Simple Summary
One of the main insect pests in protected strawberry production are thrips, but little is known about which species of thrips are present in the production system. In this study, we identified the thrips species of adults and larvae present in two strawberry cultivars at a commercial strawberry farm in Denmark. The most abundant species found were Frankliniella intonsa, followed by Thrips tabaci. The abundance of thrips peaked in July (temperature range 18–23 °C, mean humidity 65%, mean precipitation 5 mm). More thrips were found in the earlier flowering cultivar. In order to optimize control of thrips, a fundamental first step is knowing which species are present on the target crop.
Abstract
Thrips are a major pest in protected strawberry production. Knowledge of thrips species composition could be instrumental for improved thrips management, but very little is known about which species are present in strawberries grown in high-tunnels in Denmark. Thrips (adults and larvae) were sampled in two strawberry tunnels of the cultivars Murano and Furore from May to August 2018, in the middle and in the edges of the tunnels. The most abundant thrips species found in the tunnels were Frankliniella intonsa and Thrips tabaci adults. Frankliniella intonsa were also the most frequently found species of the immatures sampled, followed by T. tabaci larvae, and other species. The number of thrips differed between the two cultivars, sampling times and location in the tunnel. Frankliniella intonsa was more abundant in the middle of the tunnels, while T. tabaci was more abundant in the edge of the tunnels adjacent to the field margins. The number of thrips peaked by the end of July. Both chemical and biological control should consider species composition and occurrence; hence, a fundamental first step for thrips management is to identify the species present on the target crop.
1. Introduction
Thrips (Thysanoptera: Thripidae) are among the most important pests for a wide range of crops [1,2,3]. Damage occurs directly through feeding or indirectly by the transmission of tospoviruses [1,2,3]. Thrips can spread rapidly by flying, or by passive transport by introduced planting material or by the wind [1,4,5]. Once established in a new environment, thrips can produce several generations per year, depending on weather conditions and species [6,7].
Strawberries (Rosaceae, Fragaria × ananassa Duchesne) are grown on approximately 1200 ha in Denmark [8] and while most production is in the open field, an increasing proportion has since the early 2000s been produced in high tunnels [8,9,10]. Tunnel systems enable the growing season in the open field with harvest from June to early August to be extended until mid October [11,12]. In protected strawberry production in particular, thrips are a major pest [13]. Damage symptoms include petal browning, premature withering, fruit abortion, fruit bronzing and distortion [3,14,15]. Adult feeding on pollen causes injury to flowers, which wither and never develop into fruits [15]. The larval feeding causes fruit injury and leads to unmarketable produce, making larvae the most damaging stage.
There is a strong correlation between numbers of thrips per flower and fruit damage [16,17]. Economic injury levels (EILs) vary, and can depend on thrips developmental stage, (larval or adult), the cultivar and whether predators are present in the crop [17,18]. EILs may be higher in polythene tunnels due to higher temperatures, where thrips have a higher survival rate [15,19]. In Denmark, fruit losses up to 60–75% have been observed when none or limited crop protection has been implemented [20].
Common thrips species found in strawberries include: Frankliniella occidentalis (Pergande), Frankliniella intonsa (Trybom) and Thrips tabaci Lindeman [14,21]. All three are highly polyphagous species and occur throughout the globe including the Nordic countries [14,22,23]. Frankliniella occidentalis is an invasive species originating from the western half of North America [24,25]. It was first discovered in Europe in 1983, and in 1993 it was first recorded in northern Europe (including Denmark) where it had established in glasshouses [24]. Frankliniella intonsa is a major pest of tunnel-grown strawberries. This is in part due to its broad-spectrum insecticide resistance, high reproductive capacity and ability to colonize host plants in large numbers [15,26,27,28,29]. Frankliniella intonsa is believed to be native to Eurasia and shares similar distribution patterns, feeding habits and tospoviruses with F. occidentalis [30]. Thrips tabaci is believed to originate from the Eastern Mediterranean and was first introduced in Europe in 1889 [31]. It has been recorded as a damaging pest in strawberry in Europe by Lewis, 1997 [1] and by Benninson and Hough, 2015 [32]. Frankliniella intonsa and T. tabaci are known to compete with the more dominating F. occidentalis for resources [33,34]. In its absence, these two may be the most abundant species in the strawberry crop [35,36].
Currently, no chemical control options are available against thrips in tunnel produced strawberries in Denmark, while the use of natural enemies can be implemented [26,37,38,39]. The latter involves various predatory mites and the predatory hemipteran: Orius spp. [40,41,42,43]. Both chemical and biological control should be based on knowledge of pest biology, hence a fundamental first step for obtaining control of thrips is to identify the different species present on the targeted crop [44]. Very little is known about the species composition of thrips in strawberry produced in high tunnels in Denmark, so the aim of this study was to identify the species composition of thrips; adults and larvae, throughout a growing season in strawberry high tunnels on two common strawberry cultivars.
2. Materials and Methods
2.1. Sampling of Thrips in Strawberry Tunnels
Sampling of thrips for species identification was undertaken at a large commercial berry farm near Skælskør, Denmark (latitude: 11°19′52° E; altitude: 20 m). The farm was managed conventionally, and strawberries were grown on table tops in high tunnels covering 4 ha in total. Phytosanitary management included the use of fungicides, while no chemical pest control was applied. Besides chemical disease control, sachets of 500 Amblyseius (Neoseiulus) cucumeris were placed every 1–2 m, in the two tunnels as a biological pest control measure. This was done before flowering commenced. Naturally occurring predators such as Orius spp. and larvae of Chrysoperla spp. were also observed in the tunnels, by visual observations on-site.
