/c/412647/ (accessed on 13 November 2020).
- Campos-Vega, R.; Loarca-Pina, G.; Vergara-Castaneda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Steiner, J.; Procopio, S.; Becker, T. Brewer’s spent grain: Source of value- added polysaccharides for the food industry in reference to the health claims. Eur. Food Res. Technol. 2015, 241, 303–315. [Google Scholar] [CrossRef]
- ICO. Available online: http://www.ico.org/prices/new-consumption-table.pdf (accessed on 11 November 2020).
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Nigam, P.S. An overview: Recycling of solid barley waste generated as a by-product in distillery and brewery. Waste Manag. 2017, 62, 255–261. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recy. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Huis, A.; van Loon, J.J.A. Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. J. Insects Food Feed 2015, 1, 131–139. [Google Scholar] [CrossRef]
- Nguyen, T.-X.; Tomberlin, J.; Vanlaerhoven, S. Influence of resources on Hermetia Illucens (Diptera: Stratiomyidae) larval development. J. Med. Entomol. 2013, 50, 898–906. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia Illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Putra, J.E.N.R.E. Growth of black soldier fly (Hermetia illucens) larvae fed on spent coffee ground. IOP Conf. Ser. Earth Environ. Sci. 2018, 187, 012070. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Zimbelli, A.; Randazzo, B.; Compagni, M.D.; Truzzi, C.; Antonucci, M.; Riolo, P.; Loreto, N.; Osimani, A.; Milanović, V.; et al. Black soldier fly (Hermetia illucens) reared on roasted coffee by-product and Schizochytrium sp. as a sustainable terrestrial ingredient for aquafeeds production. Aquaculture 2020, 518, 734659. [Google Scholar] [CrossRef]
- Truzzi, C.; Giorgini, E.; Annibaldi, A.; Antonucci, M.; Illuminati, S.; Scarponi, G.; Riolo, P.; Isidoro, N.; Conti, C.; Zarantoniello, M.; et al. Fatty acids profile of black soldier fly (Hermetia illucens): Influence of feeding substrate based on coffee-waste silverskin enriched with microalgae. Anim. Feed Sci. Technol. 2020, 259, 114309. [Google Scholar] [CrossRef]
- Erickson, M.C.; Islam, M.; Sheppard, D.C.; Liao, J.; Doyle, M.P. Reduction of Escherichia coli O157: H7 and Salmonella enterica serovar enteritidis in chicken manure by larvae of the black soldier fly. J. Food Prot. 2004, 67, 685–690. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Newton, L.G.; Thompson, S.A.; Savage, S.A. Value-added manure management system using the black soldier fly. Bioresour. Technol. 1994, 50, 275–279. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Magri, M.E.; Zurbrugg, C.; Lindstrom, A.; Vinneras, B. Faecal sludge management with the larvae of the black soldier fly (Hermetia illucens)—From a hygiene aspect. Sci. Total Environ. 2013, 458–460, 312–318. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens). Entomol. Exp. Appl. 2018, 166, 761–770. [Google Scholar] [CrossRef]
- Paz, A.S.P.; Carrejo, N.S.; Rodriguez, C.H.G. Effects of larval density and feeding rates on the bioconversion of vegetable waste using black soldier fly larvae Hermetia illucens (L.), (Diptera: Stratiomyidae). Waste Biomass Valorization 2015, 6, 1059–1065. [Google Scholar]
- Bosch, G.; Oonincx, D.G.A.B.; Jordan, H.R.; Zhang, J.; van Loon, J.J.A.; van Huis, A.; Tomberlin, J.K. Standardisation of quantitative resource conversion studies with black soldier fly larvae. J. Insects Food Feed 2019, 6, 1–16. [Google Scholar] [CrossRef]
- Sheppard, D.C.; Tomberlin, J.K.; Joyce, J.A.; Kiser, B.C.; Summer, S.M. Rearing methods for the black soldier fly (Diptera: Stratiomyidae). J. Med. Entomol. 2002, 39, 695–698. [Google Scholar] [CrossRef]
- Nyakeri, E.M.; Ogola, H.J.; Ayieko, M.A.; Amimo, F.A. An open system for farming black soldier fly larvae as a source of proteins for small scale poultry and fish production. J. Insects Food Feed 2016, 3, 51–56. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.; Feng, W.; Yang, D.; ur Rehman, R.; Cai, M.; Zhang, J.; Yu, Z.; Zheng, L. Physicochemical structure of chitin in the developing stages of black soldier fly. Int. J. Biol. Macromol. 2020, 149, 901–907. [Google Scholar] [CrossRef] [PubMed]
- ISO. Available online: https://www.iso.org/standard/39145.html (accessed on 5 November 2020).
