Does the African Citrus psyllid, Trioza erytreae (Del Guercio) (Hemiptera: Triozidae), Represent a Phytosanitary Threat to the Citrus Industry in Mexico?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Information and Delimitation of the Study Area
2.2. Model Calibration
2.3. Conductance Area Model
3. Results
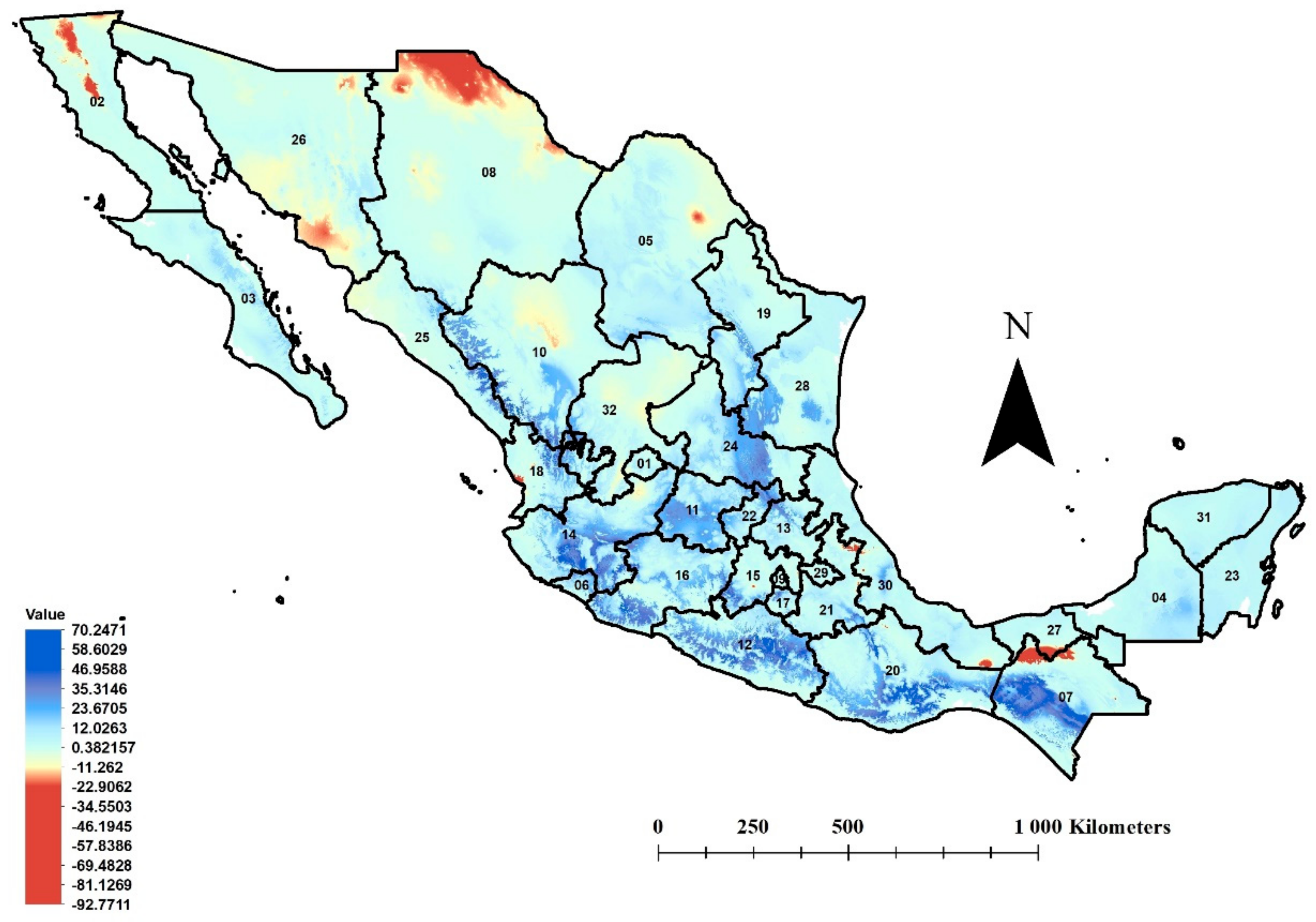
3.1. Environmental Availability for Trioza erytreae in Mexico
3.2. Potentially Affected Citrus-Producing Area
3.3. MESS Analysis
3.4. Environmental Connectivity
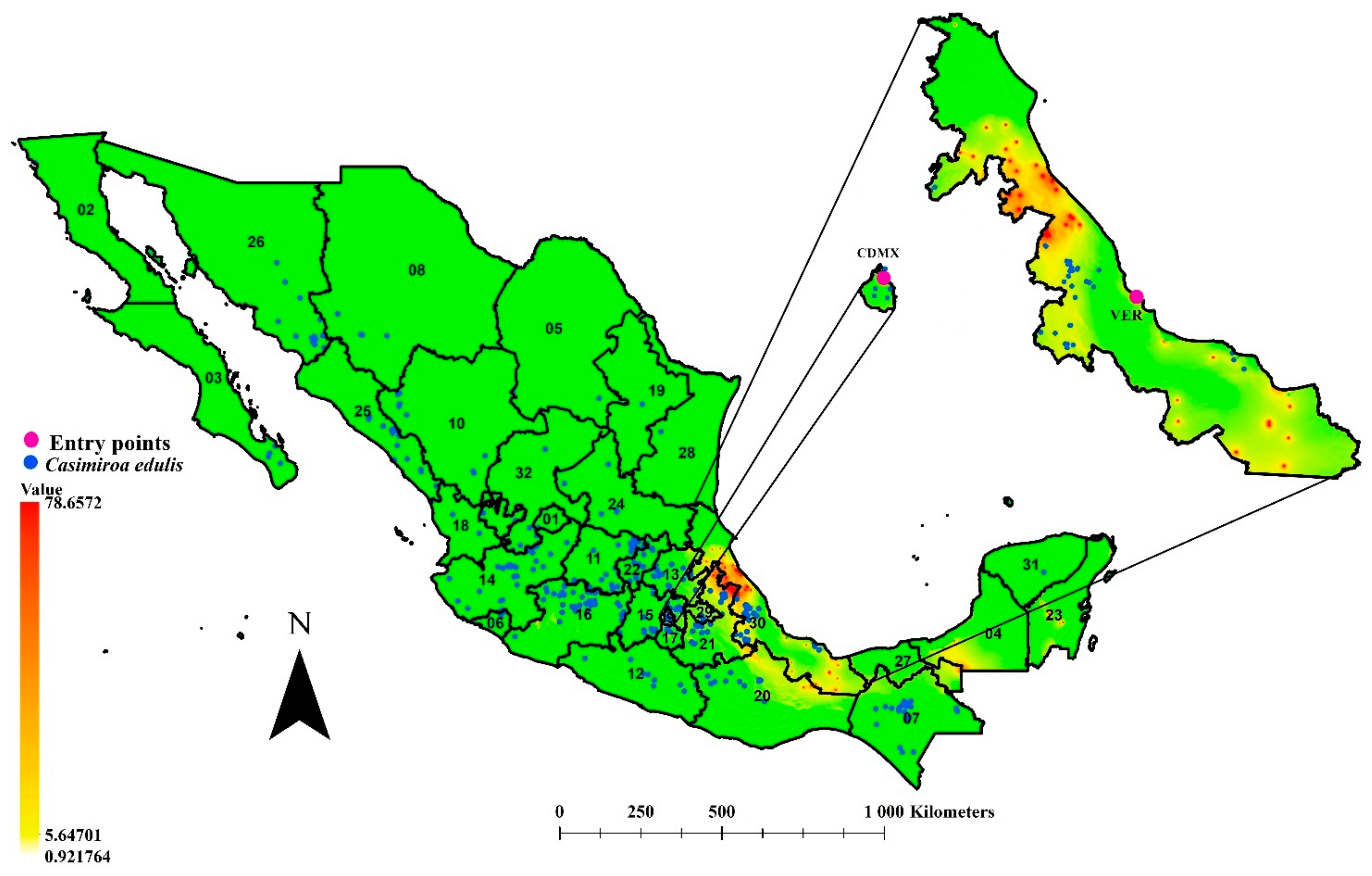
3.5. Role of Casimiroa edulis in the Potential Spreading of T. erytreae to Citrus-Producing Areas
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Folimonova, S.Y.; Achor, D.S. Early events of citrus greening (huanglongbing) disease development at the ultrastructural level. Phytopathology 2010, 100, 949–958. [Google Scholar] [CrossRef]
- Bové, J.M. Huanglongbing or yellow shoot, a disease of Gondwanan origin: Will it destroy citrus worldwide? Phytoparasitica 2014, 42, 579–583. [Google Scholar] [CrossRef]
- Gottwald, T.R.; da Graça, J.V.; Bassanezi, R.B. Citrus huanglongbing: The pathogen and its impact. Plant Health Prog. 2007, 8, 1–36. [Google Scholar] [CrossRef]
- Shimwela, M.M.; Narouei-Khandan, H.A.; Halbert, S.E.; Keremane, M.L.; Minsavage, G.V.; Timilsina, S.; Massawe, D.P.; Jones, J.B.; van Bruggen, A.H.C. First occurrence of Diaphorina citri in East Africa, characterization of the Ca. Liberibacter species causing huanglongbing (HLB) in Tanzania, and potential further spread of D. citri and HLB in Africa and Europe. Eur. J. Plant Pathol. 2016, 146, 349–368. [Google Scholar] [CrossRef]
- Halbert, S.E.; Manjunath, K.L. Asian citrus psyllids (Sternorryncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Fla. Entomol. 2004, 87, 330–353. [Google Scholar] [CrossRef]
