Peeling the Layers of Caddisfly Diversity on a Longitudinal Gradient in Karst Freshwater Habitats Reveals Community Dynamics and Stability
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Sampling and Laboratory Analysis
2.3. Data Analysis
2.3.1. Relationship between Caddisfly Communities and Environmental Properties
2.3.2. Diversity and Similarity of Caddisfly Communities Based on Common Indices
2.3.3. Naïve and Non-Naïve Diversity Profiles Developed by Leinster and Cobbold
2.3.4. Assessing the Resilience of Caddisfly Communities to Climate Change
3. Results
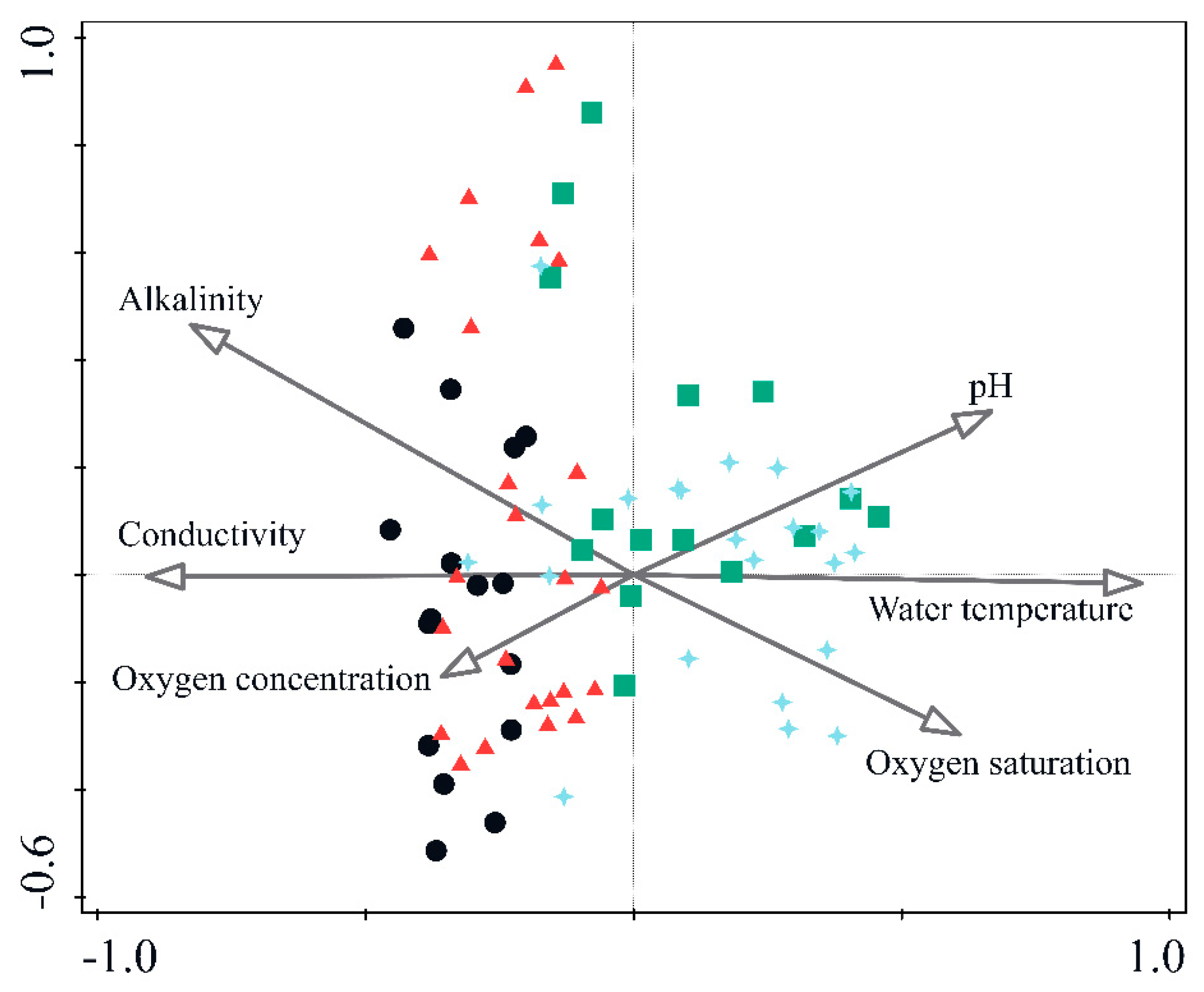
3.1. Relationship between Caddisfly Communities and Environmental Properties
3.2. Evaluation of Sampling Effort and Species Richness Estimation
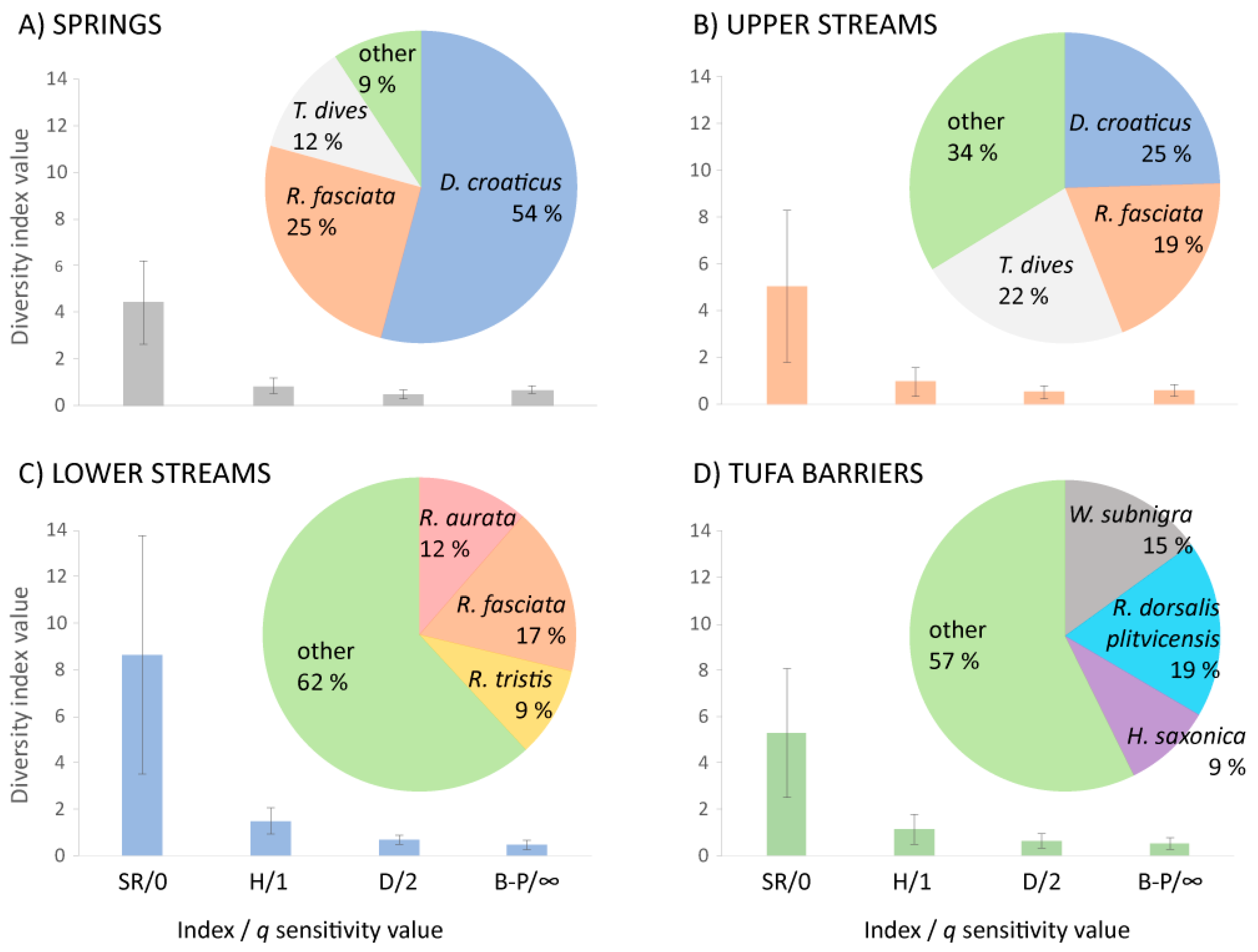
3.3. Diversity and Similarity of Caddisfly Communities: Common Indices
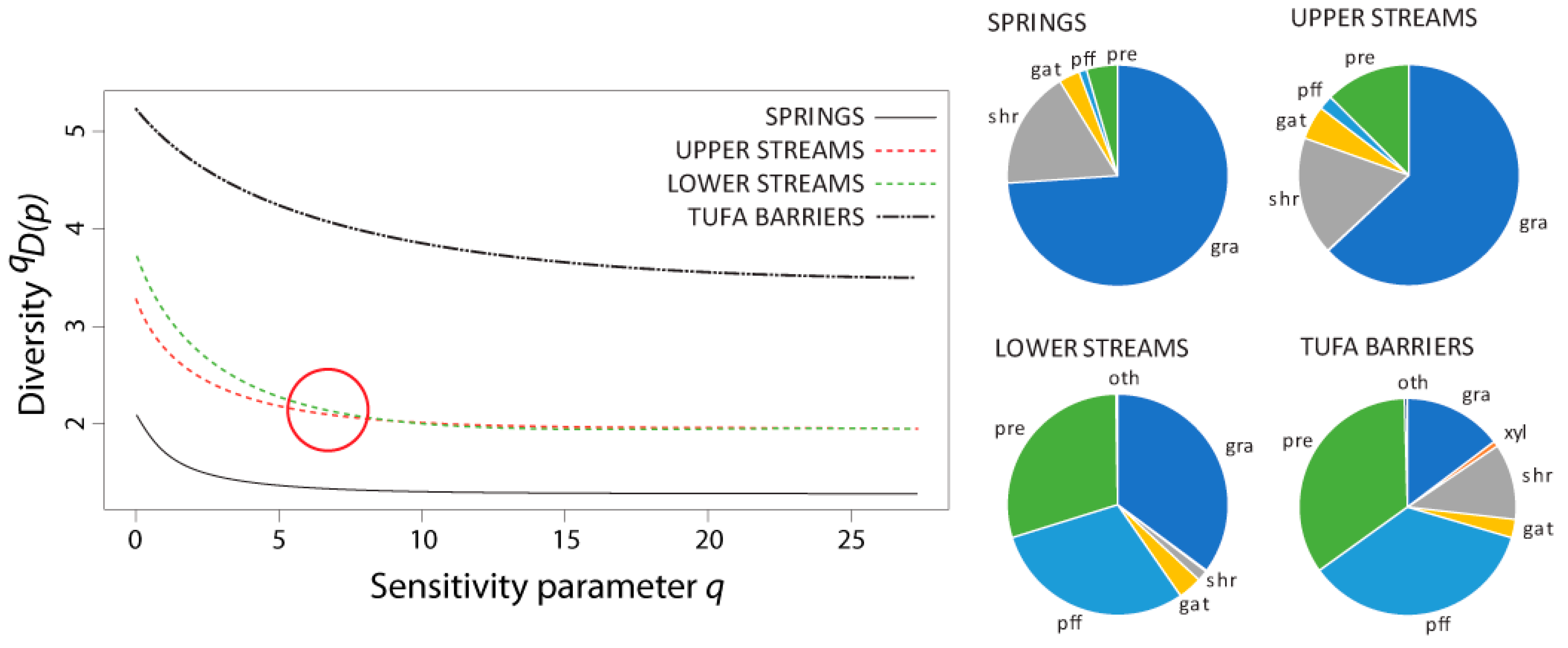
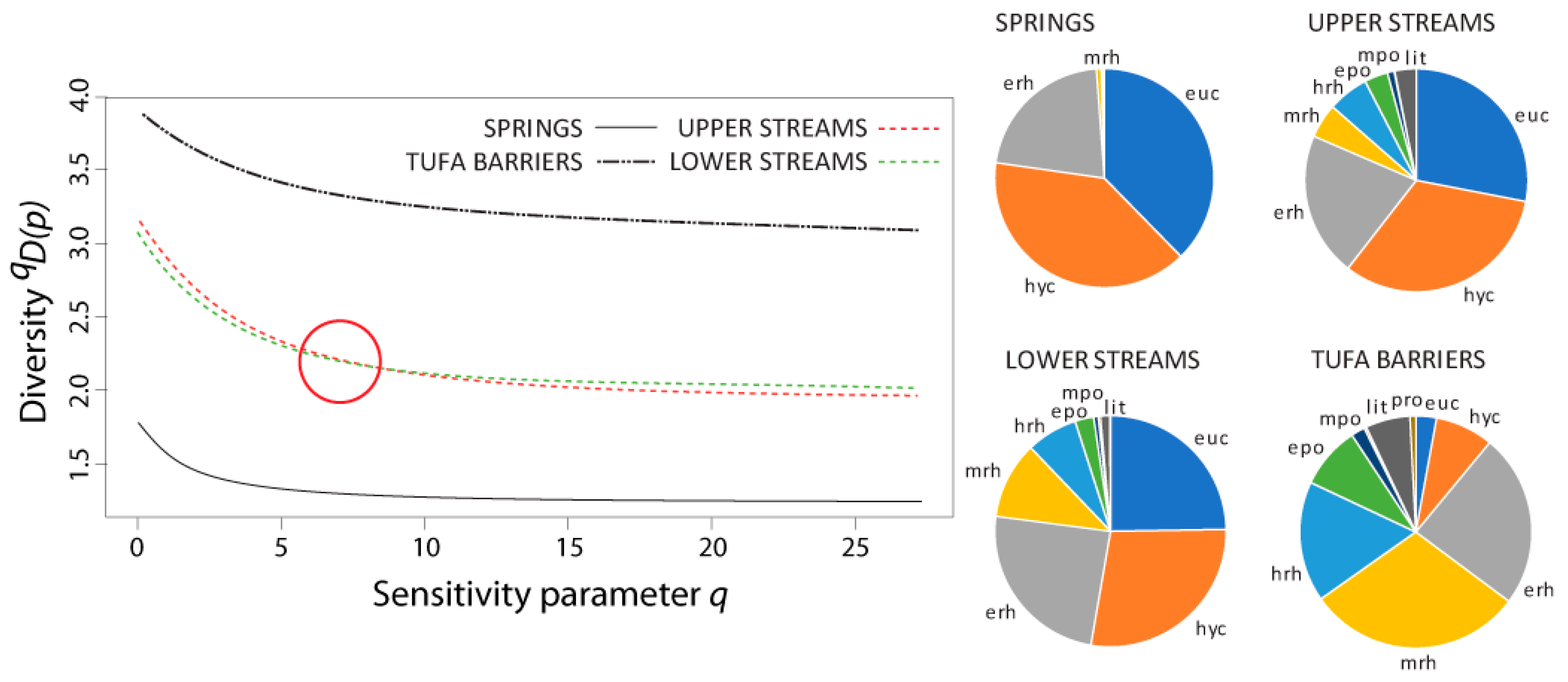
3.4. Diversity and Similarity of Caddisfly Communities: Naïve and Non-Naïve Diversity Profiles
3.5. Resilience of Caddisfly Communities to Climate Change: The Climate Score Index (CSI)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardoso, P.; Bartonb, P.S.; Birkhoferc, K.; Chichorroa, F.; Deacond, C.; Fartmanne, T.; Fukushimaa, C.S.; Gaigherd, R.; Habelf, J.C.; Hallmanng, C.A.; et al. Scientists’ warning to humanity on insect extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- Shannon, C. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Berger, W.H.; Parker, F.L. Diversity of planktonic Foraminifera in deep-sea sediments. Science 1970, 168, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Leinster, T.; Cobbold, C.A. Measuring diversity: The importance of species similarity. Ecology 2012, 93, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Graf, W.; Murphy, J.; Dahl, J.; Zamora-Muñoz, C.; Lópex-Rodríguez, M.J. Trichoptera. In Distribution and Ecological Preferences of European Freshwater Organisms; Schmidt-Kloiber, A., Hering, D., Eds.; Pensoft: Sofia, Bulgaria; Pensoft: Moscow, Russia, 2008; Volume 1, 388p, ISBN 9789546424419. [Google Scholar]
- Hershkovitz, Y.; Dahm, V.; Lorenz, A.W.; Hering, D. A multi-trait approach for the identification and protection of European freshwater species that are potentially vulnerable to the impacts of climate change. Ecol. Indic. 2015, 50, 150–160. [Google Scholar] [CrossRef]
- Schmidt-Kloiber, A.; Hering, D. freshwaterecology.info—An Online Database for European Freshwater Organisms, their Biological Traits and Ecological Preferences. Biodivers. Inform. Sci. Stand. 2019, 3. [Google Scholar] [CrossRef]
- Stanford, J.A.; Ward, J.V. Insect species diversity as a function of environmental variability and disturbance in stream systems. In Stream Ecology: Application and Testing of General Ecological Theory; Barnes, J.R., Minshall, G.W., Eds.; Plenum Press: New York, NY, USA, 1983; pp. 265–278. [Google Scholar]
- Allison, G. The influence of species diversity and stress intensity on community resistance and resilience. Ecol. Monogr. 2004, 74, 117–134. [Google Scholar] [CrossRef]
