Combination of Modified Atmosphere and Irradiation for the Phytosanitary Disinfestation of Trogoderma granarium Everts (Coleoptera: Dermestidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Experimental Design
2.3. Treatments
2.4. Insect Rearing after Treatments
2.5. Data Analyses
3. Results
3.1. Effects of Gamma Radiation
3.2. Effects of Combined Treatments
3.2.1. Effect of MA in Combination with Gamma Radiation
3.2.2. Effect of Combination MA with X-ray Radiation
3.3. Estimating Lethal Times
3.4. Synergistic Ratios
3.5. Confirmatory Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eliopoulos, P.A. New approaches for tackling the khapra beetle. CAB Rev. 2013, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Chand, P.; Vishwakarma, R.; Singh, C.K. Khapra beetle (Trogoderma granarium Everts): A food security threat. Bull. Environ. Pharmacol. Life Sci. 2017, 6, 14–19. [Google Scholar]
- Usman, K.; Muhammad, U.F.; Muhammad, F.A.; Umar, N. Khapra beetle: A review of recent control methods. Curr. Investig. Agri. Curr. Res. 2018, 5, 666–671. [Google Scholar]
- Jiang, X.L.; Wang, L.W. Study on the attractant of Trogoderma granarium (Coleoptera: Dermestidae) and warehouse monitoring. Plant Quar. 1995, 9, 8–9. [Google Scholar]
- Athanassiou, C.G.; Phillips, T.W.; Wakil, W. Biology and control of the khapra beetle, Trogoderma granarium, a major quarantine threat to global food security. Annu. Rev. Entomol. 2019, 64, 131–148. [Google Scholar] [CrossRef]
- EPPO (European and Mediterranean Plant Protection Organization). A2 List of Pests Recommended for Regulation as Quarantine Pests. Trogoderma granarium. 2020. Available online: https://gd.eppo.int/taxon/TROGGA (accessed on 3 March 2021).
- Zhang, S.F. Global distribution and chemical control of Trogoderma granarium. Plant Quar. 2004, 18, 125–128. [Google Scholar]
- Day, C.; White, B. Khapra Beetle, Trogoderma Granarium Interceptions and Eradications in Australia and Around the World; SARE Working Paper 1609; School of Agricultural and Resource Economics, University of Western Australia: Crawley, Australia, 2016. [Google Scholar]
- Myers, S.W.; Hagstrum, D.W. Quarantine. In Stored Product Protection; Hagstrum, D.W., Phillips, T.W., Cuperus, G., Eds.; Kansas State University: Manhattan, KS, USA, 2012; pp. 297–304. [Google Scholar]
- Wakil, W.; Kavallieratos, N.G.; Usman, M.; Gulzar, S.; El-Shafie, H.A.F. Detection of phosphine resistance in field populations of four key stored-grain insect pests in Pakistan. Insects 2021, 12, 288. [Google Scholar] [CrossRef]
- Finkelman, S.; Navarro, S.; Rindner, M.; Dias, R. Effect of low pressure on the survival of Trogoderma granarium Everts, Lasioderma serricorne (F.) and Oryzaephilus surinamensis (L.) at 30 °C. J. Stored Prod. Res. 2006, 42, 23–30. [Google Scholar] [CrossRef]
- Bell, C.H.; Wilson, S.M. Phosphine tolerance and resistance in Trogoderma granarium Everts (Coleoptera: Dermestidae). J. Stored Prod. Res. 1995, 31, 199–205. [Google Scholar] [CrossRef]
- Arthur, F.H.; Michael, J.; Domingue, M.J.; Deanna, S.; Sche, D.S.; Myers, S.W. Bioassays and methodologies for insecticide tests with larvae of Trogoderma granarium (Everts), the Khapra Beetle. Insects 2019, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Boukouvala, M.C.; Kavallieratos, N.G. Effect of six insecticides on egg hatching and larval mortality of Trogoderma granarium Everts (Coleoptera: Dermedtidae). Insects 2020, 11, 263. [Google Scholar] [CrossRef]
- Hallman, G.J. Control of stored product pests by ionizing radiation. J. Stored Prod. Res. 2013, 52, 36–41. [Google Scholar] [CrossRef]
- IPPC (International Plant Protection Convention). List of Topics for IPPC Standards. 2021. Available online: https://www.ippc.Int/en/core–activities/standards-setting/list-topics-ippc-standards/list (accessed on 2 April 2020).
