Habitat Specificity, Host Plants and Areas of Endemism for the Genera-Group Blepharida s.l. in the Afrotropical Region (Coleoptera, Chrysomelidae, Galerucinae, Alticini)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Flea Beetle and Plant Datasets
2.2. Ecoregions
2.3. GIS Analyses
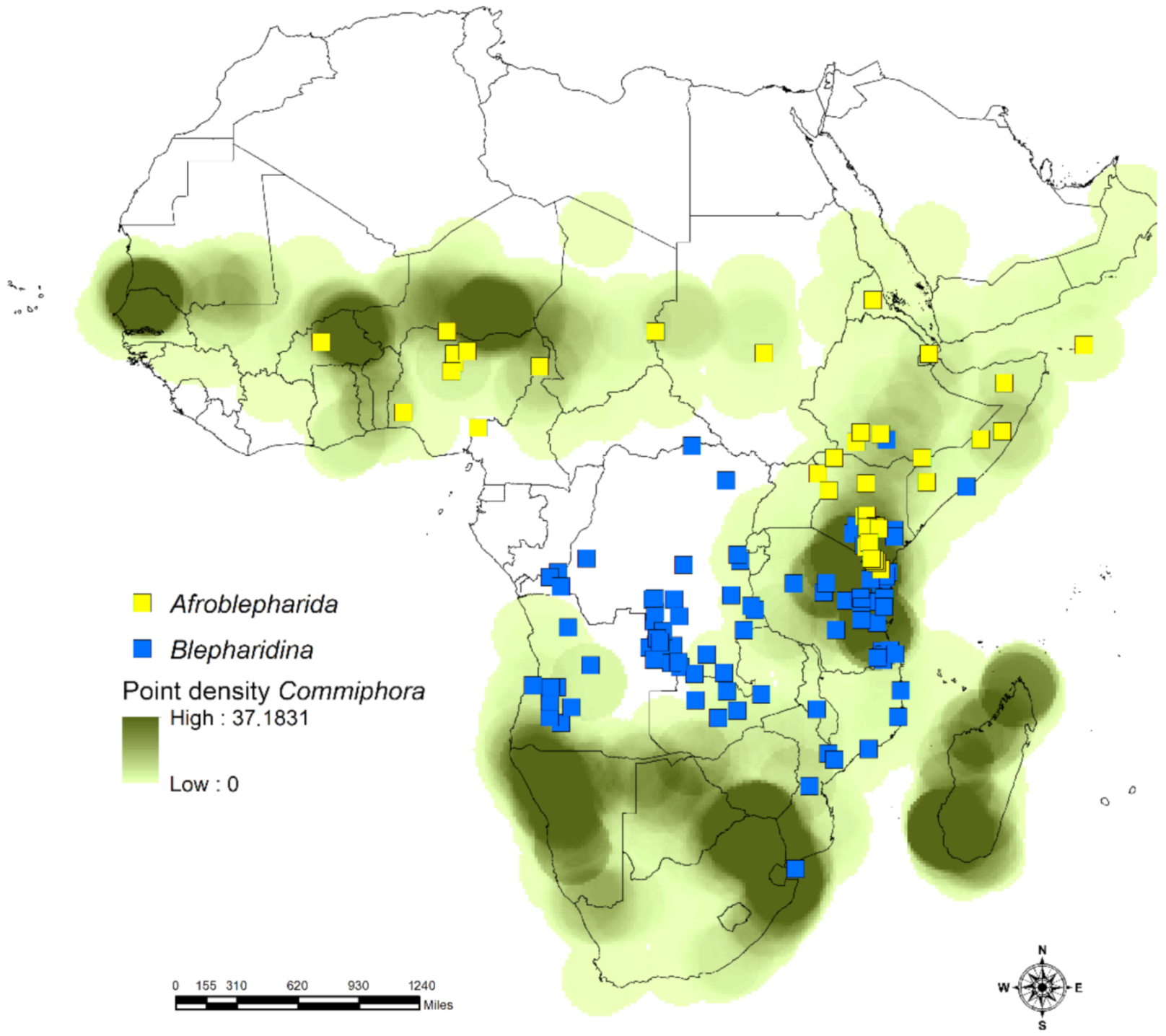
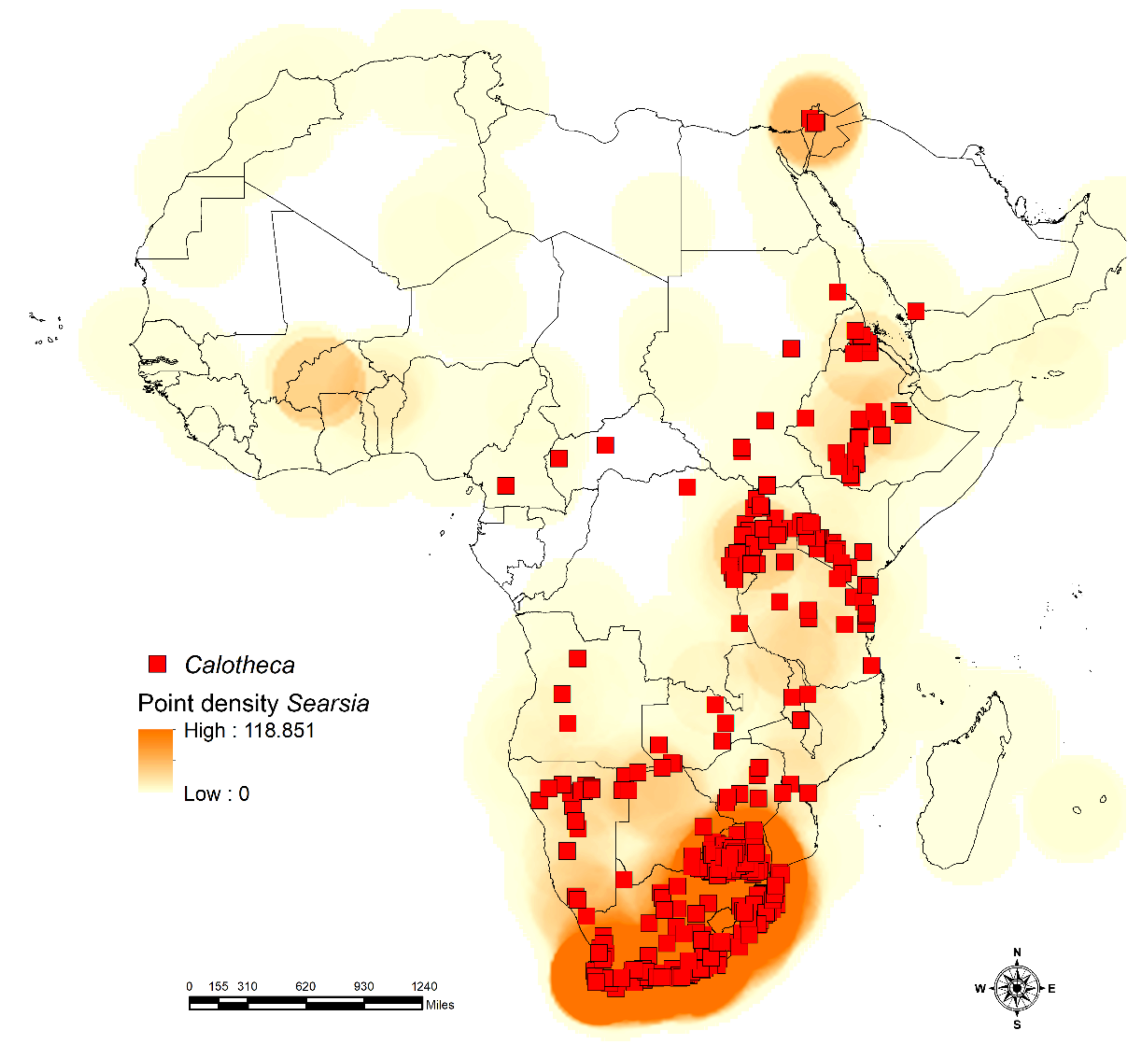
2.3.1. Density of Species Richness and Occurrence
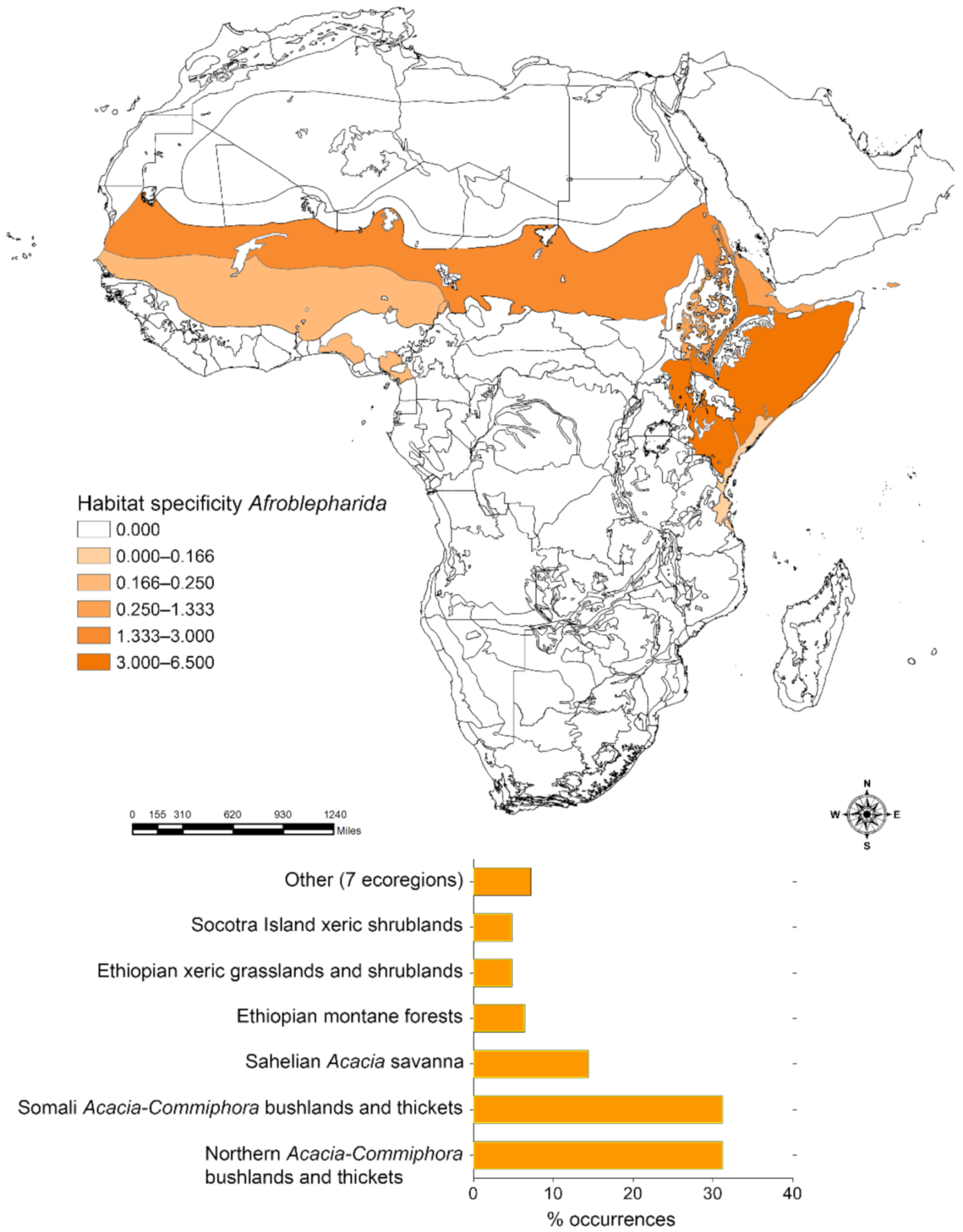
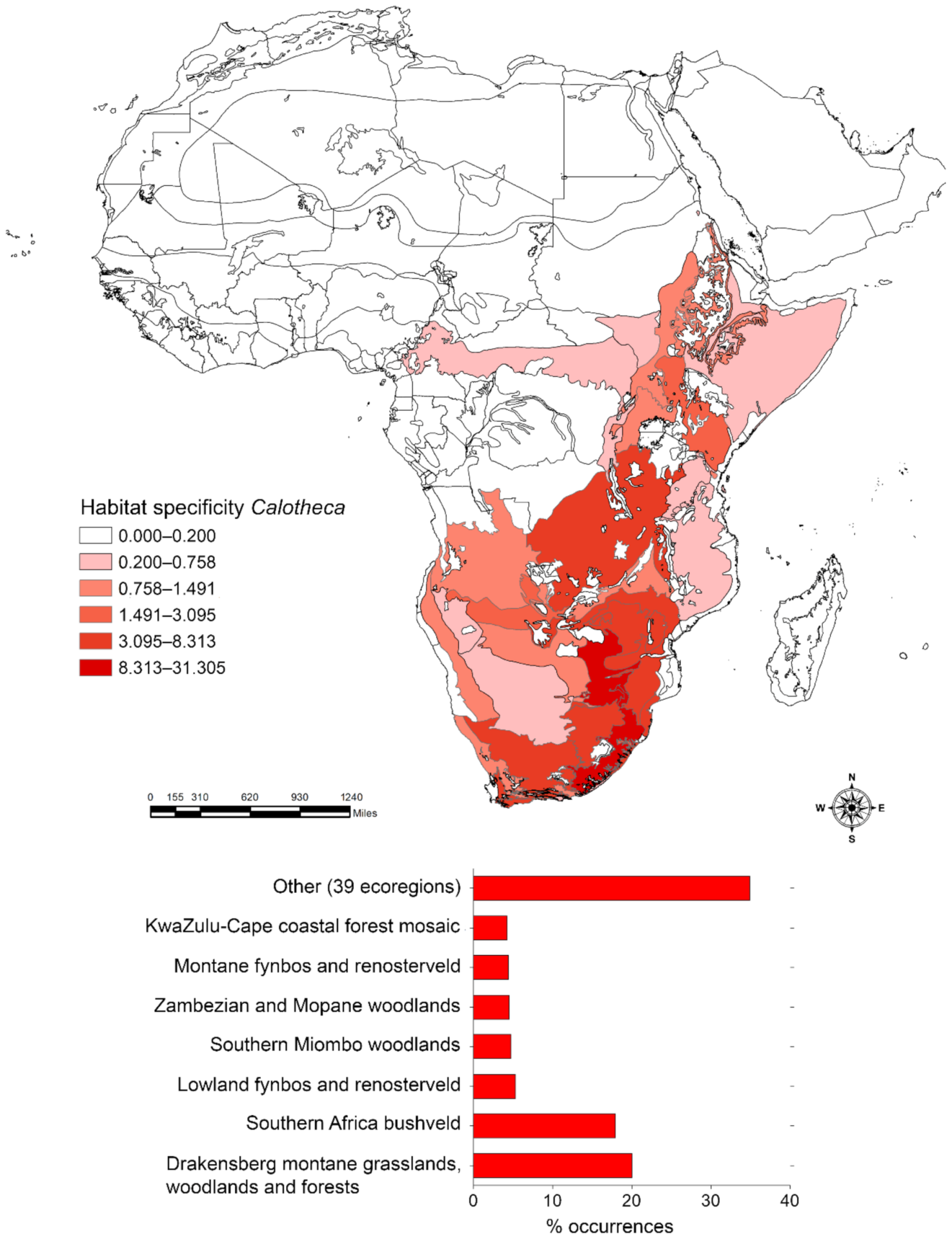
2.3.2. Habitat Specificity
2.3.3. Flea Beetle–Plant Species Associations
2.3.4. Areas of Endemism
3. Results
3.1. Habitat Specificity and Flea Beetle-Plant Species Associations
3.2. Areas of Endemism
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nadein, K.S. Catalogue of Alticini Genera of the World (Coleoptera: Chrysomelidae). Beetles and Coleopterists Website, Zoological Institute, Saint-Petersburg. Available online: http://www.zin.ru/Animalia/Coleoptera/eng/alticinw.htm (accessed on 20 February 2021).