- Heino, J. Biodiversity of Aquatic Insects: Spatial Gradients and Environmental Correlates of Assemblage-Level Measures at Large Scales. Freshw. Rev. 2009, 2, 1–29. [Google Scholar] [CrossRef]
- Conti, L.; Schmidt-Kloiber, A.; Grenouillet, G.; Graf, W. A trait-based approach to assess the vulnerability of European aquatic insects to climate change. Hydrobiologia 2014, 721, 297–315. [Google Scholar] [CrossRef]
- Moe, S.J.; De Schamphelaere, K.; Clements, W.H.; Soresnsen, M.T.; van den Brink, P.J.; Liess, M. Combined and interactive effects of global climate change and toxicants on populations and communities. Environ. Toxicol. Chem. 2013, 32, 49–61. [Google Scholar] [CrossRef]
- Morse, J.C.; Frandsen, P.B.; Graf, W.; Thomas, J.A. Diversity and Ecosystem Services of Trichoptera. Insects 2019, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Frandsen, P.B.; Prendini, E.; Zhou, X.; Holzenthal, R.W. A multigene phylogeny and timeline for Trichoptera (Insecta). Syst. Entomol. 2020, 45, 670–686. [Google Scholar] [CrossRef]
- Cerjanec, D.; Kučinić, M.; Vilenica, M.; Ćukušić, A.; Ćuk, R.; Ibrahimi, H.; Vučković, I.; Žalac, S.; Ruk, D. Ecological and faunistic features of caddisflies (Insecta: Trichoptera) in different types of habitats in the Dinaric karst area (Central Croatia). Ecol. Monten. 2020, 36, 6–39. [Google Scholar] [CrossRef]
- AQEM Consortium. Manual for the application of the AQEM system. A comprehensive method to assess. In European Streams Using Benthic Macroinvertebrates, Developed for the Purpose of the Water Framework Directive 2002, Version 1; European Commission: Duisburg, Germany, 2002; p. 198. [Google Scholar]
