Simple Summary
Bactrocera dorsalis fat body protein 1 (Bdfbp1) cDNA was cloned. The deduced amino acid sequence contains three motifs: hemocyanin N, high molecular weight glutenin (gultenin hmw), and hemocyanin C from N to C termini. The glutenin hmw allows Bdfbp1 to fold into a compact form for storage. Bdfbp1 was highly expressed in the late third instar larvae and day 0 pupae. This suggests that Bdfbp1 is stored during larval stages as a storage protein for construction of adult tissues during pupal stages, and may be associated with adult eclosion.
Abstract
Bactrocera dorsails fat body protein 1 (Bdfbp1) cDNA was cloned (GenBank accession no. MT514270), and the complete 3,749-bp cDNA encoded a 1,152-amino acid protein. The phylogenetic relationship of dipteran fbp1s was analyzed. The sequence XP_028900815 from the insect genome project for Zeugodacus cucurbitae (LOC105219342) was proposed that two fbp1 genes were present in the sequence. The developmental transcriptional expression profiles were determined. In the larval stages, Bdfbp1 mRNA had significantly higher expression in the late third instar larvae compared with first, second, and early third instar larvae. In the pupal stages, the highest expression of Bdfbp1 mRNA was found in the newly pupated pupae and then decreased with age. In the fat body of female adults, Bdfbp1 was highly expressed in newly emerged samples and decreased rapidly over the following three days. In the fat body of male adults, Bdfbp1 was highly expressed in newly eclosed samples. RNAi treatment decreased the expression level of Bdfbp1 without statistical difference. However, RNAi treatment significantly decreased the rate of eclosion. These results suggest that Bdfbp1 may function as a storage protein and be associated with adult eclosion.
1. Introduction
The fat body, an essential tissue for insect metabolism, provides energy and synthesizes proteins for insect development and reproduction. As the developmental stages change, the function of the fat body changes accordingly. At the pupal stage, insects require large amounts of energy and materials for adult development. Because pupae do not ingest any food, all substances for adult development are stored during the larval stages, especially the late larval stage.
The oriental fruit fly, Bactrocera dorsalis, is one of the most disastrous pests, leading to damages of about 450 plant species throughout the world [1]. Their high fecundity makes them preeminent, with 1000 eggs laid over the entire life-span of females [2]. Females deposit their eggs into fruit where the larvae hatch and feed. Until the late third instar, larvae drill out the fruit and enter the soil to pupate. Pesticides are most frequently employed to control B. dorsalis. The tendency to develop resistance to different types of insecticides has been reported [3,4,5]. It is necessary to develop new strategies to control B. dorsalis.
There are two forms of fat body in the B. dorsalis adult abdomen: the white, nodule-like larval-form fat body and the thin, sheet-like adult-form fat body [6]. A comparison of gene expression between the larval- and adult-form fat body in B. dorsalis adults reveals that the arylphorin receptor is expressed more abundantly in the larval form [6]. The nomenclature of the arylphorin receptor (accession no. JZ476873) was changed to fat body protein 1 (fbp1) after the release of B. dorsalis genome annotation.
The fbp1 gene of Drosophila melanogaster has long served as a model for the study of 20-hydroxyecdysone regulation. The transcriptional expression of fbp1 is the tissue specific in the fat body, and temporally specific at the end of the third instar [7,8]. A ligand binding assay and Western blot analyses demonstrated that the product of fbp1 of D. melanogaster is a larval serum protein (LSP) receptor [9]. LSPs are storage proteins generally referred to as hexamerins, which are involved in pupation and metamorphosis [10,11].
In B. dorsalis, the storage protein, hexamerin gene BdAr is expressed in third instar larvae, pupae, and adults, with especially high expression levels in late third instar larvae and day 0 adults [12]. The form of fat body is larval form in third instar larvae and day 0 adults. Because B. dorsalis fbp1 (Bdfbp1) is highly expressed in larval-form fat body as BdAr is [6], this makes us to hypothesize that fbp1 functions as a storage protein. The developmental transcription profiles of Bdfbp1 were determined through real-time quantitative polymerase chain reaction, and the function of Bdfbp1 related to adult eclosion was explored through RNA interference.
2. Materials and Methods
2.1. Insects
Colonies of the oriental fruit fly, B. dorsalis, were maintained as described elsewhere [12]. Larvae were fed on an artificial diet containing 1800 mL of water, 20 mL of HCl, 5 g of benzoic acid, 240 g of sugar, 140 g of yeast extract, and 480 g of wheat bran [13].
2.2. cDNA Cloning of fbp1 through Rapid Amplification of cDNA Ends and Sequence Analyses
A 641-bp cDNA fragment encoding partial Bdfbp1 was obtained from the day 0 (D0) fat body cDNA library [6]. The 5′ and 3′ end of the Bdfbp1 cDNA fragments were produced using the GeneRacerTM Kit (Thermal, Carlsbad, CA, USA). The total RNA was extracted from the D0 female fat body using TRIZOL® Reagent (Thermal, Carlsbad, CA, USA) according to the manufacturer’s instructions. Bdfbp1-specific primers were designed based on the sequence of the 641-bp fragment. The PCR parameters were as follows: 94 °C for 2 min; 35 cycles of 94 °C for 30 s, 56–62 °C (based on the Tm of the primers, Table 1) for 30 s, and 72 °C for 1–3 min (based on the expected size of the fragment); and 72 °C for 10 min. The PCR products were purified, cloned, and sequenced as described elsewhere [12].

Table 1.
Sequences of specific primers used for the rapid amplification of cDNA ends (RACE).
To prevent errors during assembly, the entire open reading frame in one fragment was cloned using two specific primers designed in the 5′ and 3′ untranslated regions. The sequences of sense and antisense primers were: Bdfbp1-full-01 5′-GACATTGATTGGTAGAGTCCAGCGGATAC-3′ and Bdfbp1-full-02 5′-GCTTCGGCTAATGCAACTAGCAGTGAG-3′, respectively. The PCR parameters were as follows: 94 °C for 2 min; 40 cycles of 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 4 min; and 72 °C for 10 min.