- Jayas, D.S.; Jeyamkondan, S. PH—Postharvest Technology: Modified atmosphere storage of grains, meat, fruits and vegetables. Biosyst. Eng. 2002, 82, 235–251. [Google Scholar] [CrossRef]
- Navarro, S. The use of modified and controlled atmospheres for the disinfestation of stored products. J. Pest Sci. 2012, 85, 301–322. [Google Scholar] [CrossRef]
- Gao, M.X.; Wang, C.Y.; Li, S.R.; Zhang, S.F. The effect of irradiation on Trogoderma granarium in grain and legume. J. Plant Prot. 2004, 31, 377–382. [Google Scholar]
- Mansour, M. Irradiation as a phytosanitary treatment against Trogoderma granarium (Coleoptera: Dermestidae). Fla. Entomol. 2016, 99, 138–142. [Google Scholar]
- Cornwell, P.B.; Crook, L.J.; Bull, J.O. Lethal and sterilizing effects of gamma radiation on insects infesting cereal commodities. Nature 1957, 179, 670–672. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhan, G.P. Chapter 2, Theory of phytosanitary irradiation treatment. In Phytosanitary Irradiation Treatment for Postharvest Pest Control, 1st ed.; China Agriculture Press: Beijing, China, 2016; pp. 60–80. [Google Scholar]
- Cao, Y.; Xu, K.; Zhu, X.; Bai, Y.; Yang, W.; Li, C. Role of modified atmosphere in pest control and mechanism of its effect on insects. Front. Physiol. 2019, 1, 206. [Google Scholar] [CrossRef] [Green Version]
- Bailey, S.W. Airtight storage of grain: Its effect on insect pests-Ⅳ. Rhyzopertha dominica (F.) and some other coleoptera that infest stored grain. J. Stored Prod. Res. 1965, 1, 25–33. [Google Scholar] [CrossRef]
- Burges, D.H. Diapause pest status and control of the Khapra beetle, Trogoderma granarium Everts. Ann. Appl. Biol. 1962, 50, 614–617. [Google Scholar] [CrossRef]
- Zhang, X.Y. A study of the effectiveness of Colbat-60 gamma ray irradiation on all stages of insect growth and lethality of Trogoderma granarium Everts. Plant Quar. 1991, 20, 3–8. [Google Scholar]
- Burges, H.D. Studies on the Dermestid beetle Trogoderma granarium Everts -IV. Feeding, growth, and respiration with particular reference to diapause larvae. J. Insect Physiol. 1960, 5, 317–334. [Google Scholar] [CrossRef]
- Buscarlet, L.A.; Aminian, B.; Bali, C. Effect of irradiation and exposure to nitrogen on mortality of adults Tribolium Confusum J. du V. In Proceedings of the 4th International Working Conference on Stored-Product Protection, Tel Aviv, Israel, 21–26 September 1986; Donahaye, E., Navarro, S., Eds.; Maor-Wallach Press: Jerusalem, Israel, 1987; pp. 186–193. [Google Scholar]
- Follett, P.A.; Snook, K. Cold storage enhances the efficacy and margin of security in postharvest irradiation treatments against fruit flies (Diptera: Tephritidae). J. Econ. Entomol. 2013, 106, 2035–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neven, L.G.; Rehfield-Ray, L.M.; Obenland, D. Confirmation and efficacy tests against codling moth and oriental fruit moth in peaches and nectarines using combination heat and controlled atmosphere treatments. J. Econ. Entomol. 2006, 99, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- USDA (United State Department of Agriculture). Animal and Plant Health Inspection Service, Plant Protection and Quarantine. In Treatment Manual; USDA: Washington, DC, USA, 2008. [Google Scholar]
- IPPC (International Plant Protection Convention). Phytosanitary Treatment for Regulated Pest; ISPM 28; FAO: Rome, Italy, 2007. [Google Scholar]
- Lacroix, M.; Follett, P. Combination irradiation treatments for food safety and phytosanitary uses. Stewart Postharvest Rev. 2015, 3, 4. [Google Scholar]