- Kalaninová, D.; Bulánková, E.; Šporka, F. Caddisflies (Trichoptera) as good indicators of environmental stress in mountain lotic ecosystems. Biologia 2014, 69, 1030–1045. [Google Scholar] [CrossRef]
- Prommia, T.O.; Laudeeb, P.; Chareonviriyaphap, T. Biodiversity of Adult Trichoptera and Water Quality Variables in Streams, Northern Thailand. APCBE Proc. 2014, 10, 292–298. [Google Scholar] [CrossRef][Green Version]
- Illies, J. Limnofauna Europaea; Gustav Fischer Verlag: Stuttgart, Germany; Gustav Fischer Verlag: New York, NY, USA, 1978; p. 532. [Google Scholar]
- Ridl, A.; Vilenica, M.; Ivković, M.; Popijač, A.; Sivec, I.; Miliša, M.; Mihaljević, Z. Environmental drivers influencing stonefly assemblages along a longitudinal gradient in karst lotic habitats. J. Limnol. 2018, 77. [Google Scholar] [CrossRef]
- Ivković, M.; Dorić, V.; Baranov, V.; Mihaljević, Z.; Kolcsár, L.P.; Kvifte, G.M.; Nerudova, J.; Pont, A.C. Checklist of aquatic Diptera (Insecta) of Plitvice Lakes National Park, Croatia, a UNESCO world heritage site. ZooKeys 2020, 918, 99–142. [Google Scholar] [CrossRef] [PubMed]
- Ivković, M.; Plant, A. Aquatic insects in the Dinarides: Identifying hotspots of endemism and species richness shaped by geological and hydrological history using Empididae (Diptera). Insect Conserv. Divers. 2015, 8, 302–312. [Google Scholar] [CrossRef]
- Vilenica, M.; Ivković, M. A decade-long study on mayfly emergence patterns. Mar. Freshw. Res. 2020. [Google Scholar] [CrossRef]