The signal peptide was predicted using the SignalP 4.1 Server [14] (http://www.cbs.dtu.dk/services/SignalP4.1/). The Conserved Domains Database (CDD, http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) (accessed on 25 May 2020) on the NCBI website was used to identify the conserved motifs [15]. Lalign program [16] was applied to align amino acid sequences of MT514270 and XP_029405180.
2.3. Identification of the fbp1 from the Melon Fly, Zeugodacus Cucurbitae
The blastn and blastp search algorithms in NCBI searches indicated that the sequence cloned in this study had high identity to the B. dorsalis fat body protein 1 (accession numbers: XM_029549320 and XP_029405180, respectively). The blast searches also revealed one uncharacterized gene product from the genome of the melon fly, Zeugodacus cucurbitae, (GeneID: LOC105219342; protein accession number: XP_028900815). A protein of 2380 amino acids was identified from XP_028900815. Blast results revealed that the amino acid sequence 1–1225 of XP_028900815 was highly similar to that of the Bdfbp1 cloned in this study. However, a melon fly fbp1 (accession number: JAD03259) was found in a survey of the GenBank protein database and its sequence was same as the amino acid sequence 130–2380 of XP_028900815 from LOC105219342.
2.4. Phylogenetic Analysis
Amino acid sequences of 11 fbp1s from the blast results and GenBank were used for the phylogenetic analysis. Sequences were aligned using the MUSCLE program (http://www.ebi.ac.uk/Tools/msa/muscle/) (accessed on 20 July 2020). Phylogenetic relationships were analyzed using neighbor-joining (NJ). The Poisson model was used as substitution model to construct an NJ tree using MEGA 7.0 software [17]. Bootstrapping analyses of 1000 replications were applied.
2.5. Real-Time Quantitative PCR (qPCR)
Developmental regulation of Bdfbp1 transcription was performed in larvae, pupae, and adult fat body tissues through qPCR. The sample collection of different developmental stages and qPCR were conducted [12]. Briefly, larvae were collected from the first, second, early third, and late third instars. Pupae were collected separately from days 0 to 8 after pupation. Fat bodies from 0- to 10-day-old females and males were separately isolated. Glyceraldehyde-3-phosphate dehydrogenase (gapdh) was used as the reference gene for adult samples [18]. 