- Ke, D.Y.; Kader, A.A. Tolerance and responses of fresh fruits to O2 levels at or below 1%. In Proceedings of the Fifth International Controlled Atmosphere Research Conference, Wenatchee, WA, USA, 14–16 June 1989; Volume 2, pp. 209–216. [Google Scholar]
- Hallman, G.J.; Levang-Brilz, N.M.; Zettler, J.L.; Winborne, I.C. Factors affecting ionizing radiation phytosanitary treatments, and implications for research and generic treatments. J. Econ. Entomol. 2010, 103, 1950–1963. [Google Scholar] [CrossRef] [PubMed]
- IPPC (International Plant Protection Convention). Guidelines for the Use of Irradiation as a Phytosanitary Treatment Measure; ISPM 18; FAO: Rome, Italy, 2003. [Google Scholar]
- ASTM E1026-13. Standard Practice for Using the Fricke Dosimetry System; US-ANSI: West Conshohocken, PA, USA, 2002. [Google Scholar]
- Zhan, G.P.; Zhao, J.P.; Ma, F.; Liu, B.; Zhong, Y.; Song, Z.J.; Zhao, Q.Y.; Chen, N.Z.; Ma, C. Radioprotective effects on late third-instar Bactrocera dorsalis (Diptera: Tephritidae) larvae in low-oxygen atmospheres. Insects 2020, 11, 526. [Google Scholar] [CrossRef]
- Abbott, W.S. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- DPS (Data Processing System). User’s guide. Version 13.5; Hangzhou RuiFeng Information Technology Co., Lt.: Hangzhou, China, 2010. [Google Scholar]
- LeOra Software. PoloPlus, Version 2.0. A User’s Guide to Probit or Logit Analysis; LeOra Software: Berkeley, CA, USA, 2008. [Google Scholar]
- Wheeler, M.W.; Park, R.M.; Bailer, A.J. Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ. Toxicol. Chem. 2006, 25, 1441–1444. [Google Scholar] [CrossRef]
- Myers, S.W.; Cancio-Martinez, E.; Hallman, G.J.; Fontenot, E.A.; Vreysen, M.J.B. Relative tolerance of six Bactrocera (Diptera: Tephritidae) species to phytosanitary cold treatment. J. Econ. Entomol. 2016, 109, 2341–2347. [Google Scholar] [CrossRef] [Green Version]
- Hewlett, P.S.; Plackett, R.L. A unified theory for quantal responses to mixtures of drugs: Non-interactive action. Biometrics 1959, 15, 591–610. [Google Scholar] [CrossRef]
- Chadwick, P.R. A comparison of safroxan and piperonyl butoxide as pyrethrum synergists. Pyrethrum Post. 1961, 6, 30–37. [Google Scholar]
- Lee, B.H.; Kim, H.M.; Kim, B.S.; Yang, J.O.; Moon, Y.M.; Ren, Y. Evaluation of the synergistic effect between ethyl formate and phosphine for control of Aphis gossypii (Homoptera: Aphididae). J. Econ. Entomol. 2016, 109, 143–147. [Google Scholar] [CrossRef]
- Couey, H.M.; Chew, V. Confidence limits and sample size in quarantine research. J. Econ. Entomol. 1986, 79, 887–890. [Google Scholar] [CrossRef]
- Follett, P.A.; Neven, L.G. Current trends in quarantine entomology. Annu. Rev. Entomol. 2006, 51, 359–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NAPPO (North American Plant Protection Organization). RSPM 34. Development of Phytosanitary Treatment Protocols for Regulated Arthropod Pests of Fresh Fruits or Vegetables; NAPPO: Ottawa, ON, Canada, 2011. [Google Scholar]
- Hallman, G.J. Phytosanitary applications of irradiation. Compr. Rev. Food Sci. Food Saf. 2011, 10, 143–151. [Google Scholar] [CrossRef]
- Hallman, G.J.; Yves, M.H.; Andrew, G.P.; Carl, M.B. Phytosanitary irradiation: An overview. Fla. Entomol. 2016, 99, 1–14. [Google Scholar]
- CABI (CAB International). Datasheet: Trogoderma granarium (Khapra Beetle). 2020. Available online: https://www.cabi.org/isc/datasheet/55010 (accessed on 2 April 2020).
