1. Introduction
Dengue is a hurdle to public health in Sri Lanka, causing high morbidity and mortality [
1]. Two
Aedes species that transmit the dengue virus are well established in the country;
Aedes albopictus and
Aedes aegypti. Both belong to family Culicidae and subfamily Culicinae [
2]. In Sri Lanka, eliminating dengue depends entirely on vector control or sabotaging the human–vector contact. Therefore, studying the bionomics and the behavior of the dengue vectors facilitates effective vector control.
Oviposition site selection is critical for mosquito survival and affects the longevity of eggs, larval development and growth performance, predator avoidance, intra and interspecific interactions and offspring phenotypes and fitness [
3]. Chemical cues play a crucial role in the selection of oviposition sites. The volatile chemicals from an oviposition site are sensed by mosquito olfactory receptors located on the antennae, palps, labrum and tarsi [
4]. The odors released from semiochemical substances from decaying organic matter in the oviposition sites influence the mosquito egg deposition [
5]. The
Ae. albopictus follows the hypothesis of preference performance for oviposition site selection [
3] as the adult female oviposits in the most suitable habitat to maximize offspring fitness while minimizing the resource-mediated interactions [
6].
In a previous study, the oviposition response of
Ae. aegypti and
Ae. albopictus for leaves of bamboo (
Arundinaria gigantea), white oak (
Quercus alba), live oak (
Quercus virginiana), pecan (
Carya illinoensis), hackberry (
Celtis occidentalis), red maple (
Acer rubrum), redtop panicgrass (
Panicum rigidulum) and harvested Bermuda grass (
Cynodon dactylon) have been determined [
7]. They reported that semiochemicals formed by the breaking down of organic matter through microbial metabolic activity caused the female
Aedes mosquitoes to oviposit [
7].
It is reported that
Ae. albopictus is more abundant in the rural areas than in the urban areas of Sri Lanka [
8]. In addition to the usual natural breeding sites, such as tree holes and rock pools and artificial freshwater collections,
Ae. albopictus also oviposits in groundwater bodies with brackish water [
9]. On the other hand, most rural areas have ample vertebrate excreta because of livestock practices such as poultry farming, dairy farming and pig farming. These vertebrate excreta make oviposition sites rich in nutrients and this may underlie the increased population size of
Ae. albopictus mosquitoes in these areas. Therefore, this study was carried out to investigate how different vertebrate excreta affect the oviposition and growth performance of
Ae. albopictus. Moreover, the excreta combinations were analyzed for potential synergistic effects on the oviposition of
Ae. albopictus mosquitoes.
2. Materials and Methods
2.1. Mosquito Rearing
The experiments were carried out during April to November 2018. The eggs of Ae. albopictus were obtained on paper sheets from inbred mosquito colonies at the Medical Research Institute (MRI), Sri Lanka. A pure colony consisting of only Ae. albopictus was maintained during the entire study period in the insectary (temperature: 27 °C; relative humidity 80%; photoperiod of 12 h) at the University of Kelaniya, Sri Lanka. For that, the egg sheets obtained were immersed in a 500 mL glass bottle filled with aged tap water. Following egg hatching, the larvae of second instar were transferred into 750 mL enamel trays covered with a net. The larvae were fed with larval food (crushed shrimp powder) until they pupated. The pupae were transferred to another tray containing aged tap water and kept inside mosquito rearing cages until adults emerged.
These adults were fed with a 10% sucrose solution for two days and then starved for 24 h prior to the experiments. On the experiment day, a blood-filled membrane was placed in the cage in a manner that the blood containing side was facing the interior of the cage. The blood-fed gravid females were collected using an aspirator. These blood-fed gravid females were used for all experiments.
2.2. Preparation of Excreta Infusions
The excreta solutions were prepared from three types of livestock animals: Pig (
Sus domesticus), cow (
Bos taurus) and goat (
Capra hircus). Fresh feces of pigs, cows and goats were collected from a village in Mawathagama, Sri Lanka (7°52′38.72′′ N 80°42′1.23′′ E) (Gamin etrex©, Garmin Ltd., Olathe, KS, USA). These feces were air dried under the sun for 10 days. Then, the feces were finely ground and sieved through a small mesh and stored on air-tight containers [
10]. The excreta infusions were prepared by mixing 10 g of crushed excreta in 200 mL of water.