18S ribosomal RNA genes were used as the reference genes for larval and pupal samples. The sequences of specific primers used in the qPCR are listed in Table 2. The PCR parameters were as follows: 50 °C for 2 min; 95 °C for 8.5 min; and 40 cycles of 95 °C for 15 s and 60–65 °C (based on the Tm of the primers, Table 2) for 1 min. A melting curve analysis was carried out for each test to determine the specificity of the amplification. Three independent biological replicates of each developmental stage were performed. The expression data were collected via the Bio-Rad iQ5 2.0 Standard Edition Optical System Software V2.0. The expression data were applied for quantification data analyses. The statistical analyses, ANOVA followed by a Tukey multiple comparison were performed using Prism™ 5.0 (GraphPad Software, San Diego, CA, USA). P < 0.05 was considered statistically significant.

Table 2.
Sequences of specific primers used in the real-time quantitative PCR.
2.6. RNA Interference
Synthesis of double-stranded Bdfbp1 (dsBdfbp1) RNA was conducted using MEGAscript® RNAi Kit (Thermal) following the manufacturer’s instructions. A plasmid containing the full-length cDNA of Bdfbp1 was used as the template. The two pairs of primers used to synthesize the dsRNA are listed in Table 3. The dsRNA products were electrophoresed on 1% agarose gel to confirm the size and integrity. For the feeding treatment, 40–60 eggs were placed into a Petri dish (15 cm in diameter) containing 55 g of larval artificial diet. In total, 50 μg of two fragments of dsRNA (25 μg of each) in 10 mL of water was added to the Petri dish on day 3 after egg collection. At this time, the larvae were in the early third instar. Ten mL of water was added to the Petri dish for the controls. After five days of continuous feeding (250 μg of dsRNAs or water), late third instar larvae started to jump out the artificial diet for pupation. Ten late third instar larvae were collected from each treatment and control groups for RNA extraction, and one μg of RNA was reverse-transcribed as cDNA for qPCR. The others were left for pupation. Pupae were then moved to observe the rate of eclosion. The rate of eclosion was analyzed using Student’s t test. P < 0.05 was considered statistically significant.

Table 3.