- Heather, N.W. Generalised quarantine disinfestation research protocol. In Irradiation as a Phytosanitary Treatment of Food and Agricultural Commodities; IAEA-TEC-DOC-1427; IAEA: Vienna, Austria, 2002; pp. 171–178. [Google Scholar]
- Yang, Z.; Sun, Y.; Lee, P.T. Impact of the development of the China-Europe Railway Express-A case on the Chongqing international logistics center. Transp. Res. Part A Policy Pract. 2020, 136, 244–261. [Google Scholar] [CrossRef]
- Sun, Y.P.; Johnson, E.R. Analysis of joint action of insecticides against house flies. J. Econ. Entomol. 1960, 53, 887–892. [Google Scholar] [CrossRef]
- Li, L.; Zhang, G.; Li, B.; Yang, J.O.; Park, M.G.; Liu, T. Postharvest treatment of mandarin fruit sing a combination of methyl bromide and phosphine against Bactrocera dorsalis (diptera: Tephritidae). Pest. Manag. Sci. 2019, 76, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Feroz, A. Efficacy and cytotoxic potential of deltamethrin, essential oils of Cymbopogon citratus and Cinnamonum camphora and their synergistic combinations against stored product pest, Trogoderma granarium (Everts). J. Stored Prod. Res. 2020, 87, 101614. [Google Scholar] [CrossRef]
- Hossain, F.; Follett, P.; Salmieri, S.; Vu, D.K.; Harich, M.; Lacroix, M. Synergistic effects of nanocomposite films containing essential oil nanoemulsions in combination with ionizing radiation for control of rice weevil Sitophilus oryzae in stored grains. J. Food Sci. 2019, 84, 1439–1446. [Google Scholar] [CrossRef]
- Ren, L.L.; Peng, C.Y.; Liu, B.; Li, X.Y.; Li, B.S. Research progress of controlled atmosphere treatment for phytosanitary use. Plant Quar. 2019, 33, 1–5. [Google Scholar]
