Entangling the Enemy: Ecological, Systematic, and Medical Implications of Dermestid Beetle Hastisetae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Organism
2.2. Examined Materials
2.3. Microscopy
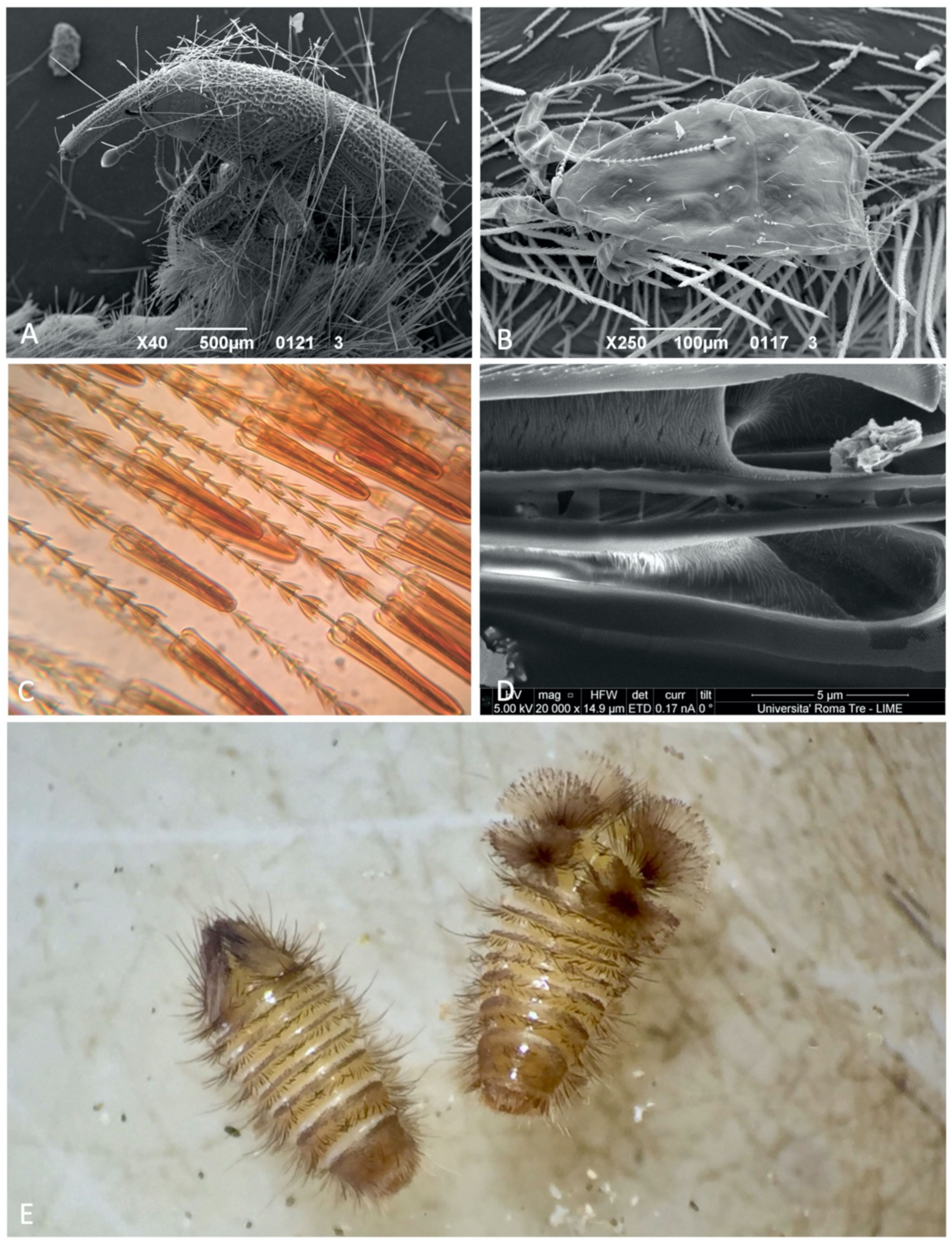
2.4. Defensive Behaviors Observation-Experiment
3. Results
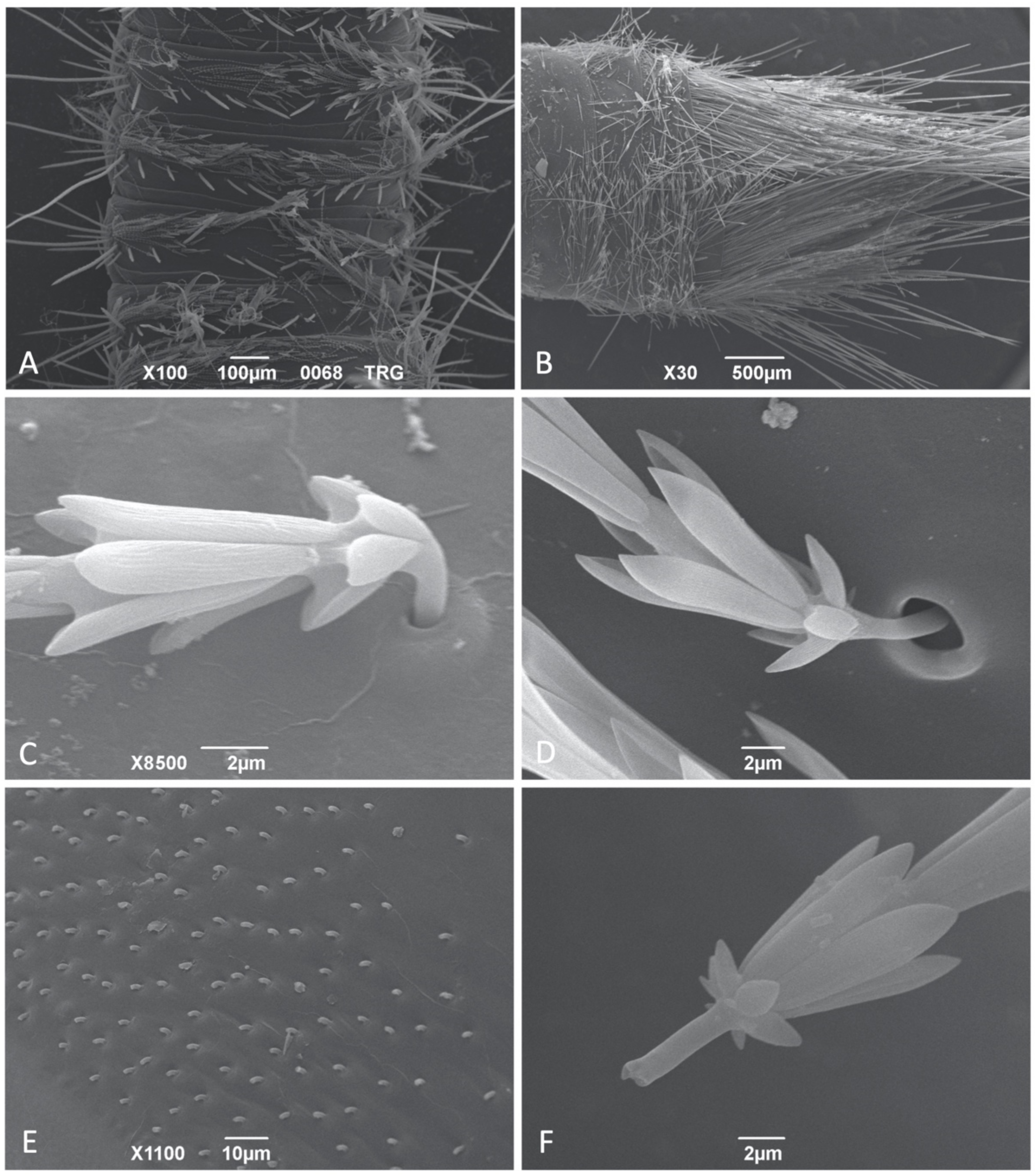
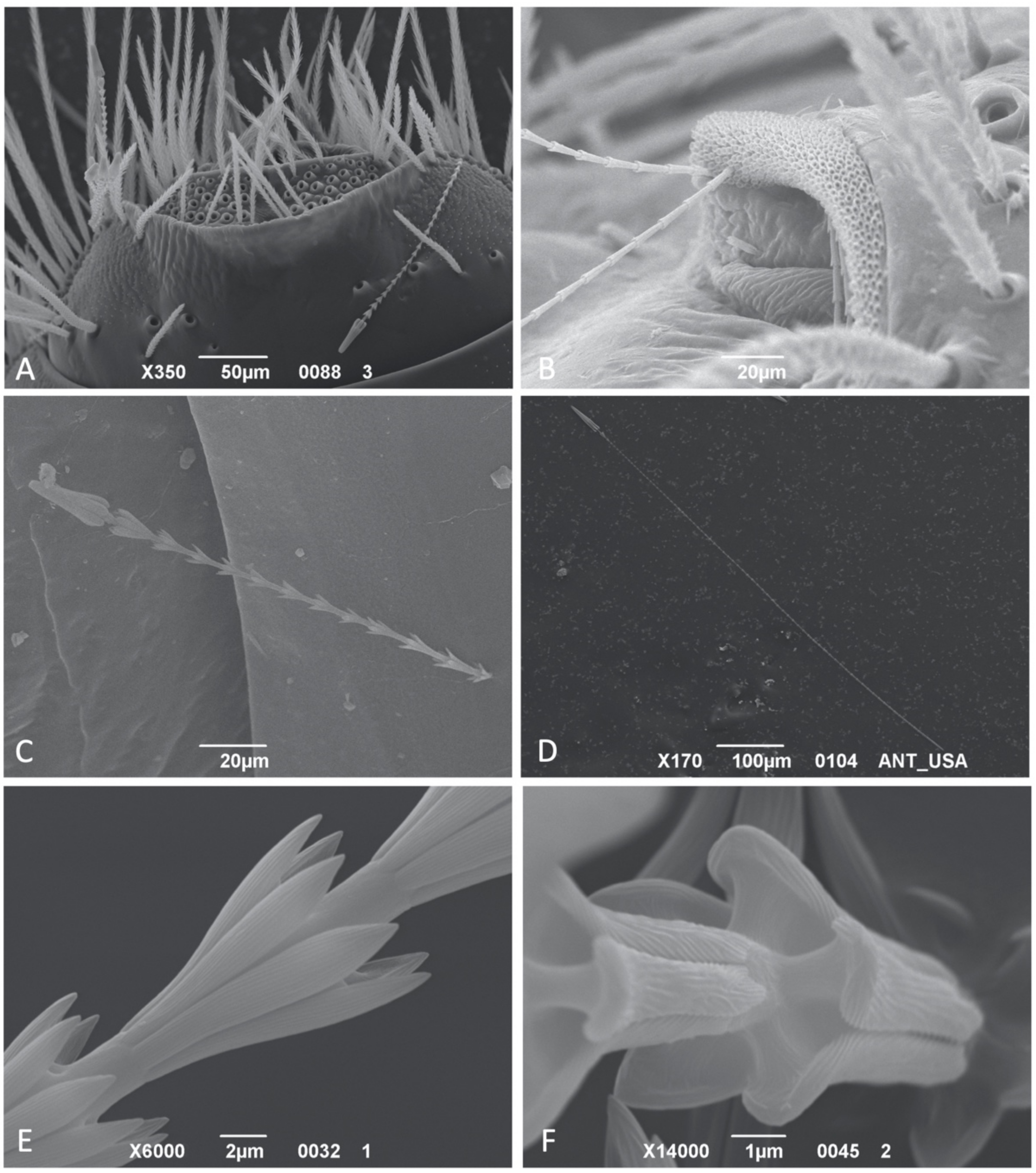
3.1. Hastiseta Morphology
3.1.1. Hastiseta Insertion and the Pedicel
3.1.2. Hastiseta Insertion and the Pedicel
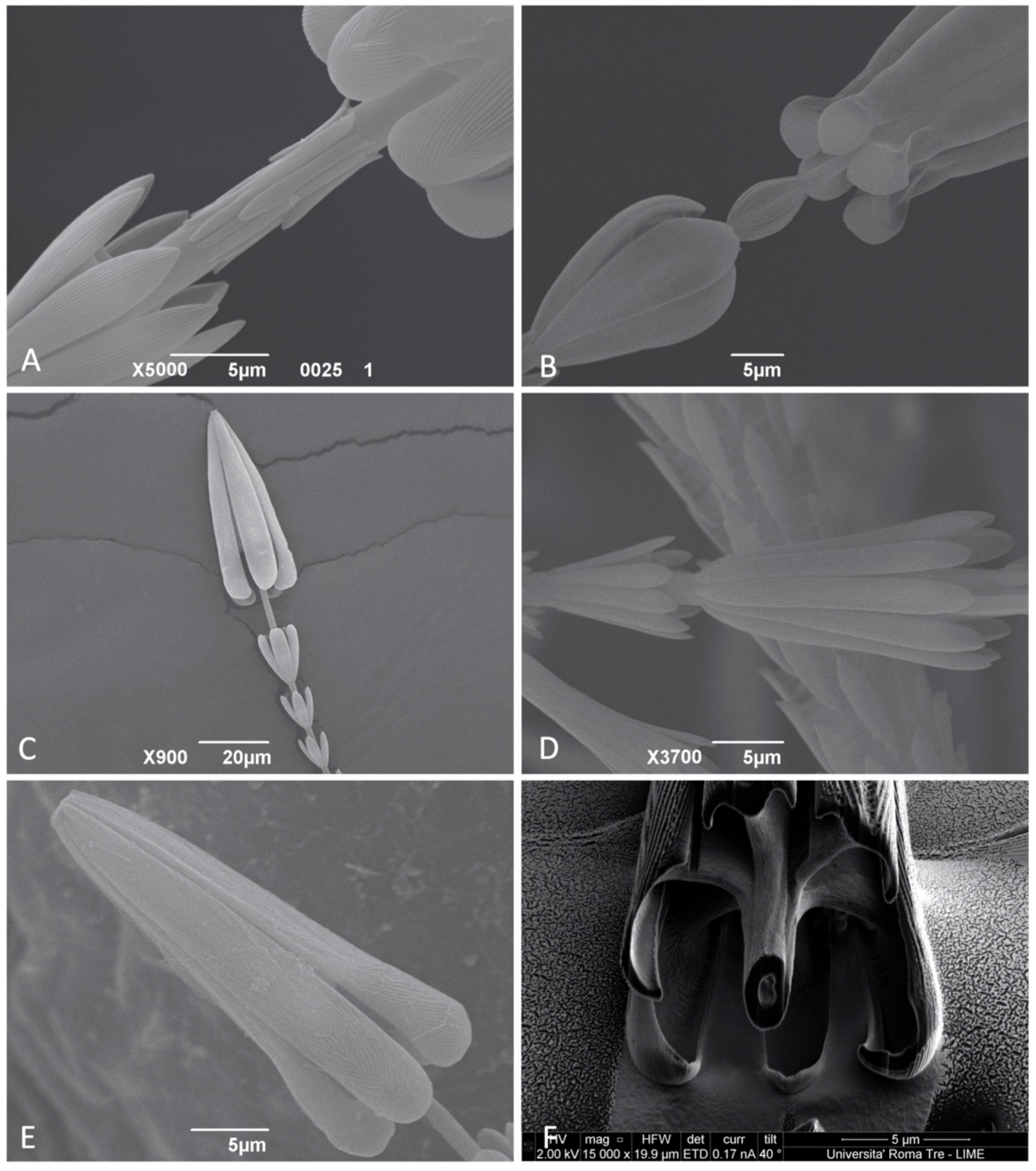
3.1.3. Apical Head
3.1.4. Internal Structure
3.2. Response to Disturbance
4. Discussion
4.1. Morphology and Biomechanics of Hastisetae
4.2. Systematics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisner, T.; Eisner, M.; Deyrup, M. Millipede defense: Use of detachable bristles to entangle ants. Proc. Natl. Acad. Sci. USA 1996, 93, 10848–10851. [Google Scholar] [CrossRef] [PubMed]