Sequences of specific primers used for the synthesis of dsBdfbp1 RNA.
3. Results
3.1. cDNA Cloning and Sequence Analyses
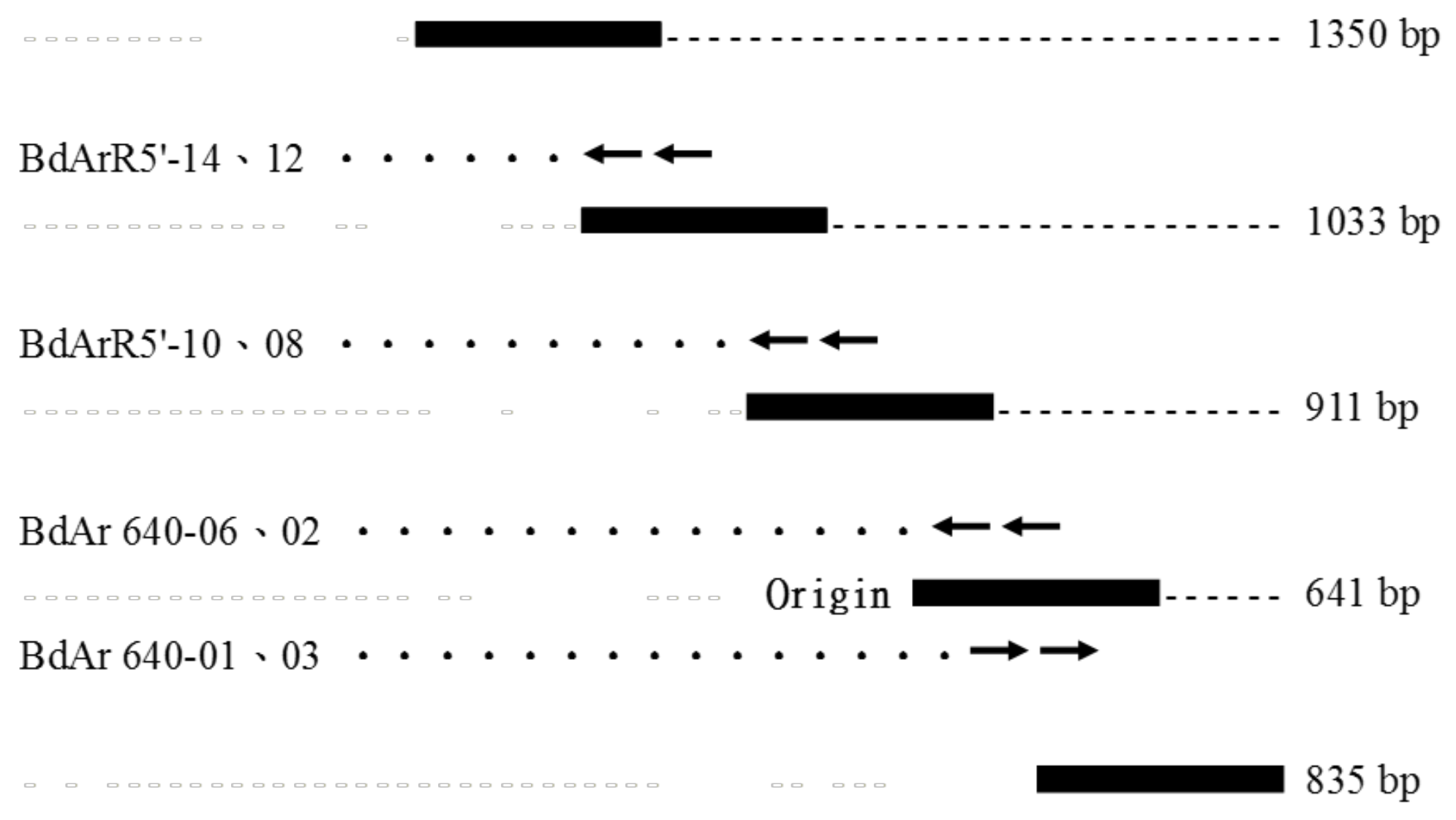
The five cDNA fragments encompassing the entire coding region of the Bdfbp1 cDNA were cloned, sequenced, and analyzed (Figure 1). The assembled 3749-bp cDNA sequence revealed an open reading frame of 3459 bp encoding a protein of 1152 amino acid residues (GenBank accession no. MT514270). Signal 4.1 predicted that the first 17 amino acids at the N-terminus produced a signal peptide and no transmembrane domain was predicted. The CDD search for the Bdfbp1 revealed that the N (amino acid sequence 50–167) and C (amino acid sequence 899–1145) termini belonged to hemocyanins N and C, respectively. The conserved motif between hemocyanins N and C was a high-molecular-weight glutenin subunit (glutenin hmw, amino acid sequence 361–618) (Figure 2).
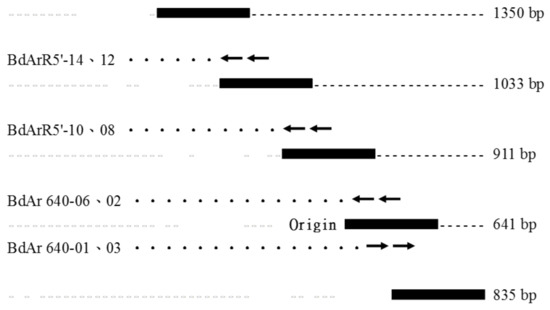
Figure 1.
Assembly of the fragments of the fbp1 cDNA cloning in B. dorsalis.

Figure 2.
Deduced amino acid sequences of the fat body protein 1 cDNA from B. dorsalis (GenBank accession number MT514270). The cleavage site of the putative signal peptide sequence is indicated by a vertical arrow. The hemocyanin N domain is underlined, the hemocyanin C domain is indicated with double underlines, and the high molecular weight glutenin subunit is marked in gray.
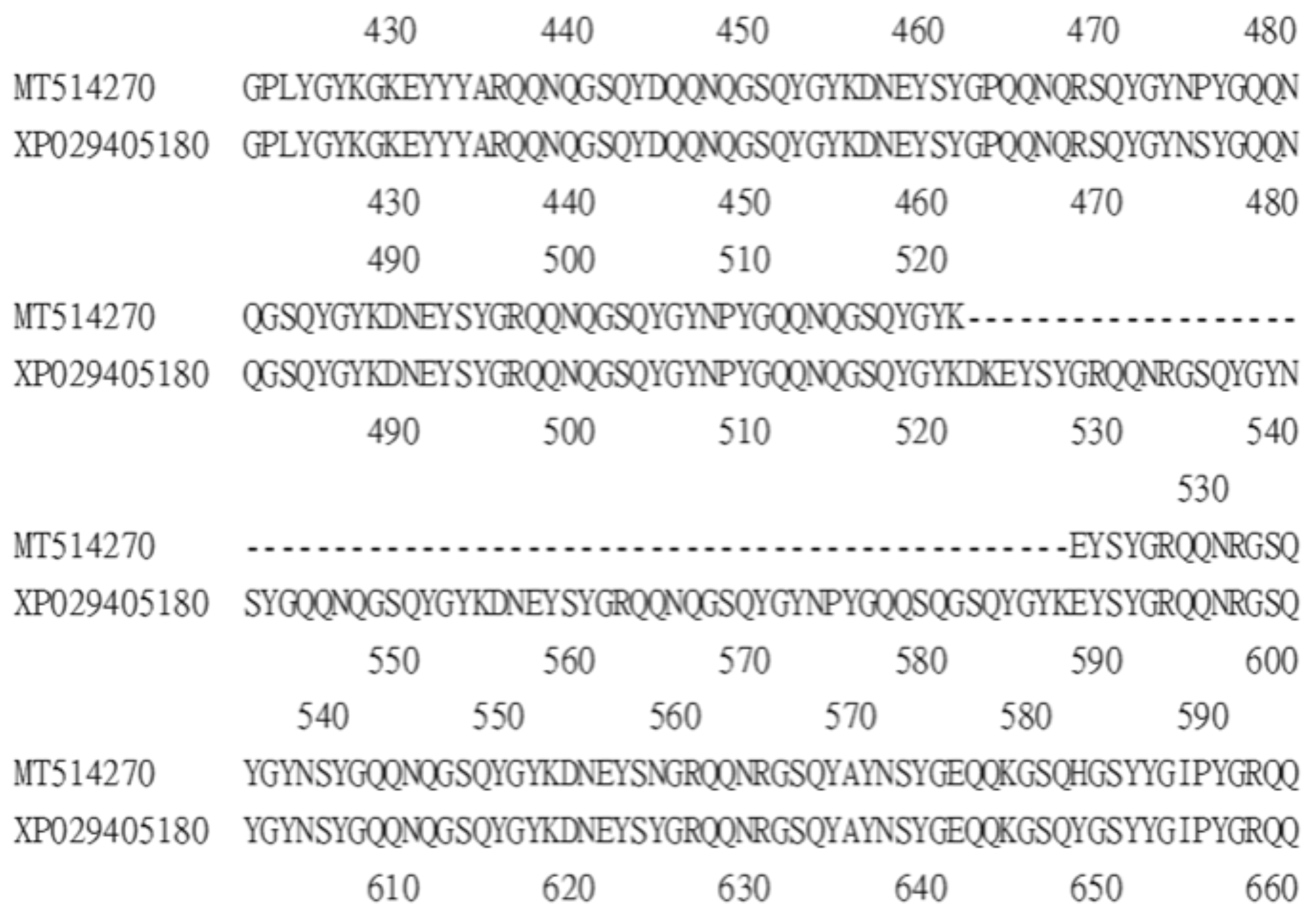
A survey of the GenBank protein database identified two Bdfbp1s (accession numbers XP_029405180 and XP_029405169). The identity between the protein cloned in this study, MT514270, and the protein from GenBank, XP_029405180, was 94%. The alignment between MT514270 and XP029405180 indicated that MT514270 was almost identical to XP_029405180, except that MT514270 was 66 amino acids shorter than XP_029405180 (amino acid sequence 522–587) (Figure 3). These 66 amino acids belong to the glutenin hmw motif. The identity between MT514270 and XP029405169 was 23.7%. No glutenin hmw motif was found in XP_029405169 (Figure 4).
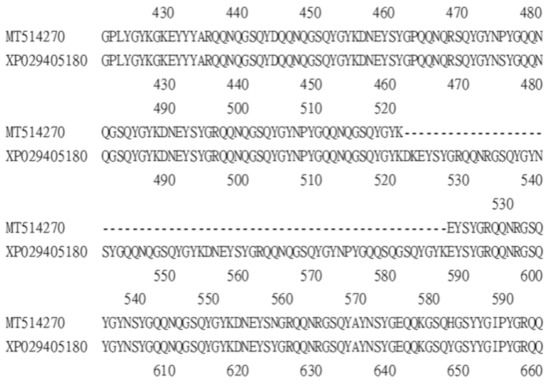
Figure 3.
Alignment between Bdfbp1 MT514207 (amino acid sequence 421–594) and Bdfpb1 XP029405180 (amino acid sequence 421–660) shows that MT514207 is 66 amino acids shorter than XP029405180.

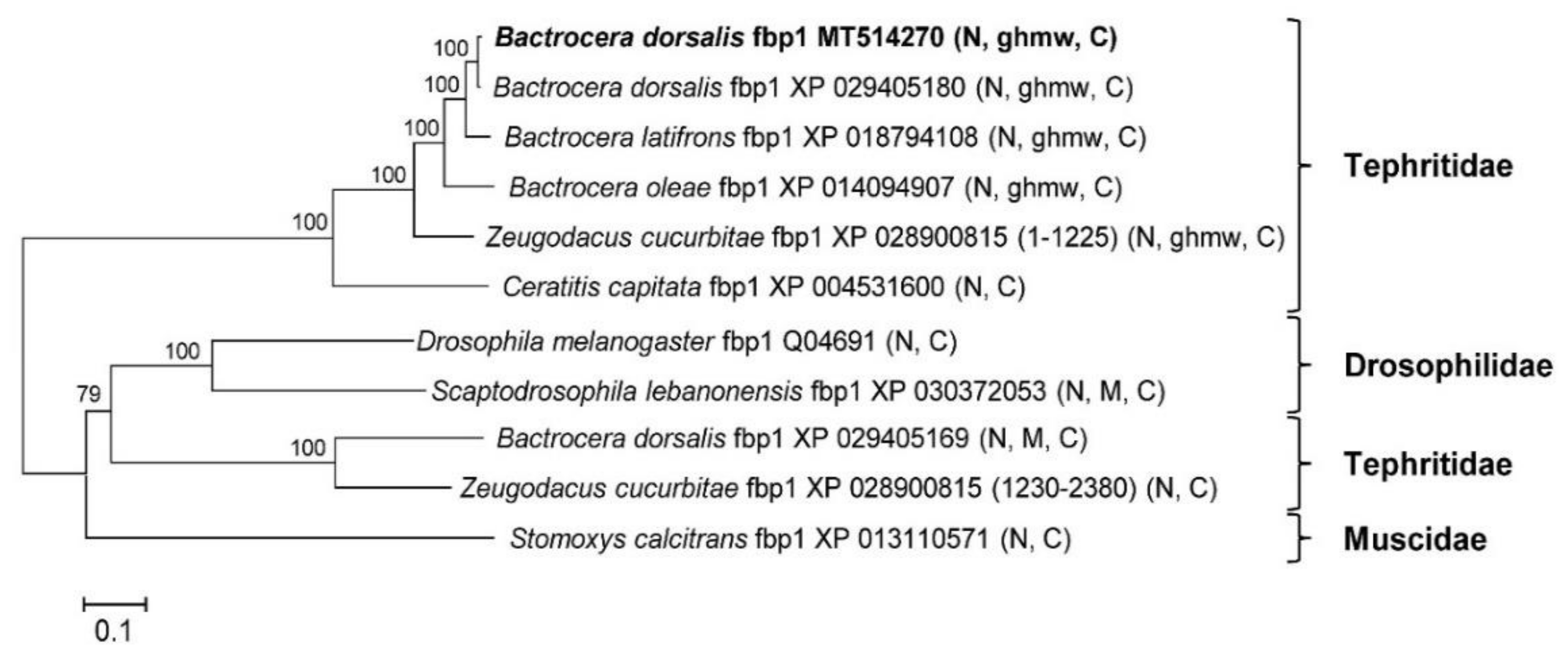
Figure 4.