- Flores-Sánchez, J.L.; Mora-Aguilera, G.; Loeza-Kuk, E.; López-Arroyo, J.I.; Domínguez-Monge, S.; Acevedo-Sánchez, G.; Robles-García, P. Pérdidas en producción inducidas por Candidatus Liberibacter asiaticus en limón persa en Yucatán, México. Rev. Mex. Fitopatol. 2015, 33, 195–210. [Google Scholar]
- Dala-Paula, B.M.; Plotto, A.; Bai, J.; Manthey, J.A.; Baldwin, E.A.; Ferrarezi, R.S.; Gloria, M.B.A. Effect of huanglongbing or greening disease on orange juice quality, a review. Front. Plant Sci. 2019, 9, 1976. [Google Scholar] [CrossRef]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Aguiar, A.M.F.; Martin, J.H. Psyllids (Homoptera: Psylloidea) from Madeira island: An updated checklist including new records. Bol. Soc. Port. Entomol. Supl. 1999, 6, 335–342. [Google Scholar]
- Ajene, I.J.; Khamis, F.M.; van Asch, B.; Pietersen, G.; Seid, N.; Rwomushana, I.; Ombura, F.L.O.; Momanyi, G.; Finyange, P.; Rasowo, B.A.; et al. Distribution of Candidatus Liberibacter species in Eastern Africa, and the first report Canndidatus Liberibacter asiaticus in Kenya. Sci. Rep. 2020, 10, 3919. [Google Scholar] [CrossRef] [PubMed]
- Hollis, D. Afrotropical jumping plant lice of the family Triozidae (Homoptera: Psylloidea). Bull. Br. Mus. Nat. Hist. 1984, 49, 1–102. [Google Scholar]
- Kilalo, D.; Olubayo, F.; Obukosia, S.; Shibairo, S.I. Farmer management practices of citrus insect pests in Kenya. Afr. J. Hortic. Sci. 2009, 2, 168–176. [Google Scholar]
- Tamesse, J.L.; Messi, J.; Soufo, E.S.; Kambou, J.; Tiago, A.B.; Ndongo, A.O.; Dzokou, V.J. Complexe des parasitoïdes de Trioza erytreae (Del Guercio) (Homoptera: Triozidae), psylle des agrumes au Cameroun. Fruits 2002, 57, 19–28. [Google Scholar] [CrossRef]
- Hailu, T.; Wakgari, M. Distribution and damage of African citrus psyllids (Trioza erytreae) in Casimiroa edulis producing areas of the eastern zone of Ethiopia. Int. J. Environ. Agric. Biotech. 2019, 4, 741–750. [Google Scholar] [CrossRef]