- Previšić, A.; Schnitzler, J.; Kučinić, M.; Graf, W.; Ibrahimi, H.; Kerovec, M.; Pauls, S.U. Microscale vicariance and diversification of western Balkan caddisflies linked to Karstification. Freshw. Sci. 2014, 33, 250–262. [Google Scholar] [CrossRef]
- Previšić, A.; Graf, W.; Vitecek, S.; Kučinić, M.; Bálint, M.; Keresztes, L.; Pauls, S.U.; Waringer, J. Cryptic diversity of caddisflies in the Balkans: The curious case of Ecclisopteryx species (Trichoptera: Limnephilidae). Arthropod Syst. Phylogeny 2014, 72, 309–329. [Google Scholar] [PubMed]
- Stilinović, B.; Božićvić, S. The Plitvice lakes. A natural phenomen in the middle of the Dinaridic Karst in Croatia. Eur. Water Manag. 1998, 1, 15–24. [Google Scholar]
- Previšić, A.; Graf, W.; Kučinić, M. Caddisly (Trichoptera) fauna of the Plitvice Lakes National Park, Croatia. Denisia 2010, 29, 287–294. [Google Scholar]
- Kučinić, M.; Previšić, A.; Vajdić, M.; Tunjić, M.; Mihoci, I.; Žalac, S.; Sviben, S.; Vučković, I.; Trupković, M.; Habdija, I. First systematic investigation of adults and second checklist of caddisflies of the Plitvice Lakes National Park with notes on research history, biodiversity, distribution and ecology. Nat. Croat. 2017, 26, 225–260. [Google Scholar] [CrossRef]
- Malicky, H. Atlas of European Trichoptera; Springer: Dordrecht, The Natherlands, 2004; p. 359. [Google Scholar]
- Malicky, H. Die Köcherfliegen Griechenlands. Denisia 2005, 17, 1–240. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial, PRIMER-E Plymouth; Plymouth Marine Laboratory: Plymouth, UK, 2015. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- R Core Team. R A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 10 November 2020).