- Nadein, K.S.; Bezdêk, J. Galerucinae Latreille 1802. In Coleoptera, Beetles. Vol. 3. Morphology and systematics (Phytophaga); Leschen, R.A.B., Beutel, R.G., Eds.; De Gruyter: Berlin, Germany, 2014; pp. 251–259. [Google Scholar]
- Furth, D.G.; Suzuki, K. Studies of Oriental and Australian Alticinae genera based on the comparative morphology of the metafemoral spring, genitalia, and hind wing venation. In Proceedings of the Fourth International Symposium on the Chrysomelidae, Firenze, Italy, 30 August 1996; Biondi, M., Daccordi, M., Furth, D.G., Eds.; Museo Regionale di Scienze Naturali: Torino, Italy, 1998; pp. 91–124. [Google Scholar]
- Ge, D.; Chesters, D.; Gómez-Zurita, J.; Zhang, L.; Yang, X.; Vogler, A.P. Anti-Predator Defence Drives Parallel Morphological Evolution in Flea Beetles. Proc. R. Soc. B. 2011, 278, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Nadein, K.; Betz, O. Jumping Mechanisms and Performance in Beetles. I. Flea Beetles (Coleoptera: Chrysomelidae: Alticini). J. Exp. Biol. 2016, 219, 2015–2027. [Google Scholar] [CrossRef] [PubMed]
- Jolivet, P.; Verma, K.K. Biology of Leaf Beetles; Intercept Limited: Andover, UK, 2002. [Google Scholar]
- Biondi, M.; Urbani, F.; D’Alessandro, P. Relationships between the Geographic Distribution of Phytophagous Insects and Different Types of Vegetation: A Case Study of the Flea Beetle Genus Chaetocnema (Coleoptera: Chrysomelidae) in the Afrotropical Region. Eur. J. Entomol. 2015, 112, 311–327. [Google Scholar] [CrossRef]
- D’Alessandro, P.; Iannella, M.; Frasca, R.; Biondi, M. Distribution Patterns and Habitat Preference for the Genera-Group Blepharida s.l. in Sub-Saharan Africa (Coleoptera: Chrysomelidae: Galerucinae: Alticini). Zool. Anz. 2018, 277, 23–32. [Google Scholar] [CrossRef]
- Biondi, M.; D’Alessandro, P. Taxonomical Revision of the Longitarsus capensis Species-Group: An Example of Mediterranean-Southern African Disjunct Distributions (Coleoptera: Chrysomelidae). Eur. J. Entomol. 2008, 105, 719–736. [Google Scholar] [CrossRef]
- Biondi, M.; D’Alessandro, P. Afrotropical Flea Beetle Genera: A Key to Their Identification, Updated Catalogue and Biogeographical Analysis (Coleoptera, Chrysomelidae, Galerucinae, Alticini). ZooKeys 2012, 253, 1–158. [Google Scholar] [CrossRef]
- D’Alessandro, P.; Urbani, F.; Biondi, M. Biodiversity and Biogeography in Madagascar: Revision of the Endemic Flea Beetle Genus Neodera Duvivier, 1891 with Description of 19 New Species (Coleoptera, Chrysomelidae, Galerucinae, Alticini). Syst. Entomol. 2014, 39, 710–748. [Google Scholar] [CrossRef]
- Chevrolat, L.A.A. Catalogue des Coléoptères de Collection de M. le Comte Dejean. Deuxième Édition, Revue, Corrigée et Augmentée, Livr. 5; Dejean, P.F.M.A., Ed.; Librairie Méquignon-Marvis Père et Fils: Paris, France, 1836; pp. 361–442. [Google Scholar]
- Furth, D.G. Revision of the New World Blepharida (Coleoptera: Chrysomelidae: Alticinae). Mem. Entomol. Soc. Wash. 1998, 21, 1–109. [Google Scholar]
- Biondi, M.; D’Alessandro, P. Genus-Group Names of Afrotropical Flea Beetles (Coleoptera: Chrysomelidae: Alticinae): Annotated Catalogue and Biogeographical Notes. Eur. J. Entomol. 2010, 107, 401–424. [Google Scholar] [CrossRef]