2.3. Screening the Attraction and Stimulatory Response of Female Mosquitoes towards the Animal Excreta for Oviposition
To evaluate the attraction and stimulatory response of ovipositing female
Ae. albopictus towards the selected vertebrate excreta, a 4-way bioassay choice chamber was prepared. As shown in
Figure 1, the bioassay setup consisted of 4 chambers, joined to the centrally placed insertion chamber with 4 hard, transparent joining tubes 9 cm × 2.5 cm (length × diameter). Three bioassay setups were set in an experiment in a random manner changing the position of the treatment. Aged tap water was used for the control chamber.
A fresh batch of 20 blood-fed gravid female Ae. albopictus were released into the insertion chamber of each bioassay set up at 5.00 pm, approximately one hour before starting night time. (The insectary lighting conditions did not allow dusk/dawn periods). The number of mosquitoes that had moved and settled on the different treatments were counted after the first hour (6.00 pm). This experiment was repeated for six consecutive days using same-aged fresh female batches.
2.4. Effect of Different Vertebrate Excreta Infusions on the Oviposition of Aedes albopictus Mosquitoes
Three different vertebrate excreta solutions described above were used for this experiment. Aged tap water was used as the control. Four ovitraps with filter paper stripes containing each vertebrate excreta and control were placed in the experimental cage (75 cm × 45 cm × 45 cm) in a random manner. Total of 16 ovitraps were placed in a cage. This was done in separate cages for each type of excreta. Considering the fermented excreta to be microenvironments for oviposition, these ovitraps were considered as microcosms [
11]. Twenty blood-fed gravid females of
Ae. albopictus were released into each cage and allowed to stay in for five days. The eggs in the microcosms were counted every day in each ovitrap and recorded separately. The whole experiment was replicated 4 times.
2.5. Effect of Vertebrate Excreta Combinations on the Oviposition Response of Aedes albopictus
In this study, four excreta combinations were used: Three combinations of two-vertebrate excreta and one combination of three-vertebrate excreta. The two-excreta combinations were Cow+Pig, Cow+Goat and Pig+Goat. For these combinations, 50% of excreta from each type were mixed. The other combination, Cow+Pig+Goat had 33% of excreta from each vertebrate combined.
To study the effect of the two-excreta combinations and the three-excreta combinations, four ovitraps for each of the combination (total of 16 ovitraps) were randomly arranged in the mosquito rearing cage and four containers of aerated distilled water were placed in the cage as the control. Then, 20 mated gravid females were released into the cage. They were allowed in the cages for 5 days. The eggs were counted every day in each ovitrap and recorded separately. This experiment was replicated 4 times.
2.6. Days of Fermentation of Vertebrate Excreta and Oviposition Preference of Aedes albopictus
A series of vertebrate excreta infusions was prepared and allowed to ferment for 14, 12, 10, 8, 6, 4, 2 and 0 days (the control). Two containers of vertebrate excreta with differing days of fermentation were placed in mosquito cages, one cage for each type of excreta (total of 16 microcosms of the same excreta type within a cage). A filter paper was placed in each container as an oviposition substrate. These microcosms were exposed to the mated blood-fed gravid females of Ae. albopictus for oviposition. The eggs were counted after 2 days of female introduction. This experiment was replicated 4 times.
2.7. Effect of Different Vertebrate Excreta on the Development of Aedes albopictus
Four different microcosms were prepared from each of the three excreta types (200 mL) and placed in separate mosquito cages. Another 4 aerated water containers were also used as the controls. Twenty first instar larvae were introduced to each microcosm (altogether 16 microcosms in a cage). Three cages were set up in an experiment. Each microcosm was observed daily until the emergence of adults. Larval mortality was measured by recording the number of dead larvae. Both larval and pupal duration were measured by counting the days from first instar introduction to formation of pupae and pupal formation to emergence of adult, respectively. Mosquito fecundity was measured by the right-wing length of the adult female. For that, the emerged adults were killed using ethyl acetate. The right wing of each female adult was removed at the time of dissection and mounted on a glass microscope slide in a small drop of distilled water. The wing length was measured from the axial incision to the apical end of the wing [
12] using a compound microscope (Olympus© CX21 Microscope, Tokyo, Japan) with high power (40× magnification. The wing was aligned along an ocular micrometer (1 ocular unit, 0.025 mm at 40×) and the wing length was measured to the nearest 0.01 m [
13]. This experiment was replicated 3 times.