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting nitrogen into protein—Beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- ISO. Available online: https://www.iso.org/standard/12865.html (accessed on 5 November 2020).
- ISO. Available online: https://www.iso.org/standard/37264.html (accessed on 5 November 2020).
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, Neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Chia, S.Y.; Tanga, C.M.; Osuga, I.M.; Mohamed, S.A.; Khamis, F.M.; Salifu, D.; Sevgan, S.; Fiaboe, K.K.M.; Niassy, S.; van Loon, J.J.A.; et al. Effects of waste stream combinations from brewing industry on performance of black soldier fly, Hermetia Illucens (Diptera: Stratiomyidae). PeerJ 2018, 6, e5885. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Bava, L.; Jucker, C.; Gislon, G.; Lupi, D.; Savoldelli, S.; Zucali, M.; Colombini, S. Rearing of Hermetia illucens on different organic by-products: Influence on growth, waste reduction, and environmental impact. Animals 2019, 9, 289. [Google Scholar] [CrossRef]
- Guillon, F.; Champ, M. Structural and physical properties of dietary fibres and consequences of processing on human physiology. Food Res. Int. 2000, 33, 233–245. [Google Scholar] [CrossRef]
- Cohen, A.C. Insect Diets: Science and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004; p. 92. [Google Scholar]
- Silva, M.A.; Nebra, S.A.; Silva, M.J.M.; Sanchez, C.G. The use of biomass residues in the Brazilian soluble coffee industry. Biomass Bioenergy 1998, 14, 457–467. [Google Scholar] [CrossRef]
- Fischer, H.; Romano, N.; Sinha, A.K. Conversion of spent coffee and donuts by black soldier fly (Hermetia illucens) larvae into potential resources for animal and plant farming. Insects 2021, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.A.; Vignoli, J.A.; Siika-aho, M.; Franco, T.T. Sediments in coffee extracts: Composition and control by enzymatic hydrolysis. Food Chem. 2008, 110, 168–176. [Google Scholar] [CrossRef]
- Laranja, A.T.; Manzatto, A.J.; de Campos Bicudo, H.E.M. Effects of caffeine and used coffee grounds on biological features of Aedes aegypti (Diptera, Culicidae) and their possible use in alternative control. Genet. Mol. Biol. 2003, 26, 419–429. [Google Scholar] [CrossRef]