- Heyden von, L. Kleine Coleopterologische Mittheilungen. Wien. Entomol. Ztg. 1887, 6, 98. [Google Scholar] [CrossRef]
- Bechyné, J. Contribution à La Faune Du Congo (Brazzaville). Mission A. Villiers et A. Descarpentries. LXXXI. Coleopteres Alticidae. Bull. L’institut Fr. d’Afrique Noire (Série A) 1968, 30, 1687–1728. [Google Scholar]
- Biondi, M.; Frasca, R.; Grobbelaar, E.; D’Alessandro, P. Supraspecific Taxonomy of the Flea Beetle Genus Blepharida Chevrolat, 1836 (Coleoptera: Chrysomelidae) in the Afrotropical Region and Description of Afroblepharida Subgen. Nov. Insect Syst. Evol. 2017, 48, 97–155. [Google Scholar] [CrossRef]
- D’Alessandro, P.; Frasca, R.; Grobbelaar, E.; Iannella, M.; Biondi, M. Systematics and Biogeography of the Afrotropical Flea Beetle Subgenus Blepharidina (Afroblepharida) Biondi & D’Alessandro, with Description of Seven New Species (Coleoptera, Chrysomelidae, Galerucinae, Alticini). Insect Syst. Evol. 2018, 49, 443–480. [Google Scholar] [CrossRef]
- D’Alessandro, P.; Iannella, M.; Grobbelaar, E.; Biondi, M. Revision of the Calotheca nigrotessellata Species Group from Southern Africa, with Description of Two New Species (Coleoptera: Chrysomelidae, Galerucinae, Alticini). Fragm. Entomol. 2020, 52, 169–182. [Google Scholar] [CrossRef]
- D’Alessandro, P.; Iannella, M.; Grobbelaar, E.; Biondi, M. Taxonomic Revision of the Calotheca parvula Species Group from Southern Africa, with Descriptions of Three New Species (Coleoptera, Chrysomelidae). Afr. Invertebr. in press.
- D’Alessandro, P.; Iannella, M.; Biondi, M. Revision of the Afrotropical Flea Beetle Subgenus Blepharidina s. Str. Bechyné (Coleoptera, Chrysomelidae). Zootaxa 2019, 4545, 32. [Google Scholar] [CrossRef]
- Biondi, M.; Iannella, M.; D’Alessandro, P. Unravelling the Taxonomic Assessment of an Interesting New Species from Socotra Island: Blepharidina socotrana sp. nov. (Coleoptera: Chrysomelidae). Acta Entomol. Musei Natl. Pragae 2019, 59, 499–505. [Google Scholar] [CrossRef]
- Furth, D.G.; Young, D.A. Relationships of Herbivore Feeding and Plant Flavonoids (Coleoptera: Chrysomelidae and Anacardiaceae: Rhus). Oecologia 1988, 74, 496–500. [Google Scholar] [CrossRef]
- Becerra, J.X.; Venable, D.L.; Evans, P.H.; Bowers, W.S. Interactions Between Chemical and Mechanical Defenses in the Plant Genus Bursera and Their Implications for Herbivores. Am. Zool. 2001, 41, 865–876. [Google Scholar] [CrossRef]
- Becerra, J.X. Ecology and evolution of new world Blepharida. In New Developments in the Biology of Chrysomelidae; Jolivet, P., Santiago-Blay, J.A., Schmitt, M., Eds.; SPB Academic Publishing bv: The Hague, The Netherlands, 2004; pp. 137–143. [Google Scholar]
- Becerra, J.X. Molecular Systematics of Blepharida Beetles (Chrysomelidae: Alticinae) and Relatives. Mol. Phylogenet. Evol. 2004, 30, 107–117. [Google Scholar] [CrossRef]
- Koch, C. Preliminary Notes on the Coleopterological Aspect of the Arrow Poison of the Bushmen. S. Afr. Biol. Soc. Pam. 1958, 20, 49–54. [Google Scholar]
- Miller, A.J.; Young, D.A.; Wen, J. Phylogeny and Biogeography of Rhus (Anacardiaceae) Based on Its Sequence Data. Int. J. Plant Sci. 2001, 162, 1401–1407. [Google Scholar] [CrossRef]