- Ruzzier, E.; Kadej, M.; Battisti, A. Occurrence, ecological function and medical importance of dermestid beetle hastisetae. PeerJ 2020, 8, e8340. [Google Scholar] [CrossRef]
- Nutting, W.L.; Spangler, H.G. The hastate setae of certain dermestid larvae: An entangling defense mechanism. Ann. Entomol. Soc. Am. 1969, 62, 763–769. [Google Scholar] [CrossRef]
- Mills, R.B.; Partida, G.J. Attachment mechanisms of Trogoderma hastisetae that make possible their defensive function. Ann. Entomol. Soc. Am. 1976, 69, 29–33. [Google Scholar] [CrossRef]
- Kokubu, H.; Mills, R.S. Susceptibility of thirteen stored product beetles to entanglement by Trogoderma hastisetae. J. Stored Prod. Res. 1980, 16, 87–92. [Google Scholar] [CrossRef]
- Elbert, A. Elektronenmikroskopische Untersuchungen der Pfeilhaare verschiedener Arten der Anthreninae (Col. Dermestidae). Anz. Schädlingskunde Pflanzenschutz Umweltschutz 1976, 49, 81–83. [Google Scholar] [CrossRef]
- Elbert, A. Die Pfeilhaare der Megatominae (Col., Dermestidae): Ein Abwehrsystem. Anz. Schädlingskunde Pflanzenschutz Umweltschutz 1978, 51, 109–110. [Google Scholar] [CrossRef]
- Hinton, H.E. A Monograph of the Beetles Associated with Stored Products; British Museum (Natural History): London, UK, 1945; Volume 1, pp. 1–443. [Google Scholar]
- Gorgojo, I.E.; De Las Heras, M.; Pastor, C.; Cuesta Herranz, J.; Sanz Maroto, A. Allergy to Dermestidae: A new indoor allergen? [Abstract AB105]. J. Allergy Clin. Immunol. 2015, 135, AB105. [Google Scholar] [CrossRef]