- Moog, O. Fauna Aquatica Austriaca; Wassserwirtschaftskataster, Bundesministerium für Land und Forstwirtschaft: Wien, Austria, 2012. [Google Scholar]
- Graf, W.; Murphy, J.; Dahl, J.; Zamora-Muñoz, C.; López-Rodríguez, M.J.; Schmidt-Kloiber, A. Dataset “Trichoptera”. The Taxa and Autecology Database for Freshwater Organisms, 2020, Version 7.0. Available online: www.freshwaterecology.info (accessed on 19 November 2020).
- Gu, W.; Swihart, R.K. Absent or undetected? Effects of non-detection of species occurrence on wildlife–habitat models. Biol. Conserv. 2004, 116, 195–203. [Google Scholar] [CrossRef]
- Daly, A.J.; Baetens, J.M.; De Baets, B. Ecological Diversity: Measuring the Unmeasurable. Mathematics 2018, 6, 119. [Google Scholar] [CrossRef]
- Šemnički, P.; Previšić, A.; Ivković, M.; Čmrlec, K.; Mihaljević, Z. Tufa Barriers from a Caddisfly’s Point of View: Streams or Lake Outlets? Int. Rev. Hydrobiol. 2012, 97, 465–484. [Google Scholar] [CrossRef]
- Vilenica, M.; Ivković, M.; Sartori, M.; Mihaljević, Z. Mayfly emergence along an oligotrophic Dinaric karst hydrosystem: Spatial and temporal patterns, and species–environment relationship. Aquat. Ecol. 2017. [Google Scholar] [CrossRef]
- Vander Vorste, R.; McElmurray, P.; Bell, S.; Eliason, K.M.; Brown, B.L. Does Stream Size Really Explain Biodiversity Patterns in Lotic Systems? A Call for Mechanistic Explanations. Diversity 2017, 9, 26. [Google Scholar] [CrossRef]
- Gerecke, R.; Meisch, C.; Stoch, F.; Acri, F.; Franz, H. Eucrenon—Hypocrenon ecotone and spring typology in the Alps of Berchtesgaden (Upper Bavaria, Germany) A study of microcrustacea (Crustacea: Copepoda, Ostracoda) and water mites (Acari:Halacaridae, Hydrachnelle). In Studies in Crenobiology. The Biology of Springs and Springbrooks; Backhuys Publishers: Leiden, The Netherlands, 1998; pp. 167–182. [Google Scholar]
- Stoch, F.; Gerecke, R.; Pieri, V.; Rossetti, G.; Sambugar, B. Exploring species distribution of spring meiofauna (Annelida, Acari, Crustacea) in the south-eastern Alps. J. Limnol. 2011, 70, 65–76. [Google Scholar] [CrossRef]
- Glaizer, D.S. Springs. In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Elsevier: Oxford, UK, 2009; Volume 1, pp. 734–755. [Google Scholar]