- Nikitin, A.G.; Navitskas, S.; Gordon, L.-A.N. Effect of varying doses of caffeine on life span of Drosophila melanogaster. J. Gerontol. Biol. Sci. Med. Sci. 2008, 63, 149–150. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Ur Rehman, K.; Rehman, A.; Cai, M.; Zheng, L.; Xiao, X.; Somroo, A.A.; Wang, H.; Li, W.; Yu, Z.; Zhang, J. Conversion of mixtures of dairy manure and soybean curd residue by black soldier fly larvae (Hermetia illucens L.). J. Clean. Prod. 2017, 154, 366–373. [Google Scholar] [CrossRef]
- Gold, M.; Cassar, C.M.; Zurbrügg, C.; Kreuzer, M.; Boulos, S.; Diener, S.; Mathys, A. Biowaste treatment with black soldier fly larvae: Increasing performance through the formulation of biowastes based on protein and carbohydrates. Waste Manag. 2019, 102, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Banks, I.J.; Gibson, W.T.; Cameron, M.M. Growth rates of black soldier fly larvae fed on fresh human faeces and their implication for improving sanitation. Trop. Med. Int. Health 2014, 19, 14–22. [Google Scholar] [CrossRef]
- Liu, Z.; Minor, M.; Morel, P.C.H.; Najar-Rodriguez, A.J. Bioconversion of three organic wastes by black soldier fly (Diptera: Stratiomyidae) larvae. Environ. Entomol. 2018, 47, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Diener, S. Valorisation of Organic Solid Waste Using the Black Soldier Fly, Hermetia Illucens, in Low and Middle-Income Countries. Doctoral Dissertation, ETH Zürich, Zürich, Switzerland, 2010. [Google Scholar]
- Roeder, K.A.; Behmer, S.T. Lifetime consequences of food protein-carbohydrate content for an insect herbivore. Funct. Ecol. 2014, 28, 1135–1143. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Sheppard, D.C. Factors influencing mating and oviposition of black soldier flies (Diptera: Stratiomyidae) in a colony. J. Entomol. Sci. 2002, 37, 345–352. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Gort, G.; Dicke, M.; van Loon, J.J.A. Effects of dietary protein and carbohydrate on life-history traits and body protein and fat contents of the black soldier fly Hermetia illucens. Physiol. Entomol. 2019, 44, 148–159. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Dicke, M.; van Loon, J.J.A. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Giannetto, A.; Oliva, S.; Ceccon Lanes, C.F.; de Araújo Pedron, F.; Savastano, D.; Baviera, C.; Parrino, V.; Lo Paro, G.; Spanò, N.C.; Cappello, T.; et al. Hermetia illucens (Diptera: Stratiomydae) larvae and prepupae: Biomass production, fatty acid profile and expression of key genes involved in lipid metabolism. J. Biotechnol. 2020, 307, 44–54. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Shelomi, M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [PubMed]

| Substrate | C. Protein% | C. Fat% | NDF% | ADF% | Ash% | NFC% |
|---|---|---|---|---|---|---|
| SCG | 10.60 ± 0.93 | 13.43 ± 0.01 | 52.12 ± 0.19 | 29.16 ± 0.06 | 1.22 ± 0.01 | 22.74 ± 0.66 |
| BSG | 18.72 ± 0.29 | 4.69 ± 0.06 | 50.05 ± 0.27 | 18.60 ± 0.23 | 3.80 ± 0.01 | 22.89 ± 0.04 |
| SCG + BSG | 14.62 ± 0.10 | 9.54 ± 0.03 | 52.09 ± 0.42 | 24.48 ± 0.28 | 2.35 ± 0.18 | 21.03 ± 0.10 |
| SCG + BY | 15.36 ± 0.40 | 10.97 ± 0.09 | 44.45 ± 0.95 | 24.48 ± 0.52 | 2.64 ± 0.60 | 26.86 ± 2.11 |
| BSG + BY | 21.12 ± 0.04 | 3.91 ± 0.05 | 38.24 ± 0.84 | 14.45 ± 0.36 | 4.59 ± 0.01 | 31.30 ± 0.16 |
| SCG + BSG + BY | 18.52 ± 0.03 | 7.66 ± 0.17 | 44.63 ± 1.71 | 20.87 ± 0.84 | 3.66 ± 0.02 | 26.79 ± 1.83 |
| CH.FEED | 14.96 ± 0.16 | 3.01 ± 0.02 | 13.50 ± 0.16 | 4.50 ± 0.07 | 11.69 ± 0.35 | 57.00 ± 0.25 |
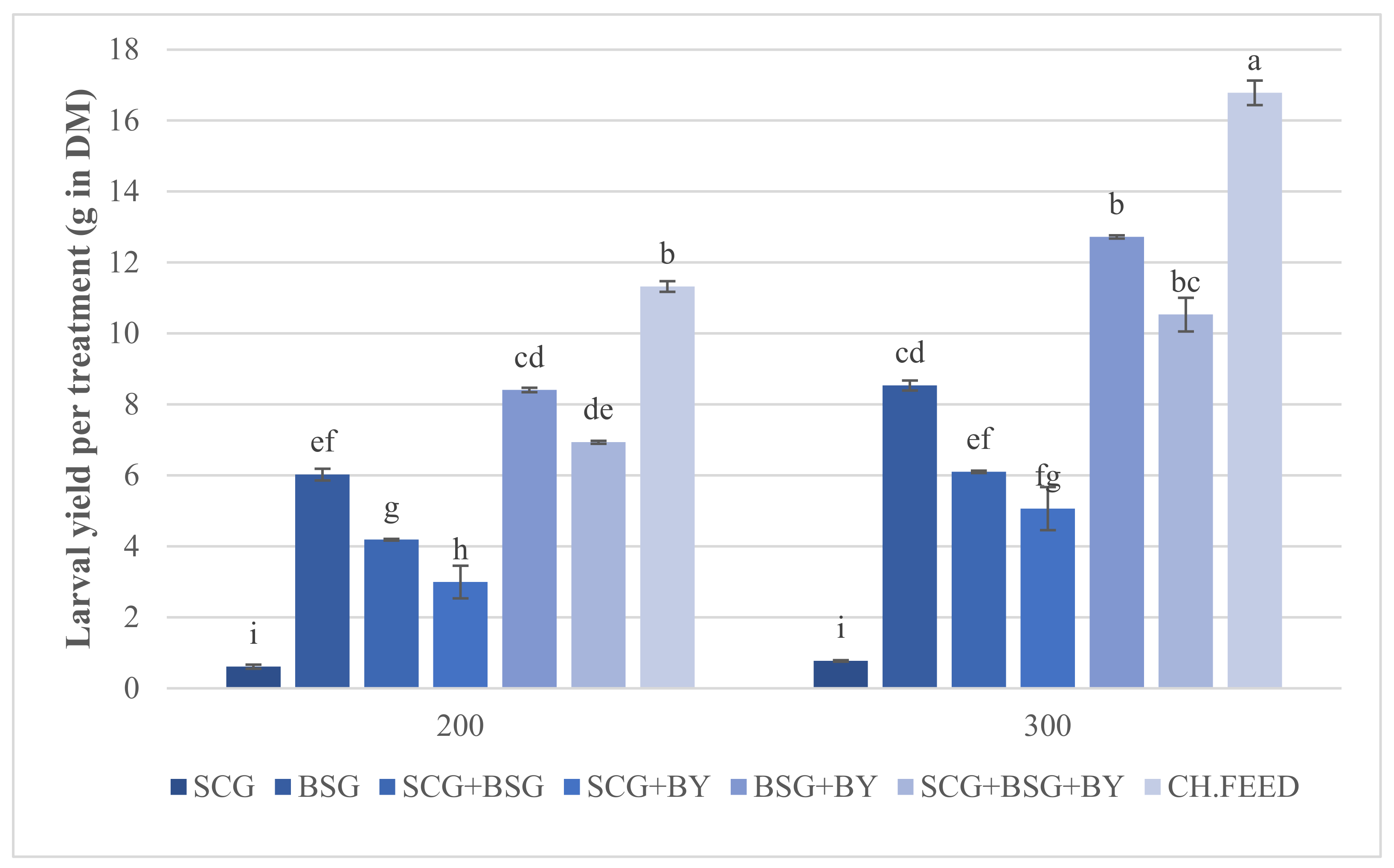
| Treatment | Development Time (Days) | Survival Rate (%) | Larval DM (%) | Larval Yield (g in DM) |
|---|---|---|---|---|
| SCG 200 | 35 | 96.37 ± 0.37 a,b | 24.68 ± 0.40 f | 0.61 ± 0.05 i |
| SCG 300 | 35 | 97.66 ± 0.59 a,b | 25.52 ± 0.41 f | 0.77 ± 0.02 i |
| BSG 200 | 9 | 99.38 ± 0.38 a | 30.29 ± 0.06 b,c | 6.02 ± 0.16 e,f |
| BSG 300 | 9 | 99.33 ± 0.41 a | 29.90 ± 0.22 b,c | 8.53 ± 0.13 c,d |
| SCG + BSG 200 | 12 | 99.38 ± 0.38 a | 25.99 ± 0.11 e,f | 4.19 ± 0.02 g |
| SCG + BSG 300 | 12 | 87.42 ± 6.79 b | 26.17 ± 0.53 e,f | 6.10 ± 0.03 e,f |
| SCG + BY 200 | 17 | 90.75 ± 3.86 a,b | 25.14 ± 0.49 f | 2.99 ± 0.46 h |
| SCG + BY 300 | 17 | 94.25 ± 2.39 a,b | 25.98 ± 0.40 e,f | 5.06 ± 0.60 f,g |
| BSG + BY 200 | 8 | 98.25 ± 1.75 a,b | 33.07 ± 0.35 a | 8.40 ± 0.06 c,d |
| BSG + BY 300 | 8 | 99.00 ± 0.58 a | 31.20 ± 0.55 a,b | 12.72 ± 0.04 b |
| SCG + BSG + BY 200 | 12 | 99.00 ± 0.20 a | 29.01 ± 0.21 c,d | 6.93 ± 0.04 d,e |
| SCG + BSG + BY 300 | 12 | 98.75 ± 0.75 a | 27.63 ± 0.16 d,e | 10.53 ± 0.47 b,c |