The study was based on seasonal and temporal sampling of thrips adults and larvae. The sampling was repeated six times between May and August 2018 (sampling dates: 25 May, 25 June, 10 July, 23 July, 13 August and 31 August), in two neighbouring, parallel tunnels; one with the cultivar Murano and one with the cultivar Furore, a slightly later blooming cultivar. Because thrips species disperse differently in crops, six different locations in each of the two tunnels were sampled; front left, front right, back left, back right, middle 1 and middle 2. Each row was 204 m long, 35 cm wide and rows were 1.5 m apart. Each sampling location was approximately 2 m wide (two rows) and 5 m long, with 4 m between locations left and right. The front of the tunnel was the open end (facing West) adjacent to a dirt road and the farm, while the back was the open end (facing East) adjacent to tunnels running horizontally, with open cereal fields on the opposite side to them.
Ten strawberry flowers and five berries were sampled at each sampling location on each of the six dates. Flowers were collected to include adult thrips, which feed on pollen and, therefore, most frequently are found here, while berries were collected to include larvae, which feed at this site. Berries were 2–4.5 cm in diameter and green to yellow in colour when sampled. Specimens were stored in plastic containers (155 mL, Ø: 69 mm) with 70% ethanol. Containers were labelled and brought back to the laboratory, where thrips were counted under a stereomicroscope and kept in 70% ethanol until slide mounting of the adult thrips.
In addition and to support the results from the two tunnels, adult thrips from flowers were sampled from tunnel-grown strawberries at three other locations in Eastern Denmark: Hoerve (latitude: 11°22′26° E; altitude: 14 m; collected 6 June), Skaelskoer (latitude: 11°21′39° E; altitude; 22 m; collected 11 June) and Broby (latitude: 10°16′08° E; altitude: 26 m; collected 2 July), from either cultivar Murano or Favori. Another sampling by the advisory service was undertaken at the same farms in cultivars Favori and Flair. All thrips specimens were identified to species.
2.2. Fast-Slide-Preparation of Adults
Fast slide preparation was undertaken to prepare and mount each of the captured adult thrips onto microscope slides for later species identification. This method was based on that provided by Silveira and Haro [45] and further adapted to exclude the use of the toxic compound phenol. In addition, puncturing of the abdomen to expel body contents as well as massaging of the specimen was not necessary. The following mixture was used: 85% lactic acid (20 parts), glacial acid (4 parts) and distilled water (1 part). First, the specimen was placed in a Petri dish containing a bit of water. Using a paintbrush, the specimen was transferred onto a watch-glass containing a few drops of NaOH (5%), and then held over a flame from an alcohol lamp for 30 s. Next, it was transferred into a test tube containing a small amount of the mixture described above and held over the flame for approximately 1 min. The hottest part of the flame was avoided, and the liquid was not allowed to boil. This was especially important with the very light-coloured specimens, as they are more easily destroyed by this treatment. The specimen was then returned to the Petri dish to be rinsed in the water, and next transferred onto the side of a microscope slide to dry off a bit. A drop of Hoyer’s medium [46] was placed in the middle of the slide and the specimen transferred onto here using a brush. Fine needles were used to spread out the wings and legs and separate the antennae of the thrips before cover glass was added. Specimens were placed in an incubator at 60 °C for approximately six days, by which time, they were sufficiently transparent to see all the structures needed for morphological identification.
2.3. Morphological Identification of Adults
A combination of taxonomic keys was used to achieve correct species identification of adult thrips. In order to key to genus, a Thysanoptera drawing key by Mound et al. [22] was used. An online photo key was used to further identify thrips to species level [47]. In addition to the pest thrips identified, nine individuals of the genus Aeolothrips were identified, but these were excluded from further analysis as this is primarily predatory thrips and not regarded as a pest [47,48,49]. Another 55 specimens from the three additional locations with tunnel-grown strawberries were identified to species level.
2.4. Molecular Identification of Two Species of Larvae
Identification of larvae to species level is very difficult with morphological methods [44,50]. For this reason, a molecular approach was applied for species identification of larvae. Quantitative real-time polymerase chain reaction (qPCR) was used for the identification of the two most abundant thrips species, based on adult identification: F. intonsa and T. tabaci. Primers of the two species were tested against the 145 specimens of immatures collected from June to August.
2.5. DNA Extraction
DNA was extracted from individual larvae using the DNeasy Blood and Tissue Extraction Kit (QIAGEN©, Hilden, Germany). First, each larva was placed in a 1.5 mL microcentrifuge tube with three glass beads (1 mm) and run in a tissue lyser machine (TissueLyser II, QIAGEN©, Hilden, Germany) for approximately 2 min. Next, 200 μL of tissue lysis buffer ATL and 20 μL of proteinase K was added and the sample was vortexed. Samples were then incubated at 56 °C for an hour to allow for a complete breakdown of cells. Then 200 μL ethanol (96–100%) was added and vortexed thoroughly. The mixture was transferred into a DNeasy spin tube and centrifuged at 8000 rpm for 1 min. Flow-through and collection tube was discarded. The spin column was transferred into a new 2 mL collection tube. Next, 500 μL buffer AW1 was added to the spin column and centrifuged at 8000 rpm for 1 min. Run-through was discarded. Hereafter, 500 μL of buffer AW2 was added and centrifuged for 3 min at 14,000 rpm. Flow-through and collection tube was discarded. The spin column with the DNA was transferred to a new 1.5 mL microcentrifuge tube. DNA was eliminated by adding 100 μL buffer AE directly onto the DNeasy membrane. The tube was left to incubate at room temperature for 1 min and then centrifuged at 8000 rpm for 1 min. This last step was repeated once more to maximize DNA output.