- MacArthur, K.M.; Richardson, V.; Novoa, R.A.; Stewart, C.L.; Rosenbach, M. Carpet beetle dermatitis: A possibly under-recognized entity. Int. J. Dermatol. 2016, 55, 577–579. [Google Scholar] [CrossRef]
- Zhantiev, R.D. Classification and phylogeny of dermestids (Coleoptera, Dermestidae). Entomol. Rev. 2000, 80, 1115–1129. [Google Scholar]
- Kiselyova, T.; McHugh, J.V. A phylogenetic study of Dermestidae (Coleoptera) based on larval morphology. Syst. Entomol. 2006, 31, 469–507. [Google Scholar] [CrossRef]
- Zhantiev, R.D. Ecology and classification of dermestid beetles (Coleoptera, Dermestidae) of the Palearctic fauna. Entomol. Rev. 2009, 89, 157–174. [Google Scholar] [CrossRef]
- Háva, J. World Catalogue of Insects. Volume 13. Dermestidae (Coleoptera); Brill: Boston, MA, USA, 2015; pp. 1–419. [Google Scholar]
- Mullen, G.; Durden, I. Medical and Veterinary Entomology, 2nd ed.; Academic Press: London, UK, 2009; pp. 1–637. [Google Scholar]
- Athanassiou, C.G.; Phillips, T.W.; Wakil, W. Biology and control of the khapra beetle, Trogoderma granarium, a major quarantine threat to global food security. Annu. Rev. Entomol. 2019, 64, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Beal, R.S. Synopsis of the economic species of Trogoderma occurring in the United States with description of a new species (Coleoptera: Dermestidae). Ann. Entomol. Soc. Am. 1956, 49, 559–566. [Google Scholar] [CrossRef]
- Beal, R.S. Descriptions, biology, and notes on the identification of some Trogoderma larvae (Coleoptera, Dermestidae); Technical Bulletin No. 1228; US Department of Agriculture: Washington, DC, USA, 1960; pp. 1–26.