| CH. FEED 200 | 11 | 99,88 ± 0,13 a | 32,83 ± 0,19 a | 11.32 ± 0.15 b |
| CH. FEED 300 | 11 | 100 a | 31,22 ± 0,64 a,b | 16.78 ± 0.34 a |
| Feed | Mean Larval Wet Mass (g) | Mean Larval Dry Mass (g) | Mean Pre-Pupal Wet Mass (g) |
|---|---|---|---|
| SCG | 0.0115 ± 0.0007 f | 0.0029 ± 0.00017 f | NM |
| BSG | 0.0989 ± 0.0019 c | 0.0294 ± 0.00058 c | 0.0871 ± 0.0012 c |
| SCG + BSG | 0.0864 ± 0.003 d | 0.0223 ± 0.001 d | 0.0744 ± 0.0047 c,d |
| SCG + BY | 0.0659 ± 0.0042 e | 0.017 ± 0.0012 e | 0.0733 ± 0.0041 d |
| BSG + BY | 0.1349 ± 0.0022 b | 0.0428 ± 0.00031 b | 0.1186 ± 0.0017 b |
| SCG + BSG + BY | 0.1248 ± 0.0027 b | 0.0352 ± 0.00065 c | 0.1202 ± 0.0035 b |
| CH.FEED | 0.1774 ± 0.002 a | 0.0563 ± 0.00066 a | 0.1601 ± 0.0032 a |
| Feed | PrCR | BCR | Substrate Mass Reduction |
|---|---|---|---|
| SCG | NM | NM | NM |
| BSG | 24.75 ± 0.44 c | 11.83 ± 0.22 d | 45.21 ± 0.38 b |
| SCG + BSG | 23.91 ± 0.18 c | 8.22 ± 0.06 e | 26.64 ± 0.35 d |
| SCG + BY | 17.55 ± 1.60 d | 6.25 ± 0.61 f | 18.48 ± 0.81 e |
| BSG + BY | 30.15 ± 0.21 b | 17.22 ± 0.07 b | 44.80 ± 0.46 b |
| SCG + BSG + BY | 28.54 ± 0.60 b | 14.16 ± 0.31 c | 33.08 ± 0.45 c |
| CH.FEED | 45.77 ± 0.64 a | 23.28 ± 0.16 a | 67.38 ± 0.31 a |
| Treatment | Total N | Protein N | NPN | C. Protein | True Protein | Ash |
|---|---|---|---|---|---|---|
| SCG 200 | NM | NM | NM | NM | NM | NM |
| SCG 300 | NM | NM | NM | NM | NM | NM |
| BSG 200 | 7.95 ± 0.01 b | 4.70 ± 0.01 d,e | 3.25 ± 0.02 a,b | 37.86 ± 0.03 b | 22.37 ± 0.05 d,e | 7.34 |
| BSG 300 | 7.98 ± 0.02 b | 4.51 ± 0.001 f | 3.48 ± 0.02 a | 38.00 ± 0.09 b | 21.45 ± 0.01 f | 7.61 |
| SCG + BSG 200 | 8.56 ± 0.004 a | 5.11 ± 0.03 b | 3.45 ± 0.04 a | 40.76 ± 0.02 a | 24.33 ± 0.15 b | 7.82 |
| SCG + BSG 300 | 8.52 ± 0.05 a | 5.03 ± 0.07 b | 3.49 ± 0.02 a | 40.54 ± 0.25 a | 23.94 ± 0.34 b | 7.67 |
| SCG + BY 200 | 8.62 ± 0.03 a | 5.59 ± 0.03 a | 3.03 ± 0.05 b,c,d | 41.03 ± 0.14 a | 26.61 ± 0.12 a | 7.35 |
| SCG + BY 300 | 8.47 ± 0.13 a | 5.18 ± 0,.2 b | 3.29 ± 0.25 a,b | 40.30 ± 0.62 a | 24.66 ± 0.57 b | 7.32 |
| BSG + BY 200 | 7.76 ± 0.01 c | 4.69 ± 0.01 d,e | 3.07 ± 0.001 b,c | 36.92 ± 0.07 c | 22.30 ± 0.06 d,e | 8.06 |
| BSG + BY 300 | 7.45 ± 0.03 d | 4.64 ± 0.04 e,f | 2.81 ± 0.01 c,d | 35.45 ± 0.15 d | 22.08 ± 0.21 e,f | 8.13 |