- Pell, S.K.; Mitchell, J.D.; Lowry, P.P.; Randrianasolo, A.; Urbatsch, L.E. Phylogenetic Split of Malagasy and African Taxa of Protorhus and Rhus (Anacardiaceae) Based on CpDNA TrnL–TrnF and NrDNA ETS and ITS Sequence Data. Syst. Bot. 2008, 33, 375–383. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Meng, Y.; Wen, J.; Sun, H.; Nie, Z.-L. Phylogenetic Analyses of Searsia (Anacardiaceae) from Eastern Asia and Its Biogeographic Disjunction with Its African Relatives. S. Afr. J. Bot. 2016, 106, 129–136. [Google Scholar] [CrossRef]
- Becerra, J.X. Synchronous Coadaptation in an Ancient Case of Herbivory. Proc. Natl. Acad. Sci. 2003, 100, 12804–12807. [Google Scholar] [CrossRef]
- Prathapan, K.; Chaboo, C. Biology of Blepharida-Group Flea Beetles with First Notes on Natural History of Podontia Congregata Baly, 1865 an Endemic Flea Beetle from Southern India (Coleoptera, Chrysomelidae, Galerucinae, Alticini). Zookeys 2011, 157, 95–130. [Google Scholar] [CrossRef]
- Bieńkowski, A.O.; Orlova-Bienkowskaja, M.J. Alien Leaf Beetles (Coleoptera, Chrysomelidae) of European Russia and Some General Tendencies of Leaf Beetle Invasions. PLoS ONE 2018, 13, e0203561. [Google Scholar] [CrossRef]
- Iannella, M.; D’Alessandro, P.; Longo, S.; Biondi, M. New Records and Potential Distribution by Ecological Niche Modeling of Monoxia obesula in the Mediterranean Area. Bull. Insectol. 2019, 72, 135–142. [Google Scholar]
- Freeman, M.; Looney, C.; Orlova-Bienkowskaja, M.J.; Crowder, D.W. Predicting the Invasion Potential of the Lily Leaf Beetle, Lilioceris lilii Scopoli (Coleoptera: Chrysomelidae), in North America. Insects 2020, 11, 560. [Google Scholar] [CrossRef]
- Iannella, M.; D’Alessandro, P.; Biondi, M. Forecasting the Spread Associated with Climate Change in Eastern Europe of the Invasive Asiatic Flea Beetle, Luperomorpha xanthodera (Coleoptera: Chrysomelidae). Eur. J. Entomol. 2020, 117, 130–138. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- GBIF.org GBIF Occurrence Download—Commiphora. Available online: https://doi.org/10.15468/dl.6bvwts (accessed on 26 March 2021).
- GBIF.org GBIF Occurrence Download—Searsia. Available online: https://doi.org/10.15468/dl.gtvuup (accessed on 26 March 2021).
- ESRI. ArcMap 10.0; ESRI: Redlands, CA, USA, 2010. [Google Scholar]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next Generation Python-Based GIS Toolkit for Landscape Genetic, Biogeographic and Species Distribution Model Analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.H.; Edwards, P.J. Quantifying Habitat Specificity to Assess the Contribution of a Patch to Species Richness at a Landscape Scale. Landsc. Ecol. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Halvorsen, R.; Edvardsen, A. The Concept of Habitat Specificity Revisited. Landsc. Ecol. 2009, 24, 851–861. [Google Scholar] [CrossRef][Green Version]
- Iannella, M.; Fiasca, B.; Di Lorenzo, T.; Biondi, M.; Di Cicco, M.; Galassi, D.M.P. Jumping into the Grids: Mapping Biodiversity Hotspots in Groundwater Habitat Types across Europe. Ecography 2020, 43, 1825–1841. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Brown, M.J.M.; Holland, B.R.; Jordan, G.J. Hyperoverlap: Detecting Biological Overlap in n-Dimensional Space. Methods Ecol. Evol. 2020, 11, 513–523. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016.