2.6. Quantitative Real-Time Polymerase Chain Reaction (qPCR) and Output
Primers of F. intonsa and T. tabaci developed by Yeh et al., [51] were used to determine if the sampled larvae were of either of these two species or of a different species. The specific primers, based on fragments of 18S rDNA and 5.8S rDNA, used for ITS1 fragment amplification were: TGCTTGAGCGGAACGAGCG (F. intonsa upstream primer sequence) + TCCACATAGCGGCGTGAAAG (F. intonsa downstream primer sequence) and TCTAAACAGAGGGAAAGGTG (T. tabaci upstream primer sequence) + AGTGTGCCAACAAGGCAATG (T. tabaci downstream primer sequence). The qPCR assay was carried out in a volume of 25 μL. The program of the qPCR machine (model: Mx3005P, Agilent Technologies©, Santa Clara, CA, USA) was set to that developed for the fluorescent dye Sybr green© (RealQ Plus2x Master Mix Green, Low ROX) (Ampliqon, Odense, Denmark) with a few modifications to fit the conditions of the selected thrips primers [51]. The first denaturation was 15 min at a temperature of 95 °C, followed by 45 cycles of 95 °C for 40 s, 50 °C for 50 s and 72 °C for 1 min.
The output was analysed with the programme MxPro qPCR Software© version 4.10 (Stratagene, Agilent Technologies©, Santa Clara, CA, USA) as follows: cycle threshold (Ct) values under 40 were accepted as positive—DNA in the sample was detected and matched that of the primer. Ct values above 40 were classed as ‘negative’—DNA was detected in the sample, but the high values were likely a result of primers having copied themselves due to the high number of cycles. At 40 cycles, amplification efficiency slows down and reaches a plateau, while below 40 cycles amplification is at its most efficient [52]. Samples containing water were used as a control. Ct values between 35 and 40 required inspection of each sample by looking at individual amplification plots. Samples containing no Ct values or being negative for both primers, were classed as ‘other’, meaning that the given sample containing DNA was neither F. intonsa nor T. tabaci, but another species of thrips. The threshold for detected fluorescence (dRn) was set to 0.15 dRn for all runs.
2.7. Statistical Analysis
A generalized linear model (GLM) was applied, as data were not normally distributed, to determine if there was a significant difference in the number of thrips in relation to sampling time: 25 May, 25 June, 10 July, 23 July, 13 August and 31 August 2018; location in tunnel: front, middle and back; and the two cultivars: Murano and Furore. A negative-binominal distribution was applied for all variables and a 3-way analysis was carried out. A pairwise comparison was undertaken of mean thrips numbers according to sampling time and location in the tunnels. Since the two tunnels had different cultivars, no distinction could be made between tunnel and cultivar. Furthermore, as counts in the same locations were repeated over time, a repeated measures analysis was undertaken, and statistical outputs are thus based on this. GLM was also applied to determine if there was a significant difference between the numbers of F. intonsa and T. tabaci with the two fixed variables sampling time and location, as described above. Tunnel was included as a random effect. A pairwise comparison of numbers of F. intonsa and T. tabaci according to sampling time and location in tunnel was also carried out. Error bars in Figure 1 and Figure 2 refer to each mean plus and minus one standard error of the mean. The model output had a degrees of freedom of one. P-values and z-scores are reported in the results.
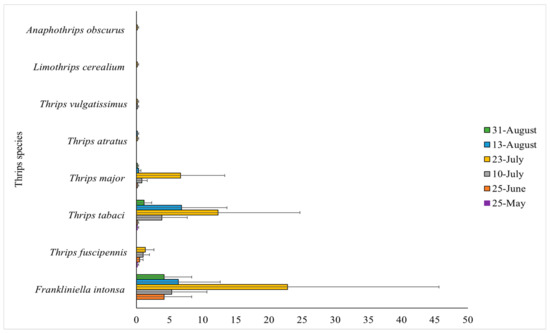
Figure 1.
Mean number of Frankliniella intonsa, Thrips fuscipennis, Thrips tabaci, Thrips major, Thrips atratus, Thrips vulgatissimus, Limothrips cerealium and Anaphothrips obscurus in Murano and Furore according to the six sampling dates. Each bar represents the three locations in the tunnel combined (front, middle and back). Error bars represent the mean number of thrips for each species at the specific sampling date ±1 standard error of the mean.
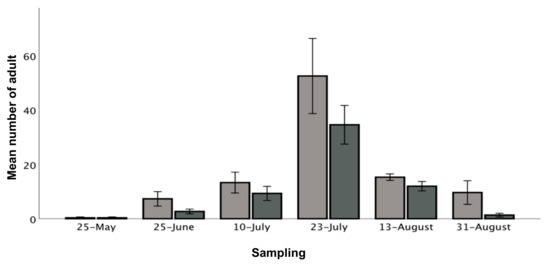
Figure 2.
Mean number of thrips in relation to sampling time in cultivar Murano (light grey) and cultivar Furore (dark grey), May to August 2018. Error bars represent the mean number of thrips for each species at the specific sampling date ±1 SE standard error of the mean.
Models were validated by goodness of fit tests and omnibus tests. Pearson chi square showed that the models were slightly over-dispersed (1.396), but, as this was marginal (below 1.5), the models were accepted. Overall, the omnibus tests showed highly statistically significant models.
A one-way analysis of variance (ANOVA) was used, as data was normally distributed, to determine if the numbers of F. intonsa, T. tabaci and ‘other’ larvae respectively, differed according to the three sampling months: June, July and August. A Tukey post hoc test was used to see how the number of F. intonsa, T. tabaci and ‘other’ larva species differed according to each of the sampling months. All analyses were carried out in IBM© SPSS© Statistics version 25 (Armonk, NY, USA).