| SCG + BSG + BY 200 | 7.73 ± 0.06 c | 4.86 ± 0.004 c | 2.87 ± 0.07 c,d | 36.78 ± 0.31 c | 23.13 ± 0.02 c | 7.22 |
| SCG + BSG + BY 300 | 7.55 ± 0.01 d | 4.81 ± 0.001 c,d | 2.74 ± 0.01 d | 35.93 ± 0.05 d | 22.89 ± 0.001 c,d | 7.75 |
| CH.FEED 200 | 6.29 ± 0.01 e | 4.52 ± 0.04 f | 1.77 ± 0.03 e | 29.95 ± 0.04 e | 21.53 ± 0.18 f | 19.10 |
| CH.FEED 300 | 5.87 ± 0.12 f | 4.30 ± 0.08 g | 1.56 ± 0.21 e | 27.92 ± 0.59 f | 20.48 ± 0.40 g | 18.45 |
| Correlation Coefficients | C. Protein% | NDF% | Larval Mean Dry Mass | Non-Fiber Carbs% | Fat% | Ash% |
|---|---|---|---|---|---|---|
| C.Protein% | 1 | NS | 0.6024 *** | NS | −0.7058 *** | NS |
| NDF% | - | 1 | −0.8026 *** | −0.9956 *** | 0.6449 *** | −0.9666 *** |
| Larval Mean Dry Mass | - | - | 1 | 0.7749 *** | −0.9255 *** | 0.8688 *** |
| NF C% | - | - | - | 1 | −0.6177 *** | 0.9615 *** |
| Fat% | - | - | - | - | 1 | −0.7617 *** |
| Ash% | - | - | - | - | - | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sideris, V.; Georgiadou, M.; Papadoulis, G.; Mountzouris, K.; Tsagkarakis, A. Effect of Processed Beverage By-Product-Based Diets on Biological Parameters, Conversion Efficiency and Body Composition of Hermetia illucens (L) (Diptera: Stratiomyidae). Insects 2021, 12, 475. https://doi.org/10.3390/insects12050475
Sideris V, Georgiadou M, Papadoulis G, Mountzouris K, Tsagkarakis A. Effect of Processed Beverage By-Product-Based Diets on Biological Parameters, Conversion Efficiency and Body Composition of Hermetia illucens (L) (Diptera: Stratiomyidae). Insects. 2021; 12(5):475. https://doi.org/10.3390/insects12050475
Chicago/Turabian StyleSideris, Vassilios, Maria Georgiadou, Georgios Papadoulis, Konstantinos Mountzouris, and Antonios Tsagkarakis. 2021. "Effect of Processed Beverage By-Product-Based Diets on Biological Parameters, Conversion Efficiency and Body Composition of Hermetia illucens (L) (Diptera: Stratiomyidae)" Insects 12, no. 5: 475. https://doi.org/10.3390/insects12050475
APA StyleSideris, V., Georgiadou, M., Papadoulis, G., Mountzouris, K., & Tsagkarakis, A. (2021). Effect of Processed Beverage By-Product-Based Diets on Biological Parameters, Conversion Efficiency and Body Composition of Hermetia illucens (L) (Diptera: Stratiomyidae). Insects, 12(5), 475. https://doi.org/10.3390/insects12050475