- Oliveira, U.; Brescovit, A.D.; Santos, A.J. Delimiting Areas of Endemism through Kernel Interpolation. PLoS ONE 2015, 10, e0116673. [Google Scholar] [CrossRef]
- Will, T.M.; Frimmel, H.E. Where Does a Continent Prefer to Break up? Some Lessons from the South Atlantic Margins. Gondwana Res. 2018, 53, 9–19. [Google Scholar] [CrossRef]
- Iannella, M.; Liberatore, L.; Biondi, M. The Effects of a Sudden Urbanization on Micromammal Communities: A Case Study of Post-Earthquake L’Aquila (Abruzzi Region, Italy). Ital. J. Zool. 2016, 83, 1–8. [Google Scholar] [CrossRef][Green Version]
- Weeks, A.; Zapata, F.; Pell, S.K.; Daly, D.C.; Mitchell, J.D.; Fine, P.V.A. To Move or to Evolve: Contrasting Patterns of Intercontinental Connectivity and Climatic Niche Evolution in “Terebinthaceae” (Anacardiaceae and Burseraceae). Front. Genet. 2014, 5. [Google Scholar] [CrossRef]





| Variables | Afroblepharida | Blepharidina | Calotheca | Searsia | Commiphora |
|---|---|---|---|---|---|
| BIO1 (°C) | 24.40 | 23.07 | 19.35 | 17.99 | 23.81 |
| BIO2 (°C) | 11.41 | 11.27 | 12.86 | 12.53 | 13.13 |
| BIO3 (%) | 68.83 | 66.89 | 61.32 | 57.43 | 61.61 |
| BIO4 (°C) | 157.48 | 151.94 | 299.71 | 337.97 | 281.59 |
| BIO5 (°C) | 33.04 | 31.12 | 29.00 | 28.17 | 33.73 |
| BIO6 (°C) | 16.22 | 14.07 | 7.53 | 6.12 | 12.06 |
| BIO7 (°C) | 16.82 | 17.05 | 21.47 | 22.05 | 21.68 |
| BIO8 (°C) | 24.87 | 23.83 | 21.28 | 19.29 | 25.59 |
| BIO9 (°C) | 23.05 | 21.43 | 16.30 | 15.70 | 20.74 |
| BIO10 (°C) | 26.30 | 24.61 | 22.58 | 21.78 | 26.83 |
| BIO11 (°C) | 22.43 | 20.98 | 15.38 | 13.60 | 19.99 |
| BIO12 (mm) | 667.36 | 1130.15 | 747.79 | 693.88 | 560.66 |
| BIO13 (mm) | 159.74 | 228.47 | 132.35 | 120.40 | 133.12 |
| BIO14 (mm) | 7.19 | 8.28 | 12.83 | 13.35 | 4.14 |
| BIO15 (%) | 96.83 | 87.83 | 71.22 | 66.86 | 106.76 |
| BIO16 (mm) | 344.36 | 575.02 | 349.32 | 326.07 | 332.65 |
| BIO17 (mm) | 29.36 | 36.01 | 48.71 | 47.84 | 16.50 |
| BIO18 (mm) | 184.91 | 305.68 | 265.82 | 239.17 | 211.67 |
| BIO19 (mm) | 60.39 | 61.76 | 88.54 | 103.94 | 34.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iannella, M.; D’Alessandro, P.; De Simone, W.; Biondi, M. Habitat Specificity, Host Plants and Areas of Endemism for the Genera-Group Blepharida s.l. in the Afrotropical Region (Coleoptera, Chrysomelidae, Galerucinae, Alticini). Insects 2021, 12, 299. https://doi.org/10.3390/insects12040299
Iannella M, D’Alessandro P, De Simone W, Biondi M. Habitat Specificity, Host Plants and Areas of Endemism for the Genera-Group Blepharida s.l. in the Afrotropical Region (Coleoptera, Chrysomelidae, Galerucinae, Alticini). Insects. 2021; 12(4):299. https://doi.org/10.3390/insects12040299
Chicago/Turabian StyleIannella, Mattia, Paola D’Alessandro, Walter De Simone, and Maurizio Biondi. 2021. "Habitat Specificity, Host Plants and Areas of Endemism for the Genera-Group Blepharida s.l. in the Afrotropical Region (Coleoptera, Chrysomelidae, Galerucinae, Alticini)" Insects 12, no. 4: 299. https://doi.org/10.3390/insects12040299
APA StyleIannella, M., D’Alessandro, P., De Simone, W., & Biondi, M. (2021). Habitat Specificity, Host Plants and Areas of Endemism for the Genera-Group Blepharida s.l. in the Afrotropical Region (Coleoptera, Chrysomelidae, Galerucinae, Alticini). Insects, 12(4), 299. https://doi.org/10.3390/insects12040299