- Moran, V.C. The development of the citrus psylla, Trioza erytreae (Del Guercio) (Homoptera: Psyllidae), on Citrus limon and four indigenous host plants. J. Entomol. Soc. S. Afr. 1986, 31, 391–402. [Google Scholar]
- Roberts, R.; Steenkamp, E.T.; Pietersen, G. Three novel lineages of ‘Candidatus Liberibacter africanus’ associated with native rutaceous hosts of Trioza erytreae in South Africa. Int. J. Syst. Evol. Microbiol. 2015, 65, 723–731. [Google Scholar] [CrossRef]
- Fernandez, E.; Franquinho-Aguiar, A. Evoluçao das pragas dequarenta Toxoptera citricida (Kirkaldy) e Trioza erytreae (Del Guercio) no Archipiélago da Madeira. Bol. San. Veg. Plagas 2001, 27, 51–58. [Google Scholar]
- Pérez-Otero, R. Detección de la psila africana de los cítricos, Trioza erytreae (Del Guercio, 1918) (Hemiptera: Psylloidea: Triozidae), en la Península Ibérica. Arq. Entomol. 2015, 13, 119–122. [Google Scholar]
- Cocuzza, G.E.M.; Alberto, U.; Hernández-Suárez, E.; Silverio, F.; Di Silvestro, S.; Tena, A.; Carmelo, R. A review on Trioza erytreae (African citrus psyllid), now in mainland Europe, and its potential risk as vector of huanglongbing (HLB) in citrus. J. Pest Sci. 2017, 90, 1–17. [Google Scholar] [CrossRef]
- Siveiro, F.; Marco-Noales, E.; Bertolini, E.; Teresani, G.R.; Peñalver, J.; Mansilla, P.; Aguín, O.; Pérez-Otero, R.; Abelleira, A.; Guerra-García, J.A.; et al. Survey of huanglongbing associated with ‘Candidatus Liberibacter’ species in Spain: Analyses of citrus plants and Trioza erytreae. Phytopathol. Mediterr. 2017, 56, 98–110. [Google Scholar]
- Pérez-Rodríguez, J.; Krüger, K.; Pérez-Hedo, M.; Ruíz-Rivero, O.; Urbaneja, A.; Tena, A. Classical biological control of the African citrus psyllid Trioza erytreae, a major threat to the European citrus industry. Sci. Rep. 2019, 9, 9440. [Google Scholar] [CrossRef]