- Ivković, M.; Kesić, M.; Mihaljević, Z.; Kúdela, M. Emergence patterns and ecological associations of some haematophagous blackfly species along an oligotrophic hydrosystem. Med. Vet. Entomol. 2014, 28, 94–102. [Google Scholar] [CrossRef]
- Erman, N.; Erman, D. Spring permanence, Trichoptera species richness, and the role of drought. J. Kans. Entomol. Soc. 1995, 68, 50–64. [Google Scholar]
- Ivković, M.; Miliša, M.; Baranov, V.; Mihaljević, Z. Environmental drivers of biotic traits and phenology patterns of Diptera assemblages in karst springs: The role of canopy uncovered. Limnol. Ecol. Manag. Inland Waters 2015, 54, 44–57. [Google Scholar] [CrossRef]
- Pozojević, I.; Brigić, A.; Gottstein, S. Water mite (Acari: Hydrachnidia) diversity and distribution in undisturbed Dinaric karst springs. Exp. Appl. Acarol. 2018, 76, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Von Fumetti, S.; Nagel, P.; Baltes, B. Where a springhead becomes a springbrook—A regional zonation of springs. Fundam Appl. Limnol. Arch. Hydrobiol. 2007, 169, 37–48. [Google Scholar] [CrossRef]
- Durance, I.; Ormerod, S.J. Climate change effects on upland stream macroinvertebrates over a 25-year period. Glob. Chang. Biol. 2007, 13, 942–957. [Google Scholar] [CrossRef]
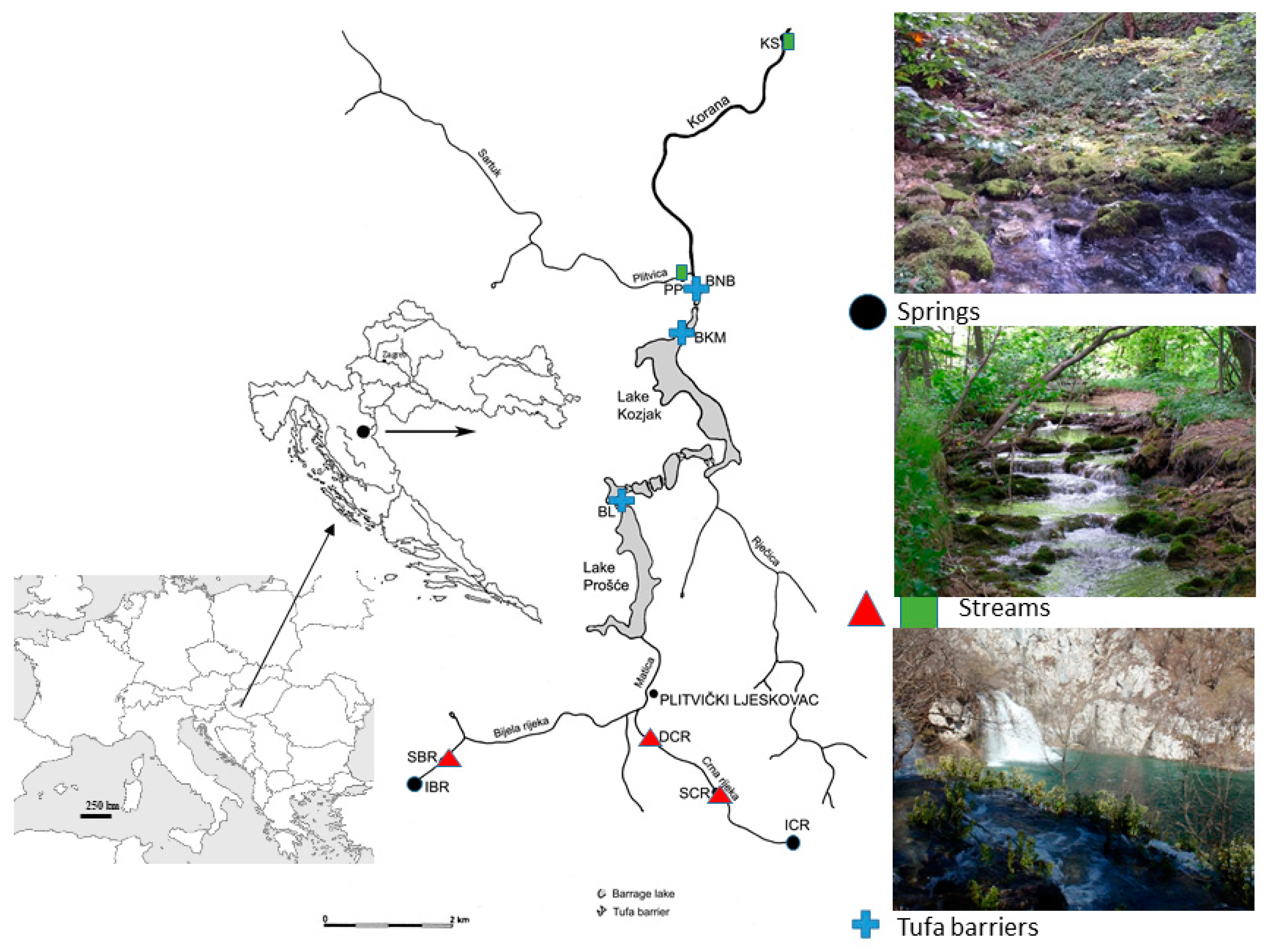

| Site | IBR | SBR | SCR | DCR | BL | BKM | BNB | PP | KS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Latitude | N 44°50′05″ | N 44°50′04″ | N 44°50′10″ | N 44°50′22″ | N 44°52′17″ | N 44°53′39″ | N 44°54′07″ | N 44°54′07″ | N 44°55′33″ | |
| Longitude | E 15°33′43″ | E 15°33′33″ | E 15°36′30″ | E 15°35′59″ | E 15°35′59″ | E 15°36′32″ | E 15°36′38″ | E 15°36′27″ | E 15°37′09″ | |
| Stream order | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 2 | |
| Altitude (m) | 720 | 716 | 670 | 667 | 630 | 546 | 504 | 556 | 390 | |
| Substrate | Pebbles and sand, Macrophytes, Moss | Pebbles and sand, Macrophytes, Moss | Pebbles and sand, Macrophytes, Moss | Pebbles and sand, Macrophytes | Pebbles, Moss on tufa, Tufa with detritus | Pebbles, Moss on tufa, Tufa with detritus, Silt | Pebbles, Moss on tufa, Tufa with detritus, Silt | Pebbles, Moss on tufa, Tufa with detritus, Silt | Pebbles, Moss on tufa, Tufa with detritus, Silt | |