3. Results
3.1. Species Composition of Thrips Adults and Larvae during the Production Season
Eight different species were morphologically identified from the 482 adult thrips sampled. These included both males and females, with the majority being females. The most abundant species in both tunnels were F. intonsa (53.3% of the total number of adult thrips) and T. tabaci (31.5% of the total number of adult thrips). Thrips fuscipennis Haliday was also recorded and made up 3.7% of the total number of adults thrips. Only a few individuals of the species Thrips atratus Haliday (0.4% of the total number of adult thrips), Thrips vulgatissimus Haliday (0.4% of the total number of adult thrips), Limothrips cerealium Haliday (0.2% of the total number of adult thrips) and Anophothrips obscurus (Mueller) (0.2% of the total number of adult thrips) were found (Figure 1). Frankliniella intonsa occurred in higher numbers earlier than other species (Figure 1). The highest density of F. intonsa and T. tabaci was in July (p < 0.001, z-scores = 7.42, 5.96 respectively). Thrips tabaci densities were higher in the middle of August compared to the end of August (p < 0.001, z-score = 4.32), while the same was not true for F. intonsa densities (p = 0.106, z-score = 1.62).
The tunnel with Murano had more F. intonsa than tunnel 2 (p < 0.001, z-score = 5.35), while numbers of T. tabaci were similar in the two tunnels (p = 0.365, z-score = 0.91). Furthermore, a higher number of F. intonsa was found in the front (14.2 ± 5.0 SE) and middle (21.7 ± 11.0 SE) of the tunnels compared to the back (7.0 ± 3.9 SE) (p < 0.001, z-scores = 3.55, 5.78 respectively). In contrast, fewer T. tabaci were found in the front (8.0 ± 3.7 SE) and middle (6.7 ± 3.4 SE) compared to the back (10.7 ± 5.5 SE) (p = 0.044, 0.020; z-scores = −2.02, −2.33, respectively).
A total of 145 larvae were collected in the two tunnels. The number of ‘positive ‘samples (matched one of the primers) was 112, the number of ‘negative’ samples (did not match any of the primers) was 24, and samples needing revision (Ct values were between 35 and 40) were 19. The revision was undertaken by inspection of individual amplification plots. Based on the molecular identification, the larvae were divided into three categories: F. intonsa, T. tabaci and ‘other’, the latter consisting of all other species of larvae. The majority of larvae sampled were identified as F. intonsa, while T. tabaci and ‘other’ occurred to a similar extent (Table 1). The numbers of F. intonsa and T. tabaci larvae, each differed according to the three sampling months (June, July and August) (p < 0.001). No larvae were found in May and no T. tabaci larvae were found in June. The number of ‘other’ larva species did not differ according to sampling time (p = 0.857) (Table 1).

Table 1.
Counts of larvae of the species: Frankliniella intonsa, Thrips tabaci and ‘Other’, including percentage of these from June until August, and p-values between months of each species. Total count per observation was 145 larvae in the two tunnels collectively. No larvae were found in May.
3.2. Species Found at Other Locations in Denmark
From the first location of the three additional sites, 29 F. intonsa and 2 T. tabaci were identified from the cultivar Murano. Ten F. intonsa, 5 T. tabaci, and 2 T. fuscipennis were identified from the second location from the cultivar Murano, and from the third location, Broby, 5 F. intonsa and 1 T. fuscipennis were identified from Favori. At all three locations, F. intonsa was found to be the most abundant. In addition, a thrips sampling at the end of June found 4 F. intonsa, 2 T. fuscipennis, and 1 T. major (Skaelskoer) from Favori, and 8 F. intonsa and 1 T. fuscipennis (Broby) from Favori. From the third location (Hoerve), 4 F. intonsa, 5 T. fuscipennis and 10 F. occidentalis was found in the cultivar Flair, indicating that the choice of cultivar has an impact on the species composition of thrips, while this remains to be investigated.
3.3. Effect of Cultivar, Time and Location on the Overall Density of Adult Thrips
Total thrips density, which may be defined as the total number of adult thrips in 10 flowers at each of the six sampling sites at each of the six sampling dates, peaked by the end of July in both tunnels (p < 0.001, z-score = 8.14). Murano had a higher number of thrips than Furore (16.4 ± 4.6 and 10.1 ± 3.1 respectively) (p < 0.001, z-score = 3.49) (Figure 2). Pairwise comparisons of densities show that there were more thrips on 10 July, 23 July and 13 August compared to the end of the season, 31 August (p = 0.003, p < 0.001, p < 0.001; z-scores = 2.96, 8.14, 3.62 respectively). There were fewer thrips on 25 May than 31 August (p = 0.000, z-score = −3.63), while there was no difference in the number of thrips on 25 June and 31 August (p = 0.854, z-score = −0.18) (Figure 2). Thrips density in the middle of tunnels was significantly higher than the back of the tunnels (means ± SE per tunnel (average for May–August): front = 12.3 ± 4.7 thrips, middle = 17.1 ± 9.2 thrips, back = 10.3 ± 5.5 thrips) (p = 0.010, z-score = 2.56). Thrips densities in the front and back of tunnels were not significantly different (p = 0.111, z-score = 1.59).
4. Discussion
4.1. Species Composition and Distribution of Thrips
Frankliniella intonsa was the most abundant thrips species found in the strawberry tunnels, both in terms of adults (53.3% of all adults sampled) and larvae (60% of all larvae sampled) (Figure 1; Table 1). The identification of adult thrips sampled from tunnel-grown strawberries at three other strawberry farms supported these findings. Globally, only few studies have reported findings of F. intonsa in strawberry, and even fewer in high tunnel systems, of which only one in Scandinavia, which also reported F. intonsa to be a dominating species [53]. A high number of F. intonsa in greenhouse strawberry was recorded by Lim and Mainali [54] in Korea, in outdoor commercial fields by Mintu and Reyes [55] in the Philippines and by Atakan [56] in the Eastern Mediterranean area of Turkey. Only Atakan [56] also found F. occidentalis, but in low numbers. The highest injury to strawberry already occurs approximately two weeks after flowering [15]. Berries are green at this time and feeding by larvae causes a limited shelf life at maturity. Here, larvae of F. intonsa occurred earlier and were more abundant than the other species found and are likely to have inflicted early damage to green berries in the tunnels and may thus be responsible for the highest injury levels to the crop.