- Khamis, F.M.; Rwomushana, I.; Ombura, L.O.; Mohamed, S.A.; Tanga, C.M.; Nderitu, P.W.; Borgermeister, C.; Sétamou, M.; Grout, T.G.; Ekesi, S. DNA barcode reference library for the African citrus triozid, Trioza erytreae (Hemiptera: Triozidae): Vector of African citrus greening. J. Econ. Entomol. 2017, 110, 2637–2646. [Google Scholar] [CrossRef]
- Kalyebi, A.; Aisu, G.; Ramathani, I.; Ogwang, J.; McOwen, N.; Russell, P. Detection and identification of etiological agents (Liberibacter spp.) associated with citrus greening disease in Uganda. Uganda J. Agric. Sci. 2015, 16, 43–54. [Google Scholar] [CrossRef][Green Version]
- Green, G.C.; Catling, H.D. Weather-induced mortality of the citrus psylla, Trioza erytreae (Del Guercio) (Homoptera: Psyllidae), a vector of greening virus, in some citrus producing areas of southern Africa. Agric. Meteorol. 1971, 8, 305–317. [Google Scholar] [CrossRef]
- Magomere, T.; Obukosia, S.D.; Mutitu, E.; Ngichabe, C.; Olubayo, F.; Shibairo, S. PCR detection and distribution of huanglongbing disease and psyllid vectors on citrus varieties with changes in elevation in Kenya. J. Biol. Sci. 2009, 9, 697–709. [Google Scholar] [CrossRef][Green Version]
- Hall, D.G.; Wenninger, E.; Hentz, M.G. Temperature studies with the Asian citrus psyllid, Diaphorina citri: Cold hardiness and temperature thresholds for oviposition. J. Insect Sci. 2011, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, T.M.; Pyšek, P.; Bacher, S.; Carlton, J.T.; Duncan, R.P.; Jarošik, V.; Wilson, J.R.U.; Richardson, D.M. A proposed unified framework for biological invasions. Trends Ecol. Evol. 2011, 26, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, J.L.; Hoopes, M.F.; Marchetti, M.P. Invasion Ecology; Wiley-Blackwell: Oxford, UK, 2013. [Google Scholar]
- Sistema de Información Agroalimentaria y Pesquera (SIAP). Producción Annual Agrícola. Available online: https://nube.siap.gob.mx/cierreagricola/2020 (accessed on 31 March 2020).