- Hagstrum, D.W.; Subramanyam, B. Stored-Product Insect Resource; AACC International: St. Paul, MN, USA, 2009. [Google Scholar]
- Kavallieratos, N.G.; Athanassiou, C.G.; Boukouvala, M.C.; Tsekos, T.T. Influence of different non-grain commodities on the population growth of Trogoderma granarium Everts (Coleoptera: Dermestidae). J. Stored Prod. Res. 2019, 81, 31–39. [Google Scholar] [CrossRef]
- Mitcham, E.; Martin, T.; Zhou, S. The mode of action of insecticidal controlled atmospheres. Bull. Entomol. Res. 2006, 96, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Calderon, M.; Navarro, S. Synergistic effect of CO2 and O2 mixtures on two stored grain insect pests. Dev. Agric. Eng. 1980, 1, 79–84. [Google Scholar]
- Vassilakos, T.N.; Riudavets, J.; Castañé, C.; Iturralde-Garcia, R.D.; Athanassiou, C.G. Efficacy of modified atmospheres on Trogoderma granarium (Coleoptera: Dermestidae) and Sitophilus zeamais (Coleoptera: Curculionidae). J. Econ. Entomol. 2019, 112, 2450–2457. [Google Scholar] [CrossRef]
- Dias, V.S.; Hallman, G.J.; Martínez-Barrera, O.Y.; Hurtado, N.V.; Cardoso, A.A.S.; Parker, A.G.; Caravantes, L.A.; Rivera, C.; Araújo, A.S.; Maxwell, F.; et al. Modified atmosphere does not reduce the efficacy of phytosanitary irradiation doses recommended for tephritid fruit flies. Insects 2020, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liu, B.; Ma, C.; Zhao, Q.Y.; Song, Z.J.; Li, Z.H.; Zhan, G.P. The impact of temperature on late-aged larvae of khapra beetle (Coleoptera: Dermestidae) treated under low-oxygen atmospheres. Plant Quar. 2021, 35, 42–46. [Google Scholar]