The second most sampled adult species was T. tabaci (31.5% of all adults sampled) (Figure 1), which also reproduced in the tunnels, with larvae found in equal numbers to “other species” (20% of all larvae sampled) (Table 1). This suggest that this species is also an established species in the strawberry tunnels. It is important to identify larvae to species level, as their presence reveals whether adults are reproducing in the tunnels, and thus become an established species. An improved method for fast slide preparation was created in this study. The method excluded the use of phenol and was overall faster due to the exclusion of abdomen puncturing and the intermediate steps of specimen massaging to evaluate transparency. It is important to state, however, that slides obtained using this method are only temporary and not permanent [45,57]. The oldest insect-mounted slides preserved by this method were 34 years old [58].
The two species F. intonsa and T. tabaci co-occur in different crops, but in most cases F. intonsa is the most frequently found species of the two [53,56,59,60,61]. The third and fourth most common adult species found were T. fuscipennis and T. major, two species of thrips that are also quite common pests of strawberry [14,15,62] (Figure 1). These species (and perhaps others) likely shared the 20% “other species” of the total percentage of sampled larvae reported in Table 1.
Additional species sampled included T. atratus and T. vulgatissimus (Figure 1), both commonly reported in tunnel-grown strawberry [21,53,63,64]. Finally, few individuals of L. cerealium and A. obscurus were sampled. These species are generally found on cereals and grasses and could have flown in from nearby fields [14]. They are mostly reported as only occasional visitors in strawberries [47,62]. Frankliniella occidentalis are commonly reported as a key pest species of thrips in strawberries [19]. In the present study, no F. occidentalis were found, likely because it is difficult for F. occidentalis to establish permanent field populations in Northern Europe, including Denmark [6,26,65]. In the UK, a recent study suggests that F. occidentalis is able to survive the winter on weeds in polythene tunnels, and this may enable them to build up large populations in second-year everbearing strawberry [19]. Such an occurrence may potentially spread to Denmark if winters become milder. Competition may also explain the absence of F. occidentalis, as F. intonsa has been reported to outcompete F. occidentalis [30,36], while T. tabaci have been shown to have lower survival rates in the presence of F. occidentalis due to competition [34,35].
Although few F. occidentalis were sampled as part of the additional collections (Hoerve), this species was absent from three other cultivars (Murano, Furore and Favori). Flair is a cultivar that may overwinter [66]. A mild winter could have caused F. occidentalis to survive the winter and build up its population that year.
4.2. Difference in Abundance of Frankliniella Intonsa and Thrips Tabaci
A combination of morphological and molecular tools allowed us to identify F. intonsa followed by T. tabaci as the two most common species in strawberry high tunnels. Thrips tabaci was more abundant in different areas than F. intonsa (back vs. front and middle, respectively). Interspecific competition between the two species may explain the spatial differences observed. Frankliniella intonsa has been reported to outcompete F. occidentalis in different ways, including higher food intake, higher reproductive activity and fecundity, as well as greater survival under fluctuating temperatures [30,36]. In addition, this species has been found to display pollen guarding when interacting with F. occidentalis on the same area of a host plant [36]. In contrast to F. intonsa, T. tabaci has been observed to have lower survival rates when interacting with F. occidentalis [34,35]. Insects are known to stay away from areas where a superior competitor is present [67,68]. One may speculate that T. tabaci may thus actively avoid areas with abundant F. intonsa.
4.3. Temporal and Spatial Patterns of Adult Thrips
The July peak of infestation is in accordance with other studies in temperate climates [19,53,63,69]. It is likely that remaining flowers in tunnels in mid-August maintained thrips numbers above those in May. The sharp decline in thrips numbers by the end of August was likely due to an observed decline in the number of flowers and larvae dropping to the ground to commence pupation [19,53,63,69]. The earlier flowering Murano had more thrips than Furore. Sampson and Kirk [69] observed that newly opened flowers and flowers standing tall are preferred by thrips due to fresh pollen and higher visibility, respectively. The cultivar Murano is in particular known to stand tall, resulting in highly visible flowers [70]. Factors such as leaf quality and plant volatiles may also impact thrips occurrence [71,72]. The highest thrips densities were found in the middle of tunnels. Similar observations have been made of the diamondback moth and the cabbage webworm in different crops [73,74]. The slope at the study site may have caused the middle of the tunnels to be warmer than the area by the tunnel openings due to the upwards wind, a factor which possibly also contributed to a higher number of thrips in the centre of the tunnels. Based on the results presented here, we found no indication of thrips entering the tunnels from outside, where an early aggregation may be expected in field edges (tunnel openings) unlike what was found in the present study. Therefore, we recommend that further investigations include studies of cultivar and spatial distribution, as well as an expansion of the number of sampling years.
5. Conclusions
This study provides new knowledge about the species composition of thrips in strawberry high tunnels in Denmark. Correct larval species identification is important, as their presence reveals whether adults are reproducing in the tunnels, and thus become an established population. Knowledge of species behaviour and interactions, and most importantly selection of efficient biocontrol agents against these thrips species, remains to be investigated in order to optimize pest control strategies.
Author Contributions
H.N., L.S. and S.K.J. conceived the research and contributed to the design of experiments. H.N. conducted experiments. H.N. analysed data and conducted statistical analyses. N.L.J. contributed with supporting data. S.K. confirmed and validated species recognition. H.N. wrote the manuscript. All authors contributed to revisions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data will be made available in a digital data repository upon acceptance of the manuscript.