| Water temperature (°C) | min | 7.3 | 7.2 | 7.1 | 6.9 | 2.5 | 3.1 | 3.3 | 3.2 | 1.7 |
| max | 7.8 | 9.9 | 9.7 | 9.6 | 20.5 | 22.9 | 22.9 | 15.4 | 19.8 | |
| O2 (mg L−1) | min | 7.6 | 8.2 | 7.9 | 8.8 | 6.7 | 8.7 | 8.4 | 8.7 | 9 |
| max | 11.8 | 11.8 | 12.5 | 13.1 | 12.3 | 12 | 12.4 | 13 | 14.1 | |
| O2 (%) | min | 65.2 | 71.2 | 68.8 | 96.7 | 59.7 | 72 | 77.3 | 75.7 | 79.6 |
| max | 101.8 | 106.6 | 115.9 | 111.1 | 139.2 | 113.6 | 117.1 | 122.5 | 121 | |
| pH | min | 6.9 | 7.5 | 7.7 | 7.9 | 6.8 | 6.9 | 8.2 | 6.8 | 6.8 |
| max | 7.8 | 8.4 | 8.6 | 8.4 | 8.7 | 8.4 | 8.7 | 8.9 | 8.7 | |
| Conductivity (μS cm−1) | min | 463 | 472 | 403 | 406 | 366 | 354 | 334 | 409 | 321 |
| max | 505 | 498 | 426 | 481 | 426 | 443 | 387 | 444 | 385 | |
| Alkalinity (mg L−1 CaCO3) | min | 235 | 230 | 210 | 215 | 210 | 200 | 185 | 225 | 180 |
| max | 295 | 295 | 290 | 280 | 260 | 220 | 230 | 280 | 215 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozojević, I.; Ivković, M.; Cetinić, K.A.; Previšić, A. Peeling the Layers of Caddisfly Diversity on a Longitudinal Gradient in Karst Freshwater Habitats Reveals Community Dynamics and Stability. Insects 2021, 12, 234. https://doi.org/10.3390/insects12030234
Pozojević I, Ivković M, Cetinić KA, Previšić A. Peeling the Layers of Caddisfly Diversity on a Longitudinal Gradient in Karst Freshwater Habitats Reveals Community Dynamics and Stability. Insects. 2021; 12(3):234. https://doi.org/10.3390/insects12030234
Chicago/Turabian StylePozojević, Ivana, Marija Ivković, Katarina Ana Cetinić, and Ana Previšić. 2021. "Peeling the Layers of Caddisfly Diversity on a Longitudinal Gradient in Karst Freshwater Habitats Reveals Community Dynamics and Stability" Insects 12, no. 3: 234. https://doi.org/10.3390/insects12030234
APA StylePozojević, I., Ivković, M., Cetinić, K. A., & Previšić, A. (2021). Peeling the Layers of Caddisfly Diversity on a Longitudinal Gradient in Karst Freshwater Habitats Reveals Community Dynamics and Stability. Insects, 12(3), 234. https://doi.org/10.3390/insects12030234