The phylogenetic tree of 11 versions of insect fat body protein 1 (fbp1) was constructed by neighbor joining. Numbers at nodes are based on bootstrapping statistical support. Scale bar = 0.1 substitution/site. Conserved domains in parentheses are preceded by the scientific name, and the accession number. N: hemocyanin N; M: hemocyanin M; C: hemocyanin C; ghmw: high molecular weight glutenin subunit.
Eleven amino acid sequences from the GenBank protein database were applied to the phylogenetic analysis, including the Z. cucurbitae sequence XP_028900815, which was separated into two sequences (amino acid sequences 1–1225 and 1230–2380). We found that Z. cucurbitae JAD03259 was 100% identical to the sequence 1230–2380 of XP_028900815 from the genome of Z. cucurbitae. A phylogenetic analysis revealed that XP_028900815 contained two genes (Figure 4). The results also showed that all fbp1s were grouped into two clades. One clade contained only Tephritidae and the other contained Drosophilidae, Tephritidae, and Muscidae (Figure 4). The conserved motifs of the distinct Tephritidae clade were hemocyanin N, glutenin hmw, and hemocyanin C from the N to C termini. Ceratitis capitata fbp1 was an exception; it contained only hemocyanins N and C. The other clade contained two types of conserved motifs: hemocyanins N and C; and hemocyanins N, M, and C from the N to C termini (Figure 4). The difference between the two clades is that the glutenin hmw.
3.2. Expression Profiles of Bdfbp1
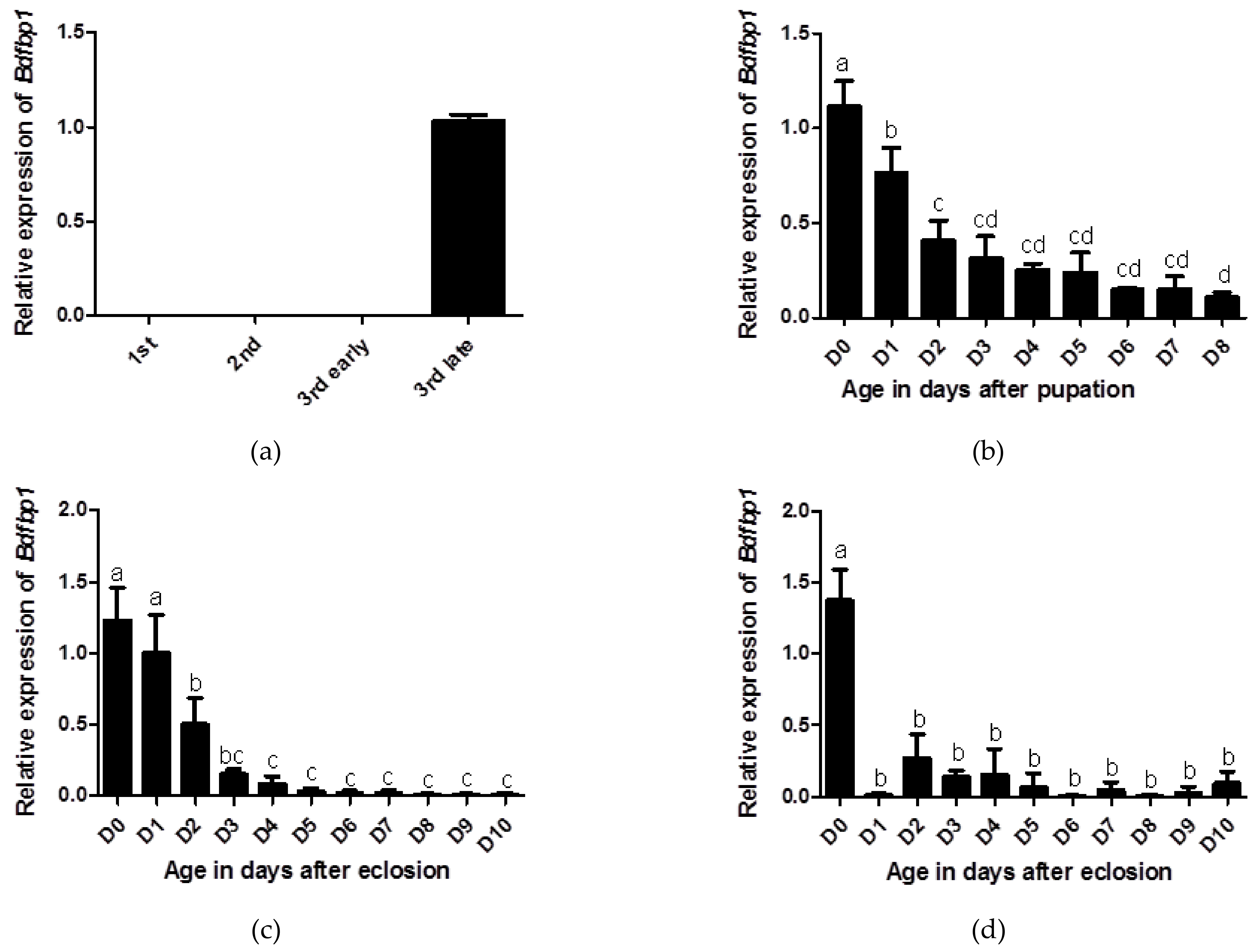
The developmental transcriptional expression profiles of Bdfbp1 were determined through qPCR. In the larval stages, Bdfbp1 transcripts were expressed at significantly higher levels in the late third instar compared with the first, second, and early third instar (Figure 5a). The expression level for day 0 pupae was the highest, followed by day 1 pupae, and these differences in expression levels were statistically significant (Figure 5b). After eclosion, Bdfbp1 transcripts were detected in the female fat body from day 0 to day 10. The expression level was highest on days 0 and 1 with significantly higher expression levels than on day 2 to day 10 (Figure 5c). Although the expression levels of Bdfbp1 were high in the day 0- and day 1-female fat body, they were still lower than the expression levels in the day 7 and day 8 pupae (data not shown). In the male fat body, Bdfbp1 transcripts were also detected from day 0 to day 10. However, Bdfbp1 had significantly higher expression levels on day 0 (Figure 5d).