Acknowledgments
This project was carried out at the University of Copenhagen. Thank you to grower Søren Olesen for making the field study possible and to lab technician Didde Hedegaard Sørensen for the assistance with the molecular work. A special thank you to advisors Frida Helgadottír and Helle Mathiasen from HortiAdvice A/S, and to Nina Jørgensen, Borregaard Bioplant ApS, for additional assistance with this project. The research was funded as an MSc project with the research group Applied Insect Plant Ecology, UCPH.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lewis, T. Thrips as Crop Pests, 1st ed.; CAB International: New York, NY, USA, 1997; pp. 11–12. [Google Scholar]
- Lewis, T. Thrips: Their Biology, Ecology and Economic Importance, 1st ed.; Academic Press: London, UK, 1973; p. 9. [Google Scholar]
- Parker, B.L.; Skinner, M.; Lewis, T. Thrips Biology and Management, 1st ed.; Springer International Publishing: New York, NY, USA, 1995; pp. 19–20. [Google Scholar]
- Mound, L. Natural and disrupted patterns of geographical distribution in Thysanoptera. J. Biogeogr. 1983, 10, 119–133. [Google Scholar] [CrossRef]
- Mound, L.; Walker, A. A Fauna of New Zealand, 1st ed.; Science Information Division: Wellington, New Zealand, 1982; p. 12. [Google Scholar]
- McDonald, J.R.; Bale, J.S.; Walters, K.F.A. Effect of temperature on development of the western flower thrips. Eur. J. Entomol. 1998, 95, 301–306. [Google Scholar]
- Stacey, D.; Fellowes, M.D.E. Temperature and the development rates of thrips: Evidence for a constraint on local adaptation? Eur. J. Entomol. 2002, 99, 399–404. [Google Scholar] [CrossRef]
- Danmarks Statestik: Det Dyrkede Areal Efter Afgrøde, Enhed, Område, og Tid. Available online: https://www.statistikbanken.dk/statbank5a/SelectVarVal/saveselections.asp (accessed on 5 June 2019).
- HortiAdvice IPM Dyrkningsvejledning for Jordbær. Report; HortiAdvice: Odense, Denmark, 2016; pp. 1–6. [Google Scholar]
- Sønsteby, A.; Karhu, S. Strawberry production, growth and development in northern climates. Int. J. Fruit Sci. 2005, 5, 107–114. [Google Scholar] [CrossRef]
- Daugaard, H. Tabletop produktion af jordbær. Det Jordbrugsvidenskabelige Fak. Århus Univ. 2007, 175, 1–6. [Google Scholar]
- Raffle, S.; Irving, R.; Moore, G. Extending the UK Strawberry Season Using a Range of Plant Types and Growing Systems; Factsheet from Horticultural Development Company: Warwickshire, UK, 2010; pp. 1–9. [Google Scholar]
- Atakan, E.; Pehlivan, S.; Kiminsu, A. Pest status of Western Flower Thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae), in tunnel-grown strawberry. Turk. Entomoloji Derg. 2016, 40, 61–71. [Google Scholar] [CrossRef]
- Alford, D. Agricultural Entomology, 1st ed.; Blackwell Science: Cambridge, UK, 1999; pp. 90–93. [Google Scholar]
- Steiner, M.Y.; Goodwin, S. Management of thrips (Thysanoptera:Thripidae) in Australian strawberry crops: Within-plant distribution characteristics and action thresholds. Aust. J. Entomol. 2005, 44, 175–185. [Google Scholar] [CrossRef]
- Sampson, C. Management of pesticide-resistant western flower thrips on tunnel- grown strawberry: A study of the reasons for successes and failures on commercial production sites. Rep. AHDB 2014, 1, 1–22. [Google Scholar]
- Coll, M.; Shakya, S.; Shouster, I.; Steinberg, Y. Decision-making tools for Frankliniella occidentalis management in strawberry: Consideration of target markets. Entomol. Exp. Appl. 2007, 122, 59–67. [Google Scholar] [CrossRef]
- Sampson, C.; Kirk, W.D.J. Predatory mites double the economic injury level of Frankliniella occidentalis in strawberry. BioControl 2016, 61, 661–669. [Google Scholar] [CrossRef]
- Sampson, C. Sustainable management of the Western Flower Thrips in strawberry crops. Out. Pest Man 2018, 10, 1–6. [Google Scholar] [CrossRef]
- Jensen, N.L. (Berry consultant at HortiAdvice, Odense, Denmark). Personal Communication, 2021.