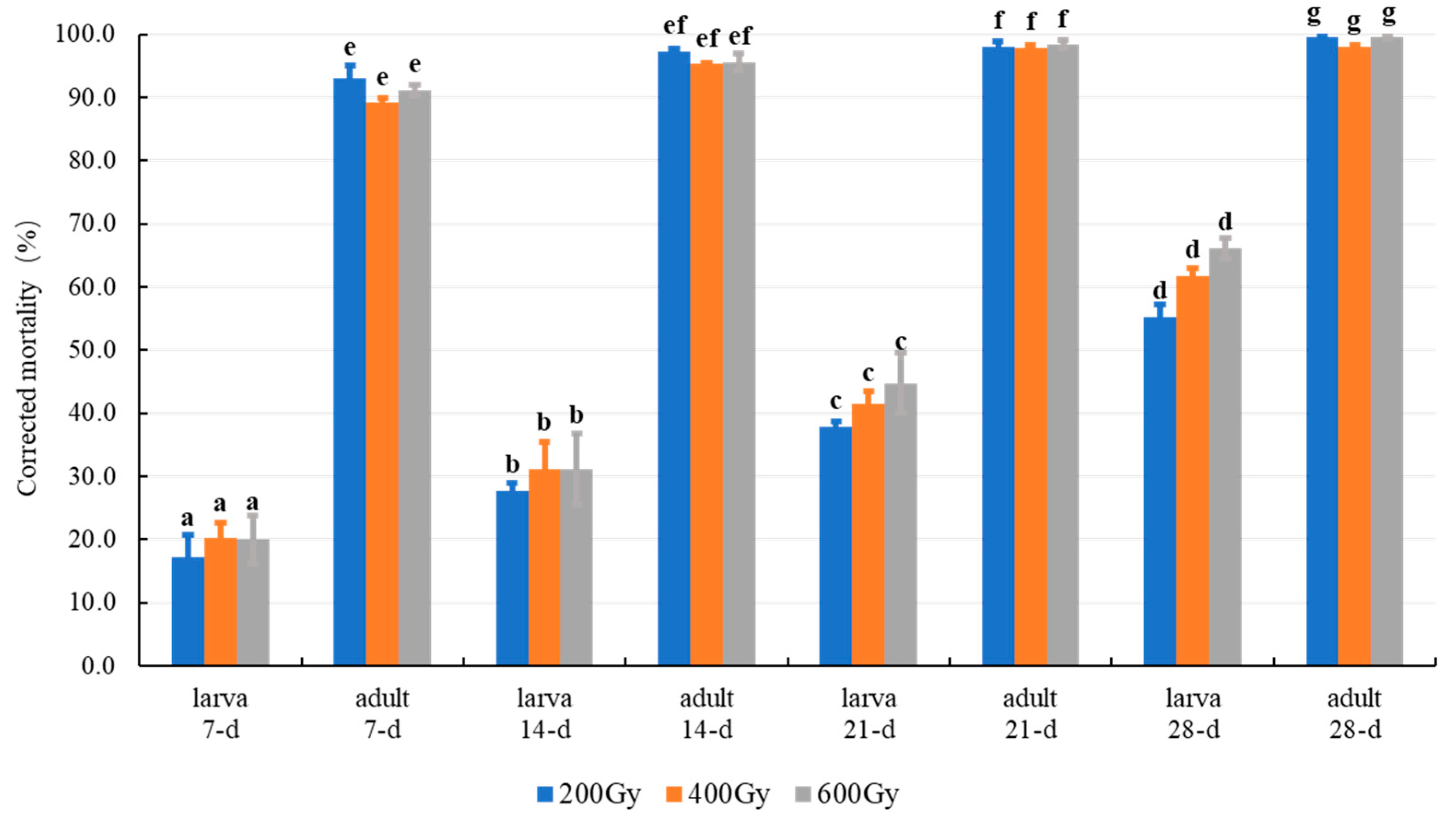
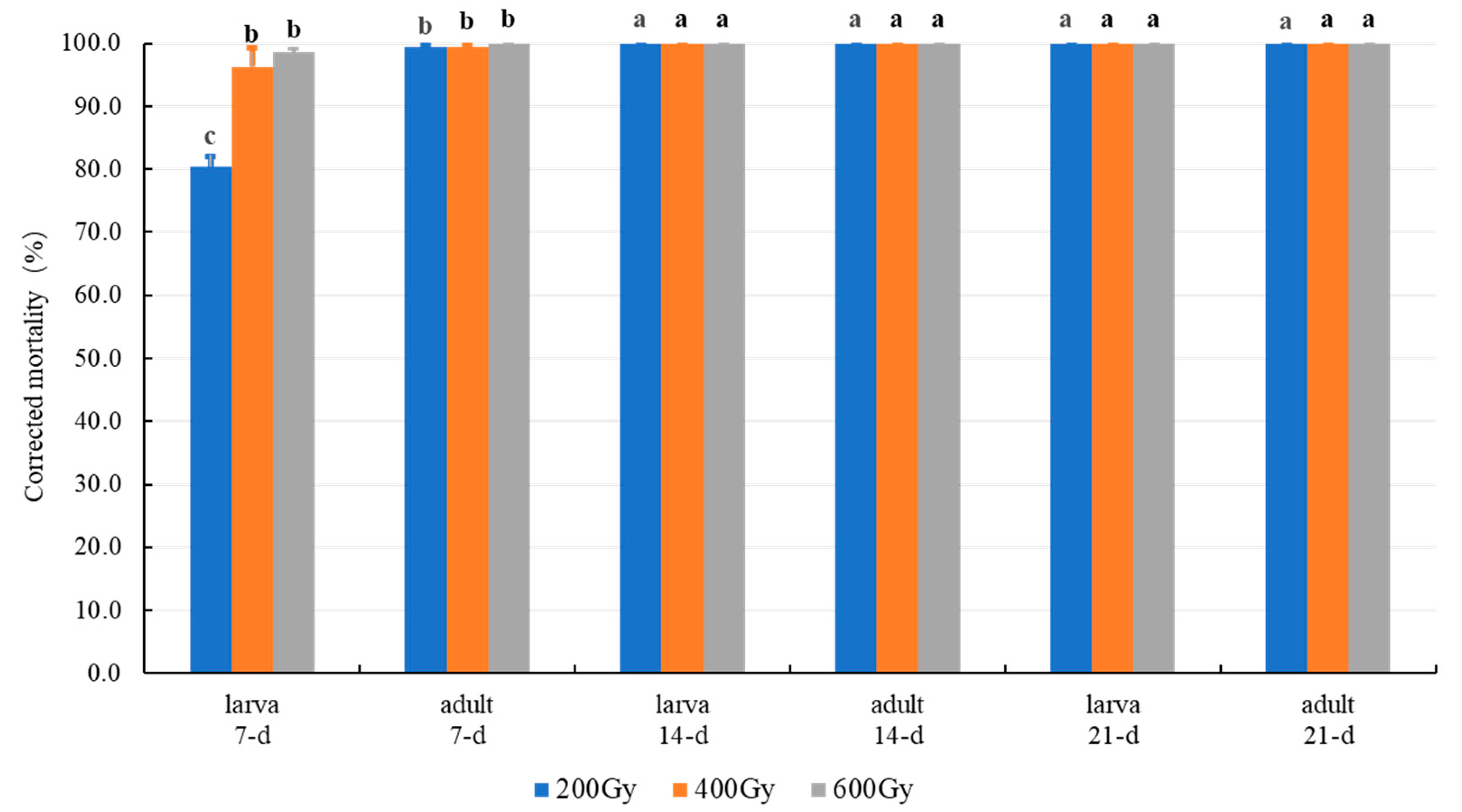
| Stage | X-ray | O2 | Exposure Times (d) |
|---|---|---|---|
| middle-stage larvae | - - | 1% | 2, 4, 6, 8, 10, 12, 14, 16 |
| 2% | 2, 6, 10, 14, 18, 20 | ||
| 200 Gy 200 Gy | 1% | 1, 2, 3, 4, 5, 6, 7 | |
| 2% | 2, 4, 6, 8,10,12 | ||
| late-stage larvae | - - | 1% | 2, 6, 10, 14, 18, 22, 24 |
| 2% | 4, 8, 12, 16, 20, 24, 28 | ||
| 200 Gy 200 Gy | 1% | 2, 3, 4, 5, 6, 7, 8, 9, 10 | |
| 2% | 2, 6, 10, 14, 18 |
| Stage | X-rays (Gy) | No. of Insects | Corrected Mortality (%) at Exposure Time of: | Stage Mortality (%) | |||
|---|---|---|---|---|---|---|---|
| 3-d | 6-d | 9-d | Mean ± SD | ||||
| Adults | 200 | 1049 | 76.6 ± 1.3 cF | 93.4 ± 3.6 bcE | 100.0 ± 0.0 aD | 90.0 ± 10.7 c | 93.5 ± 8.4 a |
| 400 | 1049 | 83.5 ± 1.8 bF | 98.2 ± 0.8 abE | 100.0 ± 0.0 aD | 94.0 ± 7.9 b | ||
| 600 | 1016 | 90.0 ± 2.2 aF | 99.7 ± 0.4 aE | 100.0 ± 0.0 aD | 96.6 ± 5.1 a | ||
| Middle-stage larvae | 200 | 1634 | 51.8 ± 2.2 cF | 94.1 ± 0.5 bE | 100.0 ± 0.0 aD | 82.0 ± 22.8 c | 87.3 ± 17.4 b |
| 400 | 1571 | 64.9 ± 0.7 bF | 96.8 ± 0.5a bE | 100.0 ± 0.0 aD | 87.2 ± 16.8 b | ||
| 600 | 1598 | 78.2 ± 1.7 aF | 99.8 ± 0.2 aE | 100.0 ± 0.0 aD | 92.7 ± 10.9 a | ||
| Late-stage larvae | 200 | 1822 | 39.8 ± 0.4 cF | 77.1 ± 1.3 cE | 90.7 ± 0.4 cD | 69.2 ± 22.8 c | 77.4 ± 20.6 c |
| 400 | 1718 | 51.6 ± 0.6 bF | 85.9 ± 1.3 bE | 95.9 ± 0.7 bD | 77.8 ± 20.1 b | ||
| 600 | 1709 | 62.3 ± 1.4 aF | 94.0 ± 0.9 aE | 99.3 ± 0.5 aD | 85.2 ± 17.4 a | ||
| Treatment | Stages | No. Insects | Slope ± SE | Intercept ± SE | Estimated Lethal Time (95% CIs) (d) * | Hetero-geneity | ||