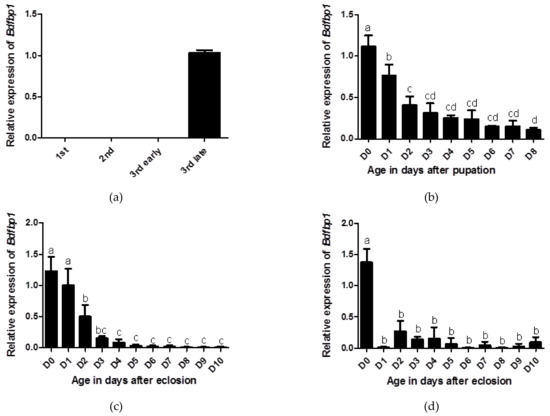
Figure 5.
Developmental profiles of Bdfbp1 transcription were determined through qPCR. (a) larval stage: first, second, early third, and late third instar; (b) pupal stage: 0–8 days after pupation; (c) adult female fat body, and (d) adult male fat body: 0–10 days after eclosion. Statistical analyses using ANOVA followed by a Tukey multiple comparison test were performed to identify differences between individual means. The same letters indicate no significant difference (p > 0.05). Standard deviation of three independent biological replicates is indicated by error bars in (a–d).
3.3. Functional Studies of Bdfbp1
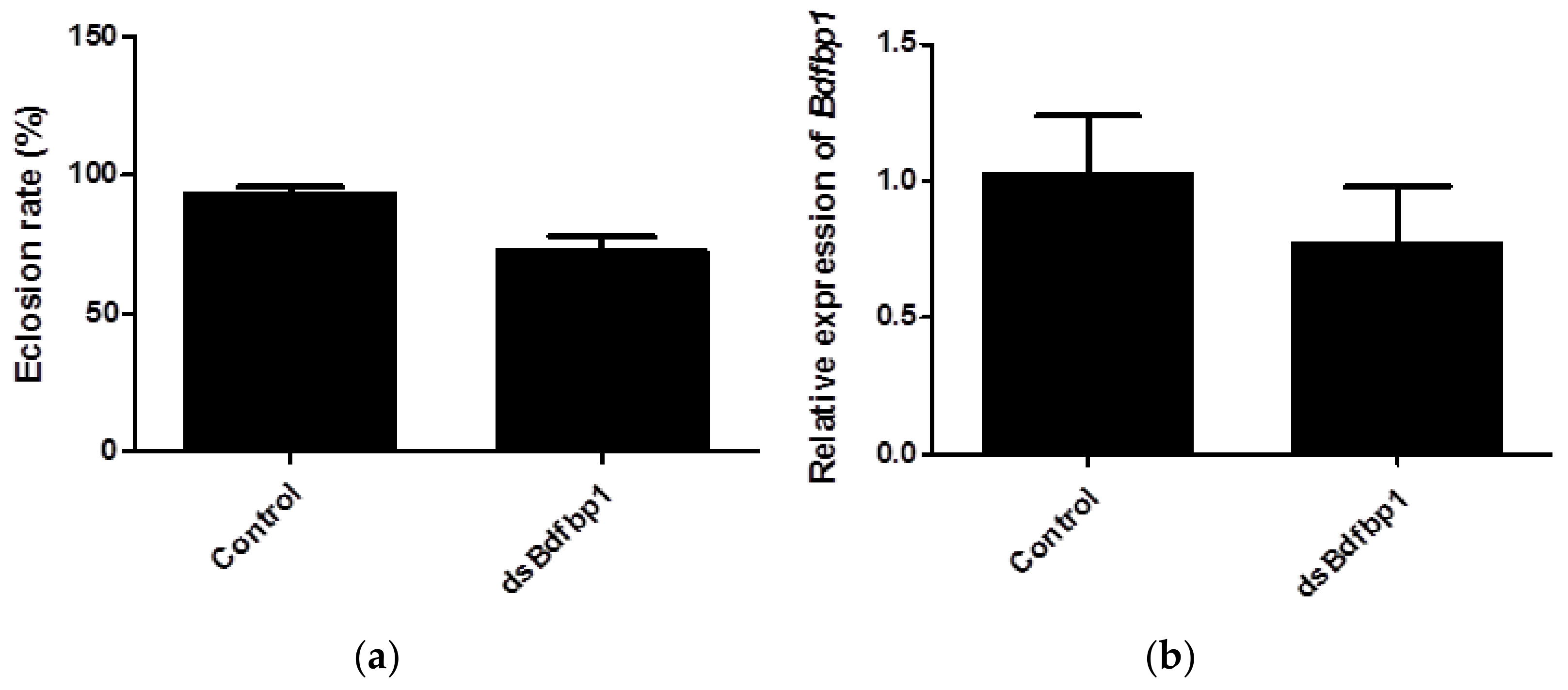
In this study, RNAi was applied to investigate the function of Bdfbp1 as a storage protein for metamorphosis. After 5 continuous days of larval feeding of dsBdfbp1 RNA or water, both treatment and control larvae were able to pupate normally. However, the eclosion rate of pupae was 72.2% in the treatment group compared with 93.2% in the control group. The difference was statistically significant (p = 0.0041; Figure 6a). The expression of Bdfbp1 was 25% lower than that of the control after dsBdfbp1 RNA application, although this difference was not statistically significant (p = 0.2240; Figure 6b).
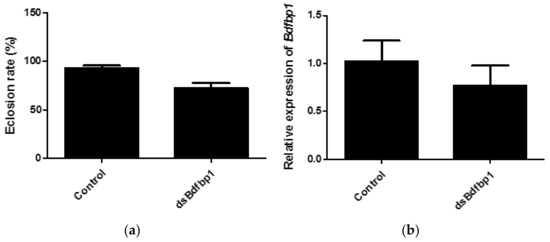
Figure 6.
(a) The eclosion rate of B. dorsalis after RNAi treatment of larvae; (b) Bdfbp1 relative transcriptional expression in B. dorsalis late third instar larvae after RNAi treatment. Statistical analyses were performed using Student’s t test. The asterisk indicates significant difference (p < 0.05). The standard deviation of each triplicate is shown by error bars in (a,b).