- Robles-González, M.M.; Velázquez-Monreal, J.J.; Medina-Urrutia, V.M.; López-Arroyo, J.I.; Flores-Virgen, R. Síntomas del huanglongbing (HLB) en árboles de limón mexicano [Citrus aurantifolia (Christm) Swingle] y su dispersión en el estado de Colima, México. Rev. Chapingo Ser. Hortic. 2013, 19, 15–31. [Google Scholar] [CrossRef]
- Andres, J.; Soto, M.; Famiani, F.; Cruz-Castillo, J.G. In situ characterization of fruits and seeds of a number of white sapote (Casimiroa edulis Llave & Lex.) accessions in Mexico. HortScience 2017, 52, 1849–1852. [Google Scholar]
- Peterson, A.T.; Soberón, J. Integrating fundamental concepts of ecology, biogeography, and sampling into effective ecological niche modeling and species distribution modeling. Plant Biosyst. 2012, 146, 789–796. [Google Scholar] [CrossRef]
- McRae, B.H.; Dickson, B.G.; Keitt, T.H.; Shah, V.B. Using circuit theory to model connectivity in ecology and conservation. Ecology 2008, 10, 2712–2724. [Google Scholar] [CrossRef]
- Fiaboe, K.K.M.; Peterson, A.T.; Kairo, M.T.K.; Roda, A.L. Predicting the potential worldwide distribution of the red palm weevil Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) using ecological niche modeling. Fla. Entomol. 2012, 95, 659–673. [Google Scholar] [CrossRef]
- Zhu, G.; Bu, W.; Gao, Y.; Liu, G. Potential Geographic Distribution of Brown Marmorated Stink Bug Invasion (Halyomorpha halys). PLoS ONE 2012, 7, e31246. [Google Scholar]
- Wei, J.; Li, X.; Lu, Y.; Zhao, L.; Zhang, H.; Zhao, Q. Modeling the potential global distribution of Phaenacoccus madeirensis Green under various climate change scenarios. Forests 2019, 10, 773. [Google Scholar] [CrossRef]
- Falaschi, M.; Mangiacotti, M.; Sacchi, R.; Scali, S.; Razzetti, E. Electric circuit theory applied to alien invasions: A connectivity model predicting the Balkan frog expansión in Northern Italy. Acta Herpetol. 2018, 13, 33–42. [Google Scholar]
- Elmes, A.; Rogan, J.; Williams, C.; Ratick, S. Modeling the potential dispersal of Asian Longhorned Beetle using circuit theory. Prof. Geog. 2019, 71, 1–15. [Google Scholar] [CrossRef]
- Venette, R.C.; Kriticos, D.J.; Magarey, R.D.; Koch, F.H.; Baker, R.H.A.; Worner, S.P.; Gómez, N.M.; McKenney, D.W.; Dobesberger, E.J.; Yemshanov, D.; et al. Pest risk maps for invasive alien species: A roadmap for improvement. BioScience 2010, 60, 349–362. [Google Scholar] [CrossRef]
- Iverson, L.R.; Prasad, A.M.; Sydnor, D.; Bossenbroek, J.; Schwartz, M.W. Modeling potential emerald ash borer spread through GIS/Cell-based/gravity models with data bolstered by web-based inputs. In Emerald Ash Borer Research and Technology Development Meeting; Mastro, V., Reardo, R., Eds.; Department of Agriculture, Forest Service/Animal and Plant Health Inspection Service: Pittsburgh, PA, USA, 2005; pp. 12–13. [Google Scholar]
- West, P.; Brown, L.; Auricht, C.; Hart, Q. Mapping actual and predicted distribution of pest animals and weeds in Australia. In GIS Applications in Agriculture: Invasive Species; Clay, S.A., Ed.; CRC Press: Oxfordshire, UK, 2011; pp. 91–128. [Google Scholar]
- Bossenbrock, J.; Croskey, A.; Finnoss, D.; Iverson, L.; McDermott, S.M.; Prasad, A.; Sims, C.; Sydnor, D. Invasive Species in a Globalized World. Ecological, Social & Legal Perspectives on Policy; Keller, R.P., Ed.; The University of Chicago Press: Chicago, IL, USA, 2014; pp. 185–208. [Google Scholar]
- Magarey, R.D.; Borchert, D.M.; Fowler, G.A.; Hong, S.C. The NCSU/APHIS Plant pest forecasting system (NAPPFAST). In Pest Risk Modelling and Mapping for Invasive Alien Species; Venette, R.C., Ed.; CAB International/USDA Forest Service: Oxfordshire, UK, 2015; pp. 82–96. [Google Scholar]
- Barbet, M.; Rome, Q.; Villemant, C.; Courchamp, F. Can species distribution models really predict the expansion of invasive species? PLoS ONE 2018, 13, e0193085. [Google Scholar]
- Soberón, J.; Peterson, A.T. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.H. WorldClim 2: New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez, A.; Lira, A.; Maher, S.P.; Peterson, A.T.; Soberón, J.; Villalobos, F. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol. Model. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.D.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on Earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. Bioscience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; PBC: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 3 August 2020).