2.8. Data Analysis
Statistical analysis was performed using SPSS V.20. One-Way ANOVA and Tukey–Kramer HSD test were performed to determine the effect of vertebrate excreta infusions on mosquito attraction for the stimulatory response, mosquito attraction for oviposition, the effect of vertebrate excreta combinations on the oviposition of Ae. albopictus mosquitoes and effect of different vertebrate excreta on the development of Ae. albopictus. Correlation, regression analysis, One-Way ANOVA and Tukey–Kramer HSD tests were performed to determine the effect of fermenting rate of vertebrate excreta on the oviposition of Ae. albopictus mosquitoes. All experiments were replicated at least 3 times. All data were expressed as mean ± standard error (SE).
4. Discussion
Previous studies have shown vertebrate excreta as potential oviposition attractants and growth enhancers for the sand fly species
Lutzomyia longipalpis and
Phlebotomus argentipes (Diptera: Psychodidae) [
10]. However, no such study has been conducted for mosquitoes other than different organic infusions that were shown to be oviposition attractants [
14,
15]. Ponnusamy et al. [
7] stated that the semiochemicals produced by microbial metabolic activity in organic matter elicited the female
Aedes mosquitoes to oviposit. In fact, the encounter of vertebrate excreta in
Ae. albopictus oviposition sites is possible as Aedes mosquitoes breed in brackish water [
9]. Considering that, this is the first comprehensive analysis of selected vertebrate excreta of goat (
Capra hircus), cow (
Bos taurus) and pig (
Sus domesticus) to identify the oviposition attraction and growth performance of
Ae. albopictus in Sri Lanka.
The feeding habits of vertebrates are different to each other. The feeding habits and physiology determine the vertebrate gut microbiota, which is a substantial component in excreta [
16,
17,
18,
19,
20,
21,
22]. The microbial communities in the vertebrate excreta may act as food sources of mosquito larvae for growth and development. They also produce nonvolatile and volatile chemicals via decomposition of detritus material in these larval habitats [
23]. Some of these compounds may serve as semiochemicals that mediate the selection of an oviposition site and are largely responsible for the spatial distribution of mosquito species in nature [
23]. Moreover, some of them act as repellents for gravid female mosquitoes while some others act as attractants [
24].
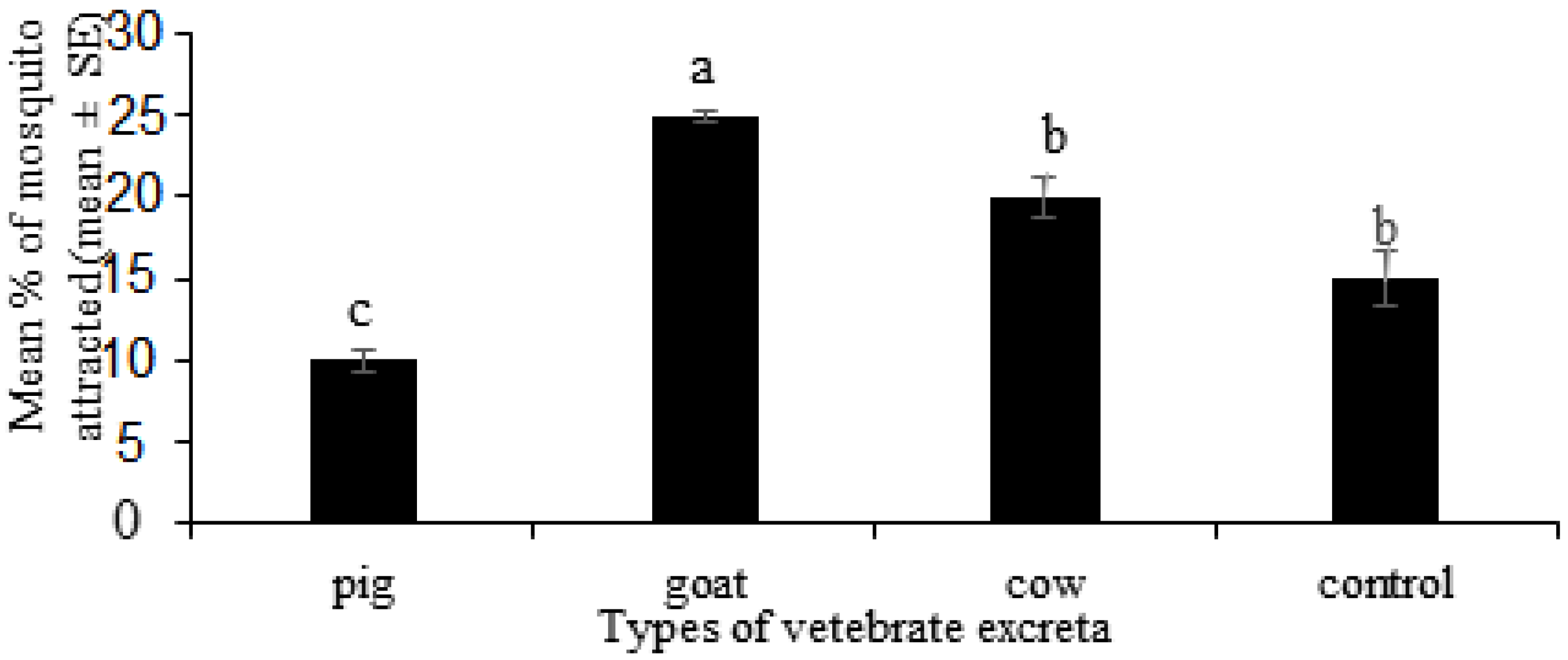