- Beal, R.S. A revisionary study of the North American dermestid beetles formerly included in the genus Perimegatoma (Coleoptera). Misc. Publ. Entomol. Am. 1967, 5, 281–312. [Google Scholar]
- Peacock, E.R. Adults and Larvae of Hide, Larder and Carpet Beetles and Their Relatives (Coleoptera: Dermestidae) and of Derodontid Beetles (Coleoptera: Derodontidae). In Handbooks for the Identification of British Insects; Royal Entomological Society: London, UK, 1993; pp. 1–144. Volume 5. [Google Scholar]
- Kiselyova, T. Description of the larval and pupal stages of Cryptorhopalum triste LeConte (Coleoptera: Dermestidae), with notes on biology and rearing. Coleopt. Bull. 2002, 56, 41–49. [Google Scholar] [CrossRef]
- Kadej, M.; Jaroszewicz, S. Detailed morphological description of the mature larva of Globicornis corticalis (Eichhoff, 1863) (Dermestidae: Megatominae) with comparisons to related species. Zootaxa 2013, 3686, 556–564. [Google Scholar] [CrossRef][Green Version]
- Kadej, M.; Jaroszewicz, S.; Tarnawski, D. Morphology of mature larvae of three species of the genus Anthrenus (Dermestidae: Megatominae: Anthrenini) with comparisons to related species. Ann. Entomol. Soc. Am. 2013, 13, 706–718. [Google Scholar] [CrossRef]
- Kadej, M.; Jaroszewicz, S.; Tarnawski, D. Comparative morphology and biology of mature larvae in the genus Anthrenus (Dermestidae: Megatominae: Anthrenini) with comparisons to related species. Ann. Société Entomol. Fr. N.S. Int. J. Entomol. 2013, 49, 244–256. [Google Scholar] [CrossRef]
- Kadej, M.; Guziak, J.; Marczak, D. A detailed updated description of the morphology of the larva of Reesa vespulae (Milliron, 1939) (Dermestidae: Megatominae: Megatomini). FL Entomol. 2017, 100, 286–291. [Google Scholar] [CrossRef]
- Kadej, M.; Guziak, J. Description of the larva of Globicornis emarginata (Gyllenhal, 1808) (Dermestidae: Megatominae). Ann. Zool. 2017, 67, 749–757. [Google Scholar] [CrossRef]
- Kadej, M. Detailed morphological description of the mature larva of Anthrenus latefasciatus Reitter, 1892 (Dermestidae: Megatominae: Anthrenini) with comparisons to related species. Zootaxa 2012, 3270, 31–40. [Google Scholar] [CrossRef]
- Kadej, M. Detailed description of the morphology of the last instar larva of Trogoderma megatomoides Reitter, 1881 (Dermestidae: Megatominae: Megatomini) with comparison to related species. J. Kans. Entomol. Soc. 2012, 85, 5–13. [Google Scholar] [CrossRef]
- Kadej, M. Larva and pupa of Megatoma (s. str.) undata (Linnaeus, 1758) (Coleoptera: Dermestidae) with remarks on biology and economic importance. Zookeys 2017, 698, 59–74. [Google Scholar] [CrossRef][Green Version]
- Kadej, M. Larva and pupa of Ctesias (s. str.) serra (Fabricius, 1792) (Coleoptera: Dermestidae) with remarks on biology and economic importance, and larval comparison of co-occurring genera. Zookeys 2018, 758, 115–135. [Google Scholar] [CrossRef]
- Kadej, M. Contribution to the knowledge of the immature stages of Dermestidae with special emphasis on the larval morphology of the genus Anthrenus Geoffroy, 1762 (Megatominae: Anthrenini). In Polish Entomological Monographs; Polish Entomological Society: Poznań, Poland, 2018; Volume 16, pp. 1–180. [Google Scholar]