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio, L. Kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Philips, S.J.; Hastie, T.; Miroslav, D.; Chee, Y.E.; Colin, J.Y. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Sweets, K.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Owens, H.L.; Campbell, L.P.; Dornak, L.L.; Saupe, E.E.; Barve, N.; Soberón, J.; Ingenloff, K.; Lira, A.; Hensz, C.M.; Myers, C.E.; et al. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol. Modell. 2013, 263, 10–18. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 1–7. [Google Scholar] [CrossRef]
- Préau, C.; Grandjean, F.; Sellier, Y.; Gailledrat, M.; Bertrand, R.; Isselin, F. Habitat patches for newts in the face of climate change: Local scale assessment combining niche modelling and graph theory. Sci. Rep. 2020, 10, 3570. [Google Scholar] [CrossRef]
- Elith, J.; Kearny, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Garrido, T.; Vázquez, E. Métodos de análisis genéticos, espaciales y de conectividad en genética del paisaje. Rev. Mex. Biodiv. 2013, 84, 1031–1054. [Google Scholar] [CrossRef]
- O’Donnell, M.S.; Ignizio, D.A. Bioclimatic Predictors for Supporting Ecological Applications in the Conterminous United States; U.S. Geological Survey: Reston, VA, USA, 2012; p. 10. [Google Scholar]
- López-Collado, J.; López-Arroyo, J.I.; Robles-García, P.L.; Márquez-Santos, M. Geographic distribution of hábitat, development, and population groth rates of the Asian citrus psyllyd, Diaphorina citri, in Mexico. J. Insect Sci. 2013, 13, 114. [Google Scholar] [CrossRef]
- Catling, H.D.; Green, G.C. The influence of weather on the survival and population fluctuations of Trioza erytreae (Del Guercio)-A vector of greening. Proc. IOCV 1972, 5, 58–64. [Google Scholar]
- Rindermann, R.S.; Sangerman-Jarquín, D.M. Desempeño competitivo de la fruticultura mexicana, 1980–2011. Rev. Mex. Cienc. Agrí. 2014, 5, 1287–1300. [Google Scholar]
- Ruiz, R.; Vela, G.V.; Moreno, R.G. Exportación de cítricos mexicanos, alternativas para el mercado de exportación. Horiz. Cont. Cienc. Soc. 2017, 6, 77–85. [Google Scholar]
- Almaguer, G.; Ayala, A.V. Adopción de innovaciones en limón ‘Persa’ (Citrus latifolia tan.) en Tlapacoyan, Veracruz: Uso de bitácora. Rev. Chapingo Ser. Hortic. 2014, 20, 89–100. [Google Scholar] [CrossRef]
- Bada, L.M.; Rivas, L.A.; Littlewood, H.F. Modelo de asociatividad en la cadena productiva en las Mipymes agroindustriales. Contab. Adm. 2017, 62, 1100–1117. [Google Scholar]
- Ramírez, J.E.; Badano, E.I.; Flores, J.; Flores, J.L.; Yáñez, L. Scientific literature on invasive alien species in a megadiverse country: Advances and challenges in Mexico. NeoBiota 2019, 48, 113–127. [Google Scholar] [CrossRef]
- Rubí-Arriaga, M.; González-Huerta, A.; Martínez-De lLa Cruz, I.; Franco-Mora, O.; Ramírez-Dávila, J.F.; López-Sandoval, J.A.; Hernández-Flores, G.V. Inventario de especies frutales y aspectos etnobotánicos en Sultepec, Estado de México, México. Pyton 2014, 83, 203–211. [Google Scholar]
- Lascurain, M.; Avendaño, S.; del Amo, S.; Niembro, A. Guía de Frutos Silvestres Comestibles en Veracruz; Instituto de Ecología, A.C.: Xalapa, Mexico, 2010; p. 144. [Google Scholar]