In the present study, it was observed that goat excreta infusion was the most attractive stimulus for Ae. albopictus mosquitoes, followed by that of cow and pig excreta, respectively. These results suggest chemical composition and microorganisms inhabiting in goat excreta infusion may greatly differ from the other tested excreta types, thus resulted in semiochemicals that are highly attractive for Ae. albopictus. The pig infusions had a deterrent effect for Ae. albopictus mosquitoes. It indicates that chemical composition and microorganisms inhabiting pig excreta may differ and may form semiochemicals that adversely affect Ae. albopictus. The goat and cow are herbivorous while the pig is omnivorous. The microbial communities within herbivorous intestines are different to those of omnivorous intestines. The microbes in the herbivorous excreta may produce semiochemicals which can act as attractants for the gravid female mosquitoes of Ae. albopictus. The microbial communities in the intestine of omnivorous vertebrates may produce semiochemicals which can act as a repellent for the gravid female mosquitoes of Ae. albopictus.
Oviposition site selection by
Ae. albopictus follows the predictions of the preference performance hypothesis for oviposition site selection. Females allocate most of their eggs in an array of habitats that confer high offspring performance and fitness [
3]. According to the optimal oviposition theory, the larval success of insects depends on the oviposition site selection by females. Females are expected to choose a site with many resources and few competitors or predators to allow the best performance for their progeny, assuming “mother knows best” [
25].
Interestingly, the results of the effect of different vertebrate excreta on the oviposition of
Ae. albopictus were different compared to the effect of vertebrate excreta for the stimulatory response of
Ae. albopictus. Even though goat excreta showed the highest stimulatory response, cow excreta showed the highest oviposition response. This suggests that mosquito attraction sites do not necessarily act as mosquito oviposition sites and vice versa. Gravid female mosquitoes may lay eggs in high nutritional habitats for high offspring performance and fitness. Hence, higher number of eggs were found in cow excreta infusions. This is aligned with the preference performance hypothesis which states that female insects evolve to oviposit so that such their offspring thrive at their best [
26]. Cow excreta may decompose to provide more nutrients for the neonate larvae. The minimum mosquito attraction and oviposition was observed in pig excreta infusions. This is mainly due to low stimulatory response towards the pig excreta. Moreover, the present study showed that control water is preferred for oviposition over pig excreta, indicating repellence for pig excreta. There are only low amounts of nutrients in pig excreta [
27], which may be due to the low amounts of semiochemicals and nutrients produced by the specific microbial communities inhabiting the pig excreta. It is suggestive that the nutrient and mineral content, as well as the digestibility of animal excreta, greatly influence the selection of ovipositing sites by mosquitoes.