4. Discussion
The present studies were undertaken to clone fbp1 cDNA from B. dorsalis as a first step in investigating the transcriptional expression profiles and the function of Bdfbp1. The deduced amino acid sequence of Bdfbp1 indicated that it secretes into the hemolymph because there was a signal peptide predicted at the N terminus and no transmembrane domain was predicted (Figure 2). Bdfbp1 contained the hemocyanin N, glutenin hmw, and hemocyanin C domains from N to C termini. Glutenin hmw has an extensive central elastomeric domain flanked by two nonelastic domains. The central elastomeric domain is characterized by three repeated motifs, namely PGQGQQ, GYYPTSPQQ, and GQQ [19]. These motifs confer elasticity to the fbp1, allowing it to fold into a more compact storage protein.
In the GenBank protein database, all fbp1s are from insects and most of them are from Brachycera, Diptera. The nomenclature for fbp1 is rather disordered. We found that two fbp1 genes were included in Z. cucurbitae sequence XP_028900815. One contains glutenin hmw motif, and the other does not (Figure 4). The one without the glutenin hmw motif is also assigned as another accession number JAD03259. There are two Bdfbp1s found in the GenBank, XP_029405180 and XP_029405169. Based on the phylogenetic analysis, these two Bdfbp1s are belonged to different clades (Figure 4). Bdfbp1 cloned in this study, MT514270, is more related to or same as XP_029405180 than it is to XP_029405169 (Figure 4). The aforementioned results suggest that the phylogeny, nomenclature, and evolution of these molecules are complex. It would be difficult to correctly summarize the characteristics of fbp1 without further studies being conducted.
Bdfbp1 transcriptional expression is developmentally regulated (Figure 5). The transcriptional expression of Bdfbp1 in the first, second, and early third instar was difficult to detect but the expression level was the highest in the late third instar. This expression profile was similar to that of Drosophila [7,8]. During the pupal stages, Bdfbp1 transcripts were expressed constantly from day 0 to day 8 after pupation, showing a trend to decrease with age. To date, no studies have reported transcriptional expression of fbp1 in the pupal stages. On the basis of expression profiles of larvae and pupae, it has been suggested that Bdfbp1 is stored during larval stages as a storage protein and used during pupal stages for metamorphosis [9,20,21]. In both females and males, on days 0 and 1, the fat body in adults is the larval-form fat body [6]. The high expression levels of Bdfbp1 in adults were consistent with the appearance of the larval-form fat body. The developmental transcriptional expression profiles suggest that Bdfbp1 is mainly synthesized from the larval-form fat body for energy storage [6].
The study of fbp1 has mainly focused on the ecdysone regulation of fbp1 expression [7,8]. In this study, knockdown of Bdfbp1 using RNAi was performed to understand the possible function of Bdfbp1. There are three main methods to apply double strand RNA: injection, topical application, and feeding [22]. Topical application and feeding were applied in this study since larvae were immersed in and digested the artificial diet mixed with dsBdfbp1 RNA. DsBdfbp1 RNA was added to the artificial diet at the time before the expression of Bdfbp1 (Figure 5a). At this time larvae were in the early third instar which were sensitive to RNAi treatment [23]. After RNAi treatment, the expression level of Bdfbp1 was decreased without statistical difference (Figure 6b). It is possible that the concentration of dsBdfbp1 RNA applied is not enough. The eclosion rate was significantly decreased after RNAi treatment. The results suggest that Bdfbp1 may be associated with the eclosion rate. Knockdown of larval storage proteins should result in a shortage of materials for adult tissue formation, and this further inhibits adult development. The larval storage proteins are mostly hexamerin [11,24,25,26]. In Riptortus pedestris, hexmerin-β is highly expressed in the last instar nymphs. Knockdown of hexmerin-β by RNAi in second instar nymphs delays adult emergence time [27].
Microinjection of dsRNA is useful to investigate the function of a gene. Most studies of gene functions in B. dorsalis larvae are performed in microinjection [23,28,29,30]. However, microinjection methods are not applicable for pest control. RNAi by feeding in B. dorsalis larvae is spare. Li et al. (2017) [31] fed B. dorsalis larvae dsBdTrys RNA on solid artificial diets, which was inconvenient for dsRNA. In this study, wet artificial diets were applied. It was easy to operate but the concentration of dsRNA would be higher to achieve the knockdown effect. Mixed dsBdTrys RNA treatment significantly decreased the size of B. dorsalis larvae [31]. Effect of mixed of larval storage protein dsRNA on adult development could be the future study. This should be a useful candidate for pest control based on RNAi.