- Martínez-De la Cruz, I.; Rubí-Arriaga, M.; González-Huerta, A.; Pérez-López, D.J.; Franco-Mora, O.; Castañeda-Vildózola, Á. Frutas y semillas comestibles en el Estado de México. Rev. Mex. Cienc. Agric. 2015, 6, 331–346. [Google Scholar]
- Barrera-Catalán, E.; Herrera-Castro, N.D.; Catalán-Heverástico, C.; Ávila-Sánchez, P. Plantas medicinales del municipio de Tixtla de Guerrero, México. Rev. Fitotec. Mex. 2015, 38, 109–111. [Google Scholar] [CrossRef]
- Magos, G.A.; Vidiro, H.; Reynolds, W.F.; Enríquez, R.G. Pharmacology of Casimiroa edulis IV. Hypotensive effects of compounds isolated from methanolic extracts in rats and guinea pigs. J. Ethnopharmacol. 1999, 64, 35–44. [Google Scholar] [CrossRef]
- Yonemoto, Y.; Chowdhury, A.K.; Kato, H.; Macha, M.M.; Okuda, H. Characterization of White sapote (Casimiroa edulis Llave & Lex.) germplasm using floral morphology, RAPD and AFLP markers. Sci. Hortic. 2007, 112, 366–375. [Google Scholar]
- d’Eeckenbrugge, G.C.; Schiavo, M.; Caron, E.; Ongwen, D.; Kamau, J.I.; Rono, B.; Leclerc, C. Worldwide interconnections of Africa using crops as historical and cultural markers. In La Difussiom des Plantes Américaines dans la Región des Grands Lacs. Approcges Générale et Sous-Régionale, l’Ouest Kényan; Vignati, E., Ed.; Institut Français de Recherche en Afrique: Nairobi, Kenya, 2019; pp. 7–41. [Google Scholar]
- Hérnandez, L.; López, J.; García, C.G.; Osorio, F.; Nava, M.E. Dinámica espacio-temporal de Diaphorina citri Kuwayama (Hemiptera: Psyllidae) en Murraya paniculata (L.) Jack en Cuitláhuac, Veracruz. Acta Zool. Mex. 2013, 29, 334–345. [Google Scholar]
- Zilch, J.F.; Rodríguez, R.; Ramírez, C. Informe Mensual No. 12 de la Campaña contra Huanglongbing de los Cítricos; SENASICA/Dirección General de Sanidad Vegetal: Mexico City, Mexico, 2015; p. 12. [Google Scholar]


| State | Lemon | Orange | Tangerine | Grapefruit | Total |
|---|---|---|---|---|---|
| Veracruz | 47,895.08 | 169,965.50 | 9118.90 | 7921.00 | 234,900.48 |
| Michoacan | 63,741.95 | 350.00 | 0.00 | 6046.00 | 70,137.95 |
| Tamaulipas | 7954.90 | 33,238.81 | 850.31 | 2196.84 | 44,240.86 |
| San Luis Potosi | 2033.50 | 32,778.59 | 2350.50 | 8.00 | 37,170.59 |
| Puebla | 2829.20 | 29,019.55 | 4248.80 | 424.05 | 36,521.60 |
| Nuevo Leon | 630.00 | 25,576.50 | 3607.00 | 1840.80 | 31,654.30 |
| Oaxaca | 21,500.90 | 4528.50 | 0.00 | 90.00 | 26,119.40 |
| Colima | 19,244.85 | 344.00 | 0.00 | 15.50 | 19,604.35 |
| Yucatan | 4261.83 | 13,163.76 | 996.69 | 721.71 | 19,143.99 |
| Tabasco | 7227.32 | 8163.50 | 62.00 | 110.00 | 15,562.82 |
| Sonora | 291.00 | 6889.00 | 458.00 | 500.50 | 8138.50 |
| Guerrero | 6956.79 | 569.08 | 12.00 | 0.50 | 7538.37 |
| Jalisco | 6557.97 | 609.25 | 0.00 | 60.00 | 7227.22 |
| Hidalgo | 279.60 | 5762.90 | 30.00 | 0.00 | 6072.50 |
| Campeche | 2099.30 | 2577.70 | 70.00 | 652.50 | 5399.50 |
| Chiapas | 2814.85 | 1905.85 | 51.90 | 0.00 | 4772.60 |
| Quintana Roo | 2375.50 | 1473.00 | 20.00 | 0.00 | 3868.50 |
| Sinaloa | 1293.20 | 1626.00 | 55.00 | 95.00 | 3069.20 |
| Baja California Sur | 57.00 | 2959.55 | 4.00 | 20.15 | 3040.70 |
| Nayarit | 2850.81 | 77.66 | 3.25 | 0.00 | 2931.72 |
| Durango | 255.81 | 283.60 | 0.00 | 199.00 | 738.41 |
| Zacatecas | 625.50 | 0.00 | 0.00 | 0.00 | 625.50 |