|---|---|---|---|---|---|---|---|---|
| LT90 | LT99 | LT99.9968 | ||||||
| 1%O2 | middle- | 1970 | 0.261 ± 0.012 | −1.188 ± 0.080 | 9.5 (9.0–9.9) e | 13.5 (12.7–14.4) e | 19.9 (18.5–21.5) de | 1.26 |
| late- | 1829 | 0.168 ± 0.007 | −1.474 ± 0.096 | 16.4 (15.5–17.5) b | 22.6 (21.1–24.5) b | 32.6 (29.2–37.5) b | 2.21 | |
| 2%O2 | middle- | 3492 | 0.281 ± 0.008 | −1.953 ± 0.060 | 11.5 (11.0–12.1) c | 15.2 (14.5–16.1) cd | 21.2 (20.0–22.6) d | 2.09 |
| late- | 1948 | 0.152 ± 0.007 | −1.774 ± 0.109 | 20.1 (19.4–20.9) a | 27.0 (25.7–28.4) a | 38.0 (35.1–41.7) a | 1.30 | |
| 1%O2 +200Gy | middle- | 3550 | 0.583 ± 0.018 | −1.516 ± 0.063 | 4.8 (4.6–5.1) h | 6.6 (6.2–7.1) h | 9.5 (8.8–10.3) h | 3.26 |
| late- | 2412 | 0.432 ± 0.014 | −1.710 ± 0.074 | 6.9 (6.4–7.6) g | 9.3 (8.6–10.4) g | 13.2 (11.9–15.1) g | 6.28 | |
| 2%O2 +200Gy | middle- | 2878 | 0.432 ± 0.014 | −2.108 ± 0.076 | 7.8 (7.5–8.2) f | 10.3 (9.8–10.8) f | 14.1 (13.4–15.0) f | 1.67 |
| late- | 2333 | 0.222 ± 0.009 | −1.180 ± 0.078 | 11.1 (10.1–12.4) cd | 15.8 (14.2–18.2) c | 23.4 (21.5–27.7) c | 5.67 | |
| Treatment | Larval Stage | Synergistic Ratios Based on: | ||
|---|---|---|---|---|
| LT90 | LT99 | LT99.9968 | ||
| 1%O2 + 200Gy | middle- | 1.98 | 2.05 | 2.09 |
| late- | 2.38 | 2.43 | 2.47 | |
| 2%O2 + 200Gy | middle- | 1.47 | 1.48 | 1.50 |
| late- | 1.81 | 1.71 | 1.62 | |
| Date of Treatment | Treatment | No. of Insects | Exposure Time (d) | No. of Survivor |
|---|---|---|---|---|
| 11 August 2020 | X-rays | 10,000 | 13 | 1 |
| X-rays | 10,000 | 14 | 0 | |
| X-rays | 10,000 | 15 | 0 | |
| control | 3400 | 15 | 3325 | |
| 5 September 2020 | X-rays | 25,374 | 15 | 0 |