In the present study, different combinations of selected vertebrate excreta were also evaluated for the oviposition preference of
Ae. albopictus. Even though cow excreta showed the highest oviposition responses, it received a substantially lower number of eggs when combined with pig excreta, suggesting highly repellent characteristics of pig excreta. However, this did not happen when goat excreta were combined with cow excreta. Instead, Cow+Goat excreta showed enhanced oviposition of
Ae. albopictus, possibly due to formation of a highly favorable semiochemical for the mosquito offspring. This combination may decompose rapidly and provide nutrients than excreta per se. The combination of cow excreta and goat excreta showed the highest oviposition. Still, it cannot be considered a synergistic increase compared to the oviposition preference per se. These results are in line with a previous study in which some combinations of leaves act synergistically, resulting in a higher total yield of mosquitoes while certain leaf combinations act antagonistically for
Ae. albopictus [
28].
When cow and goat excreta were combined with pig excreta (Cow+Pig+Goat), a minimum oviposition response was shown. Furthermore, it was observed oviposition of Ae. albopictus was reduced in combinations which include pig excreta. This indicates the chemical compounds produced from pig excreta can reduce the oviposition-enhancing features of both cow and goat excreta by damaging their microbial community. On the other hand, the least nutrients provided by the pig excreta do not support the selection of the oviposition site by Ae. albopictus.
This study further revealed duration of fermenting of different vertebrate excreta had a significant effect on the oviposition preference of gravid female Ae. albopictus mosquitoes. In all experiments, it was clearly observed that maximum numbers of eggs were oviposited after 12th and 14th days of fermentation. The fermentation process accelerated when the number of fermenting days increased. Therefore, the present study indicated the gravid female Ae. albopictus have a greater affinity to the oviposition sites that encompass highly fermented vertebrate excreta.
Cow excreta hold a large amount of water, facilitating the bacterial colonies to decompose down and give off their nutrients slowly [
5]. However, it is also reported that cow excreta decompose more rapidly than goat excreta [
29]. It may be due to the composition of food (grass). This may be a possible reason for the higher number of eggs in the cow excreta infusions. Conversely, the fewer number of eggs in the pig excreta infusions suggests the oviposition choice of gravid females of
Ae. albopictus is low on pig excreta. This observation suggests that pig excreta may ferment and decay slowly, providing only low amounts of nutrients into pig excreta infusions. Moreover, compared to cow and goat excreta, which have a pH of 8.5−9.0 [
30], pig excreta are acidic, with a pH of 6.85 [
31]. On the other hand, the optimal pH for
Aedes larvae is reported as 7.4 [
32]. Therefore, the slight acidity of pig excreta could be a reason for it to receive the lowest number of eggs in the present study. Additionally, it should be of note that in Sri Lanka, rural pig farming does not make use of antibiotics and other growth hormones; therefore, these factors do not underlie the reduced oviposition for pig excreta.
The immature stages of container-dwelling mosquitoes are placed in their habitat by ovipositing females that lay their eggs in habitats that maximize the performance of their offspring [
6]. In the present study, it was shown that growth performance of
Ae. albopictus varied among different vertebrate excreta.
The current study revealed a significant difference between total larval mortality of Ae. albopictus with the different vertebrate excreta tested. The minimum larval mortality was observed in cow excreta infusions, while maximum larval mortality was recorded for the pig excreta infusion. The experimental values of the larval mortality for the control (aged tap water) were fairly high. Even though aged tap water was used as the control, maybe because it was first instar larvae that were introduced, the agitation caused during handling could have been fatal to the larvae. On the other hand, because it is not a natural habitat but artificially induced laboratory conditions, a base level of larval mortality can be expected.
The chemical substances present in pig excreta may adversely affect the survival of
Ae. albopictus larvae. A study inferenced that rabbit excreta enhance the survival of the
Phlebotomus sand fly [
10]. Only a few experiments have been conducted to assess the excreta-based performances of mosquitoes or other medically important insects.
The vertebrate excreta tested here had significant effects on the larval duration times. Minimum larval duration was recorded for goat excreta infusion, while maximum larval duration was recorded for both cow and pig excreta infusions. These results, while seemingly counterintuitive, require further research and confirmation.
Fecundity should be critically defined in female mosquitoes as they are responsible for producing eggs and maintaining the population size. The current study revealed a significant difference between the wing length measurements and the type of vertebrate excreta. The adult mosquitoes that emerged from cow excreta infusion had a high wing length, indicating the influence of cow excreta for the greater fecundity of Ae. albopictus. It is mainly because of the rapidly decomposing cow excreta that produce nutrient-rich microcosms. The nutrients may enhance the growth of the larvae, resulting in an adult mosquito population with a larger body size.