- Cross, J. Integrated Control of Thrips on Strawberries; Factsheet from Horticultural Development Company: Kent, CT, USA, 2003; pp. 1–8. [Google Scholar]
- Mound, L.A.; Morison, G.D.; Pitkin, B.R.; Palmer, J.M. Handbook for the identification of British insect: Thysanoptera, 1st ed.; Royal Entomological Society of London: London, UK, 1976; pp. 14–18, 29–32, 46, 56. [Google Scholar]
- Kobro, S. Checklist of Nordic Thysanoptera. Nor. J. Entomol. 2011, 58, 20–26. [Google Scholar]
- Kirk, W.D.J.; Terry, I. The spread of the Western Flower Thrips Frankliniella occidentalis (Pergande). Agric. For. Entomol. 2003, 5, 301–310. [Google Scholar] [CrossRef]
- Reitz, S.R.; Gao, Y.; Kirk, W.D.J.; Hoddle, M.S.; Leiss, K.A.; Funderburk, J.E. Invasion biology, ecology, and management of Western Flower Thrips. Annu. Rev. Entomol. 2020, 65, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S. Insecticide resistance in Western Flower Thrips Frankliniella occidentalis. Integr. Pest Manag. Rev. 2000, 5, 131–146. [Google Scholar] [CrossRef]
- Molnár, A.; Pap, Z.; Fail, J. Observing population changes of thrips (Thysanoptera) species damaging forced pepper and their natural enemies. Int. J. Hortic. Sci. 2008, 14, 55–60. [Google Scholar] [CrossRef]
- Shipp, J.L.; Whitfield, G.H. Functional response of the predatory mite, Amblyseius cucumeris (Acari: Phytoseiidae), on Western Flower Thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Environ. Entomol. 1991, 20, 694–699. [Google Scholar] [CrossRef]
- Shipp, J.L.; Wang, K.; Binns, M.R. Economic injury levels for Western Flower Thrips (Thysanoptera: Thripidae) on greenhouse cucumber. J. Econ. Entomol. 2009, 93, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.S.; Lim, U.T. Life history characteristics of Frankliniella occidentalis and Frankliniella intonsa (Thysanoptera: Thripidae) in constant and fluctuating temperatures. J. Econ. Entomol. 2015, 108, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Montano, J.; Fuchs, M.; Nault, B.A.; Fail, J.; Shelton, A.M. Onion thrips (Thysanoptera: Thripidae): A global pest of increasing concern in onion. J. Econ. Entomol. 2011, 104, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bennison, J.; Hough, G. Maintaining the Expertise for Developing and Communicating Practical Integrated Pest Management (IPM) Solution for Horticulture. Annual Report from AHDB. 2015, pp. 1–44. Available online: https://ahdb.org.uk/cp-089-maintaining-the-expertise-for-developing-and-communicating-practical-integrated-pest-management-ipm-solutions-for-horticulture-emt-hdc-hta-fellowship (accessed on 18 January 2021).
- Li, W.D.; Zhang, P.J.; Zhang, J.M.; Zhang, Z.J.; Huang, F.; Bei, Y.W.; Lin, W.C.; Lu, Y.B. An evaluation of Frankliniella occidentalis (Thysanoptera: Thripidae) and Frankliniella intonsa (Thysanoptera: Thripidae) performance on different plant leaves based on life history characteristics. J. Insect Sci. 2015, 15, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, P.C.J.; Mollema, C.; Steenhuis-Broers, G.M. Comparative life history studies of Frankliniella occidentalis and Thrips tabaci (Thysanoptera: Thripidae) on cucumber. Bull. Entomol. Res. 1995, 85, 285–297. [Google Scholar] [CrossRef]
- Deligeorgidis, P.; Ipsilandis, C.; Vaiopoulou, M.; Deligeorgidis, N.; Stavridis, D.; Sidiropoulos, G. The competitive relation between Frankliniella occidentalis and Thrips tabaci: The impact on life-cycle and longevity. J. Entomol. 2006, 3, 143–148. [Google Scholar] [CrossRef][Green Version]
- Bhuyain, M.M.H.; Lim, U.T. Interference and exploitation competition between Frankliniella occidentalis and F. intonsa (Thysanoptera: Thripidae) in laboratory assays. Fla. Entomol. 2019, 102, 322–328. [Google Scholar] [CrossRef]
- Helyer, N.L.; Brobyn, P.J. Chemical control of Western Flower Thrips (Frankliniella occidentalis Pergande). Ann. Appl. Biol. 1992, 121, 219–231. [Google Scholar] [CrossRef]
- Riudavets, J. Predators of Frankliniella occidentalis and Thrips tabaci: A review. Wagening. Agric. Univ. Pap. 1995, 95, 46–78. [Google Scholar]
- MacIntyre Allen, J.K.; Scott-Dupree, C.D.; Tolman, J.H.; Harris, C.R. Resistance of Thrips tabaci to pyrethroid and organophosphorus insecticides in Ontario, Canada. Pest Manag. Sci. 2005, 61, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Frescata, C.; Mexia, A. Biological control of thrips (Thysanoptera) by Orius laevigatus (Heteroptera: Anthocoridae) in organically-grown strawberries. Biol. Agric. Hortic. 1996, 13, 141–148. [Google Scholar] [CrossRef]
- Xu, X.; Borgemeister, C.; Poehling, H.M. Interactions in the biological control of Western Flower Thrips Frankliniella occidentalis (Pergande) and Two-Spotted Spider Mite Tetranychus urticae Koch by the predatory bug Orius insidiosus say on beans. Biol. Control 2006, 36, 57–64. [Google Scholar] [CrossRef]
- Messelink, G.J.; Van Steenpaal, S.E.F.; Ramakers, P.M.J. Evaluation of phytoseiid predators for control of Western Flower Thrips on greenhouse cucumber. BioControl 2006, 51, 753–768. [Google Scholar] [CrossRef]
- Messelink, G.J.; van Maanen, R.; van Steenpaal, S.E.F.; Janssen, A. Biological control of thrips and whiteflies by a shared predator: Two pests are better than one. Biol. Control 2008, 44, 372–379. [Google Scholar] [CrossRef]
- Mehle, N.; Trdan, S. Traditional and modern methods for the identification of thrips (Thysanoptera) species. J. Pest Sci. 2012, 85, 179–190. [Google Scholar] [CrossRef]
- Silveira, L.C.P.; Haro, M.M. Fast slide preparation for thrips (Thysanoptera) routine identifications. Eur. J. Entomol. 2016, 113, 403–408. [Google Scholar] [CrossRef]
- Walter, D.; Krantz, G. Collectiong, rearing, and preparing specimens. In A Manual of Acarology, 2nd ed.; Texas Tech University: Texas, TX, USA, 2009; pp. 83–96. [Google Scholar]
- Mound, L.; Collins, D.; Hastings, A. Thysanoptera Britannica et Hibernica-Thrips of the British Isles. Available online: https://keys.lucidcentral.org/keys/v3/british_thrips/the_key/britishthysanoptera_2017.html (accessed on 1 February 2019).