| control | 3600 | 15 | 3518 | |
| 28 September 2020 | X-rays | 21,868 | 15 | 0 |
| control | 2200 | 15 | 2130 | |
| 21 October 2020 | γ-rays | 13,200 | 15 | 0 |
| control | 1032 | 15 | 1012 | |
| 31 October 2020 | X-rays | 19,904 | 15 | 0 |
| control | 3100 | 15 | 3015 | |
| 21 November 2020 | X-rays | 11,020 | 15 | 0 |
| control | 2300 | 15 | 2240 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.-Y.; Li, T.-X.; Song, Z.-J.; Sun, T.; Liu, B.; Han, X.; Li, Z.-H.; Zhan, G.-P. Combination of Modified Atmosphere and Irradiation for the Phytosanitary Disinfestation of Trogoderma granarium Everts (Coleoptera: Dermestidae). Insects 2021, 12, 442. https://doi.org/10.3390/insects12050442
Zhao Q-Y, Li T-X, Song Z-J, Sun T, Liu B, Han X, Li Z-H, Zhan G-P. Combination of Modified Atmosphere and Irradiation for the Phytosanitary Disinfestation of Trogoderma granarium Everts (Coleoptera: Dermestidae). Insects. 2021; 12(5):442. https://doi.org/10.3390/insects12050442
Chicago/Turabian StyleZhao, Qing-Ying, Tian-Xiu Li, Zi-Jiao Song, Tao Sun, Bo Liu, Xin Han, Zhi-Hong Li, and Guo-Ping Zhan. 2021. "Combination of Modified Atmosphere and Irradiation for the Phytosanitary Disinfestation of Trogoderma granarium Everts (Coleoptera: Dermestidae)" Insects 12, no. 5: 442. https://doi.org/10.3390/insects12050442
APA StyleZhao, Q.-Y., Li, T.-X., Song, Z.-J., Sun, T., Liu, B., Han, X., Li, Z.-H., & Zhan, G.-P. (2021). Combination of Modified Atmosphere and Irradiation for the Phytosanitary Disinfestation of Trogoderma granarium Everts (Coleoptera: Dermestidae). Insects, 12(5), 442. https://doi.org/10.3390/insects12050442