| Morelos | 399.50 | 186.40 | 8.50 | 4.00 | 598.40 |
| Baja California | 126.55 | 274.00 | 2.50 | 13.20 | 416.25 |
| Estado de Mexico | 133.50 | 26.00 | 5.00 | 0.00 | 164.50 |
| Guanajuato | 86.00 | 0.00 | 0.00 | 0.00 | 86.00 |
| Aguascalientes | 57.20 | 3.00 | 0.00 | 0.00 | 60.20 |
| Variable | Bioclimatic Predictor | % Contribution to Explain the Model |
|---|---|---|
| BIO1 | Annual mean temperature | 0.5 |
| BIO2 | Mean diurnal range | 0.8 |
| BIO3 | Isothermality | 1.9 |
| BIO4 | Temperature seasonality | NA |
| BIO5 | Maximum temperature of warmest month | NA |
| BIO6 | Minimum temperature of coldest month | NA |
| BIO7 | Temperature annual range | NA |
| BIO8 | Mean temperature of wettest quarter | 69.0 |
| BIO9 | Mean temperature of driest quarter | NA |
| BIO10 | Mean temperature of warmest quarter | NA |
| BIO11 | Mean temperature of coldest quarter | NA |
| BIO12 | Annual precipitation | NA |
| BIO13 | Precipitation of wettest month | NA |
| BIO14 | Precipitation of driest month | 25.3 |
| BIO15 | Precipitation seasonality (coefficient of variation) | 0.5 |
| BIO16 | Precipitation of wettest quarter | NA |
| BIO17 | Precipitation of driest quarter | NA |
| BIO18 | Precipitation of warmest quarter | 2.1 |
| BIO19 | Precipitation of coldest quarter | NA |
| >60% of Environmental Availability, States with More Than 10 Thousand Cultivated Hectares | Risk | 30–59% Environmental Availability, States with 1–10 Thousand Hectares Cultivated | Risk | <29% Environmental Availability, States with Less Than a Thousand Hectares Cultivated | Risk |
|---|---|---|---|---|---|
| Veracruz Michoacan Tamaulipas San Luis Potosi Puebla Nuevo Leon Oaxaca Colima Yucatan Tabasco | 1 2 2 1 1 2 1 3 3 1 | Sonora Guerrero Jalisco Hidalgo Campeche Chiapas Quintana Roo Sinaloa Baja California Sur Nayarit | 3 3 1 1 3 2 2 3 3 3 | Durango Zacatecas Morelos Baja California Mexico Guanajuato Aguascalientes | 1 1 3 3 1 1 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Zaragoza, S.; Pérez-De la O, N.B.; Aguirre-Medina, J.F.; López-Martínez, V. Does the African Citrus psyllid, Trioza erytreae (Del Guercio) (Hemiptera: Triozidae), Represent a Phytosanitary Threat to the Citrus Industry in Mexico? Insects 2021, 12, 450. https://doi.org/10.3390/insects12050450
Espinosa-Zaragoza S, Pérez-De la O NB, Aguirre-Medina JF, López-Martínez V. Does the African Citrus psyllid, Trioza erytreae (Del Guercio) (Hemiptera: Triozidae), Represent a Phytosanitary Threat to the Citrus Industry in Mexico? Insects. 2021; 12(5):450. https://doi.org/10.3390/insects12050450
Chicago/Turabian StyleEspinosa-Zaragoza, Saúl, Nidia Bélgica Pérez-De la O, Juan Francisco Aguirre-Medina, and Víctor López-Martínez. 2021. "Does the African Citrus psyllid, Trioza erytreae (Del Guercio) (Hemiptera: Triozidae), Represent a Phytosanitary Threat to the Citrus Industry in Mexico?" Insects 12, no. 5: 450. https://doi.org/10.3390/insects12050450
APA StyleEspinosa-Zaragoza, S., Pérez-De la O, N. B., Aguirre-Medina, J. F., & López-Martínez, V. (2021). Does the African Citrus psyllid, Trioza erytreae (Del Guercio) (Hemiptera: Triozidae), Represent a Phytosanitary Threat to the Citrus Industry in Mexico? Insects, 12(5), 450. https://doi.org/10.3390/insects12050450