Author Contributions
Conceptualization, M.-E.C.; methodology, Y.-C.Y., H.L., Y.-C.C., C.-L.T., Y.-H.Z.; formal analysis, Y.-C.Y. and M.-E.C.; data curation, M.-E.C.; writing—original draft preparation, Y.-C.Y. and M.-E.C.; writing—review and editing, M.-E.C.; supervision, M.-E.C.; project administration, Y.-C.Y. and M.-E.C.; funding acquisition, M.-E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Council, Taiwan (NSC 98-2313-B-005-029-MY3), and the Bureau of Animal and Plant Health Inspection and Quarantine, Council of Agriculture, Executive Yuan, Taiwan, to M.-E. Chen.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wei, D.; He, W.; Lang, N.; Miao, Z.; Xiao, L.; Dou, W.; Wang, J. Recent research status of Bactrocera dorsalis: Insights from resistance mechanisms and population structure. Arch. Insect Biochem. Physiol. 2019, 102, e21601. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.I.; Walsh, W.A.; Kanehisa, D.; Stark, J.D.; Nishida, T. Comparative demography of three Hawaiian fruit flies (Dip-tera: Tephritidae) at alternating temperatures. Ann. Entomol. Soc. Am. 2000, 93, 75–81. [Google Scholar] [CrossRef]
- Hsu, J.-C.; Feng, H.-T.; Wu, W.-J. Resistance and synergistic effects of insecticides in Bactrocera dorsalis (Diptera: Tephritidae) in Taiwan. J. Econ. Èntomol. 2004, 97, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Zeng, L.; Lin, Y.; Lu, Y.; Liang, G. Insecticide resistance of the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidae), in mainland China. Pest Manag. Sci. 2011, 67, 370–376. [Google Scholar] [CrossRef]
- Khan, H.A.A.; Akram, W. Trichlorfon and spinosad resistance susrvey and preliminary determination of the resistance mechanism in Pakistani field strains of Bactrocera dorsalis. Sci. Rep. 2018, 8, 11223. [Google Scholar] [CrossRef]
- Zuo, Y.-H.; Lu, K.-H.; Chen, M.-E. Characteristics and gene expression of fat body in adult oriental fruit fly, Bactrocera dorsalis. Formos. Entomol. 2013, 33, 91–106. [Google Scholar]
- Maschat, F.; Dubertret, M.-L.; The’rond, P.; Claverie, J.-M.; Lepesant, J.-A. Structure of the ecdysone-inducible P1 gene of Drosophila melanogaster. J. Mol. Biol. 1990, 214, 350–372. [Google Scholar] [CrossRef]
- Lapie, P.; Nasr, F.; Lepesant, J.-A.; Deutsch, J. Deletion scanning of the regulatory sequences of the Fbp1 gene of Drosophila melanogaster using P transposase-induced deficiencies. Genetics 1993, 155, 801–816. [Google Scholar] [CrossRef]
- Burmester, T.; Antoniewski, C.; Lepesant, J.-A. Ecdysone-regulation of synthesis and processing of fat body protein 1, the larval serum protein receptor of Drosophila melanogaster. Eur. J. Biol. Inorg. Chem. 1999, 262, 49–55. [Google Scholar] [CrossRef]
- Roberts, D.B.; Wolfe, J.; Akam, M.E. The developmental profile of two major haemolymph proteins from Drosophila melano-gaster. J. Insect Physiol. 1977, 23, 871–878. [Google Scholar] [CrossRef]
- Telfer, W.H.; Kunkel, J.G. The function and evolution of insect storage hexamers. Annu. Rev. Entomol. 1991, 36, 205–228. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-C.; Tsai, C.-L.; Chen, M.-E. cDNA cloning and transcriptional expression profiles of a hexamerin in the oriental fruit fly, Bactrocera dorsalis. Arch. Insect Biochem. Physiol. 2014, 86, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.T. Studies on the improvement of mass rearing for oriental fruit flies. Plant Prot. Bull. 1978, 20, 87–92. (In Chinese) [Google Scholar]
- Nielsen, H. Predicting secretory proteins with SignalP. In Protein Function Prediction (Methods in Molecular Biology); Kihara, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1611, pp. 59–73. [Google Scholar]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48D1, 265–268. [Google Scholar] [CrossRef]
- Huang, X.; Miller, W. A time-efficient, linear-space local similarity algorithm. Adv. Appl. Math. 1991, 12, 337–357. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Shen, G.-M.; Jiang, H.-B.; Wang, X.-N.; Wang, J.-J. Evaluation of endogenous references for gene expression profiling in different tissues of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae). BMC Mol. Biol. 2010, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.; Halford, N.; Tatham, A. High molecular weight subunits of wheat glutenin. J. Cereal Chem. 1992, 15, 105–120. [Google Scholar] [CrossRef]
- Burmester, T.; Scheller, K. Complete cDNA-sequence of the receptor responsible for arylphorin uptake by the larval fat body of the blowfly, Calliphora vicina. Insect Biochem. Mol. Biol. 1995, 25, 981–989. [Google Scholar] [CrossRef]
- Chung, S.O.; Kubo, T.; Natori, S. Molecular cloning and sequencing of arylphorin-binding protein in protein granules of the sarcophaga fat body. J. Biol. Chem. 1995, 270, 4624–4631. [Google Scholar] [CrossRef]
- Kunte, N.; McGraw, E.; Bell, S.; Held, D.; Avila, L. Prospects, challenges and current status of RNAi through insect feeding. Pest Manag. Sci. 2019, 76, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-J.; Xu, K.-K.; Cong, L.; Wang, J.-J. Identification, mRNA expression, and functional analysis of chitin synthase 1 gene and its two alternative splicing variants in oriental fruit fly, Bactrocera dorsalis. Int. J. Biol. Sci. 2013, 9, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Burmester, T.; Scheller, K. Ligands and receptors: Common theme in insect storage protein transport. Naturwissenschaften 1999, 86, 468–474. [Google Scholar] [CrossRef]
- Martins, J.R.; Nunes, F.M.F.; Simões, Z.L.P.; Bitondi, M.M.G. A honeybee storage protein gene, hex 70a, expressed in developing gonads and nutritionally regulated in adult fat body. J. Insect Physiol. 2008, 54, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Luan, Y.-X. Evolutionary implications of dipluran hexamerins. Insect Biochem. Mol. Biol. 2014, 46, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Park, K.-E.; Lee, S.A.; Jang, S.H.; Eo, H.J.; Jang, H.A.; Kim, C.-H.; Ohbayashi, T.; Matsuura, Y.; Kikuchi, Y.; et al. Gut symbiotic bacteria stimulate insect growth and egg production by modulating hexamerin and vitellogenin gene expression. Dev. Comp. Immunol. 2017, 69, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Bai, P.-P.; Xie, Y.-F.; Shen, G.-M.; Wei, D.-D.; Wang, J.-J. Phenoloxidase and its zymogen are required for the larval–pupal transition in Bactrocera dorsalis (Diptera: Tephritidae). J. Insect Physiol. 2014, 71, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-J.; Wu, Y.-B.; Chen, L.; Xu, K.-K.; Xie, Y.-F.; Wang, J.-J. Two chitin biosynthesis pathway genes in Bactrocera dorsalis (Diptera: Tephritidae): Molecular characteristics, expression patterns, and roles in larval–pupal transition. J. Econ. Èntomol. 2015, 108, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-B.; Yang, W.-J.; Xie, Y.-F.; Xu, K.-K.; Tian, Y.; Yuan, G.-R.; Wang, J.-J. Molecular characterization and functional analysis of BdFoxO gene in the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Gene 2016, 578, 219–224. [Google Scholar] [CrossRef]
- Li, Y.-L.; Hou, M.-Z.; Shen, G.-M.; Lu, X.-P.; Wang, Z.; Jia, F.-X.; Wang, J.-J.; Dou, W. Functional analysis of five trypsin-like protease genes in the oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Pestic. Biochem. Physiol. 2017, 136, 52–57. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).