- Ma, M.; Burkholder, W.E.; Carlson, S.D. Supra-anal Organ: A Defensive Mechanism of the Furniture Carpet Beetle, Anthrenus flavipes (Coleoptera: Dermestidae). Ann. Entomol. Soc. Am. 1978, 71, 718–723. [Google Scholar] [CrossRef]
- Battisti, A.; Holm, G.; Fagrell, B.; Larsson, S. Urticating hairs in arthropods: Their nature and medical significance. Annu. Rev. Entomol. 2011, 56, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Castalanelli, M.; Baker, A.; Munyard, K.; Grimm, M.; Groth, D. Molecular phylogeny supports the paraphyletic nature of the genus Trogoderma (Coleoptera: Dermestidae) collected in the Australasian ecozone. Bull. Entomol. Res. 2012, 102, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Poinar, G.O., Jr.; Poinar, R. Ancient hastisetae of Cretaceous carrion beetles (Coleoptera: Dermestidae) in Myanmar amber. Arthropod Struct. Dev. 2016, 45, 642–645. [Google Scholar] [CrossRef]

| Species | Stage | Origin | Supp. File |
|---|---|---|---|
| Anthrenocerus australis (Hope, 1843) | Larva | Australia | S1 |
| Anthrenus (Anthrenus) latefasciatus Reitter, 1892 | Exuvia | Kazakhstan | S2 |
| Anthrenus (Anthrenus) picturatus makolskii Mroczkowski, 1950 | Larva (ex ovo) | Poland | S3 |
| Anthrenus (Anthrenus) scrophulariae scrophulariae (Linnaeus, 1758) | Exuvia | Poland | S4 |
| Anthrenus (Florilinus) olgae Kalík, 1946 | Larva (ex ovo) | Poland | S5 |
| Anthrenus (Helocerus) fuscus Olivier, 1789 | Larva (ex ovo) | Poland | S6 |
| Anthrenus (Helocerus) polonicus Mroczkowski, 1950 | Larva (ex ovo) | Poland | S7 |
| Anthrenus spp. 1 | Larva | USA (California) | S8 |
| Anthrenus spp. 2 | Exuvia | Portugal | S9 |
| Ctesias serra (Fabricius, 1792) | Larva | Poland | S10 |
| Megatoma (Megatoma) undata (Linnaeus, 1758) | Exuvia | Poland | S11 |
| Thaumaglossa rufocapillata Redtenbacher, 1867 | Larva | China | S12 |
| Trogoderma granarium Everts, 1898 | Larva | Czech Republic | S13 |
| Trogoderma simplex Jayne, 1882 | Larva | USA (California) | S14 |
| Trogoderma variabile Ballion, 1878 | Larva | USA (California) | S15 |
| Trogoderma versicolor (Creutzer, 1799) | Larva (ex ovo) | Poland | S16 |
| Trogoderma spp. 1 | Larva | Italy | S17 |
| Trogoderma spp. 2 | Larva | Romania | S18 |
| Trogoderma spp. 3 | Larva | Italy | S19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruzzier, E.; Kadej, M.; Di Giulio, A.; Battisti, A. Entangling the Enemy: Ecological, Systematic, and Medical Implications of Dermestid Beetle Hastisetae. Insects 2021, 12, 436. https://doi.org/10.3390/insects12050436
Ruzzier E, Kadej M, Di Giulio A, Battisti A. Entangling the Enemy: Ecological, Systematic, and Medical Implications of Dermestid Beetle Hastisetae. Insects. 2021; 12(5):436. https://doi.org/10.3390/insects12050436
Chicago/Turabian StyleRuzzier, Enrico, Marcin Kadej, Andrea Di Giulio, and Andrea Battisti. 2021. "Entangling the Enemy: Ecological, Systematic, and Medical Implications of Dermestid Beetle Hastisetae" Insects 12, no. 5: 436. https://doi.org/10.3390/insects12050436
APA StyleRuzzier, E., Kadej, M., Di Giulio, A., & Battisti, A. (2021). Entangling the Enemy: Ecological, Systematic, and Medical Implications of Dermestid Beetle Hastisetae. Insects, 12(5), 436. https://doi.org/10.3390/insects12050436