- Trdan, S.; Andjus, L.; Raspudić, E.; Kač, M. Distribution of Aeolothrips intermedius Bagnall (Thysanoptera: Aeolothripidae) and its potential prey Thysanoptera species on different cultivated host plants. J. Pest Sci. 2005, 78, 217–226. [Google Scholar] [CrossRef]
- Stopar, K.; Trdan, S.; Bartol, T. Thrips and natural enemies through text data mining and visualization. Plant Prot. Sci. 2020, 57, 47–58. [Google Scholar] [CrossRef]
- Brunner, P.; Flemming, C.; Frey, J. A molecular identification key for economically important thrips species (Thysanoptera: Thripidae) using direct sequencing and a PCR-RFLP-based approach. Agric. For. Entomol. 2002, 4, 127–136. [Google Scholar] [CrossRef]
- Yeh, W.B.; Tseng, M.J.; Chang, N.T.; Wu, S.Y.; Tsai, Y.S. Agronomically important thrips: Development of species-specific primers in multiplex PCR and microarray assay using internal transcribed spacer 1 (ITS1) sequences for identification. Bull. Entomol. Res. 2015, 105, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Taberlet, P.; Bonin, A.; Zinger, L.; Coissac, E. Single-species detection. In Environmental DNA: For Biodiversity Research and Monitoring, 1st ed.; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Tuovinen, T.; Lindqvist, I. Effect of introductions of a predator complex on spider mites and thrips in a tunnel and an open field of pesticide-free everbearer strawberry. J. Berry Res. 2014, 4, 203–216. [Google Scholar] [CrossRef]
- Lim, U.T.; Mainali, B.P. Optimum density of chrysanthemum flower model traps to reduce infestations of Frankliniella intonsa (Thysanoptera: Thripidae) on greenhouse strawberry. Crop Prot. 2009, 28, 1098–1100. [Google Scholar] [CrossRef]
- Mintu, C.; Reyes, C.P. Frankliniella intonsa (Trybom), a thrips species infesting strawberry in La Trinidad, Benguet Province, Philippines. Asia Life Sci. 2018, 27, 377–383. [Google Scholar]
- Atakan, E. Population densities and distributions of the Western Flower Thrips (Thysanoptera: Thripidae) and its predatory bug, Orius niger (Hemiptera: Anthocoridae), in strawberry. Int. J. Agric. Biol. 2011, 13, 638–644. [Google Scholar]
- Upton, M. Aqueous gum-chloral slide mounting media: A historical review. Bull. Entomol. Res. 1993, 83, 267–274. [Google Scholar] [CrossRef]
- Kobayashi, T. Regeneration of old slide specimens mounted in Hoyer’s medium. Chronomus J. 2013, 1. [Google Scholar] [CrossRef][Green Version]
- Raspudić, E.; Ivezić, M.; Brmež, M.; Trdan, S. Distribution of Thysanoptera species and their host plants in Croatia. Acta Agric. Slov. 2009, 93, 275–283. [Google Scholar] [CrossRef][Green Version]
- Atakan, E.; Uygur, S. Winter and spring abundance of Frankliniella spp. and Thrips tabaci Lindeman (Thysan., Thripidae) on weed host plants in Turkey. J. Appl. Entomol. 2005, 129, 17–26. [Google Scholar] [CrossRef]
- Orosz, S. Investigation of Thysanoptera Populations in Sweet Pepper Greenhouses and in Their Surroundings. Ph.D. Thesis, Szent István University, Budapest, Hungary, 2012. [Google Scholar]
- Brown, S.; Bennison, J.; Boxworth, A. Determining the Threat of Rose Thrips (Thrips fuscipennis) in UK Strawberry Crops. Report from AHDB. 2018, pp. 1–28. Available online: https://www.linkedin.com/authwall?trk=gf&trkInfo=AQEOlTEmRQybDwAAAXfxku0Qt6RYm8eE85v7Bzr98x83Z_JKEoqS1t83RqWztl7JJHQ4vaIrF_fUwiu05J_jz_m2CgWlcZ8bazzLYGHfrVRpgtm4VMwcgARyTciF8za-BIYcEx0=&originalReferer=&sessionRedirect=https%3A%2F%2Fuk.linkedin.com%2Fpublic-profile%2Fin%2Fsam-brown-1873ab12b%3FchallengeId%3DAQG-Ny0GZ4yZzQAAAXfxMn20efHLtHctJGaX11eEXByRYMwtpGsZCiEa7iz4englsrE4DsF2r-b-S1KqBfYOnNEM0oR39rhsEg%26submissionId%3D89eaffa1-206c-6816-8465-ff2634451651 (accessed on 18 January 2021).
- Sampson, C.; Kirk, W.D.J. Can mass trapping reduce thrips damage and is it economically viable? Management of the Western Flower Thrips in strawberry. PLoS ONE 2013, 8, 238–245. [Google Scholar] [CrossRef]
- Sampson, C. Management of the Western Flower Thrips on Strawberry. Ph.D. Thesis, Keele University, Keele, UK, 2014. [Google Scholar]
- Brødsgaard, H. Cold hardiness and tolerance to submergence in water in Frankliniella occidentalis. Environ. Entomol. 1993, 22, 647–653. [Google Scholar] [CrossRef]
- Factsheet from Norsk Landbruksrådgivning; Jordbærsorter: Ås, Norway, 2016; pp. 1–12.
- Crawley, R.; Pattrasudhi, R. Interspecific competition between insect herbivores: Asymmetric competition between Cinnabar Moth and the Ragwort Seed-Head Fly. Ecolo. Entomol. 1988, 13, 243–249. [Google Scholar] [CrossRef]
- Denno, R.F.; McClure, M.S.; Ott, J.R. Interspecific interactions in phytophagous insects-competiton reexamined and resurrected. Ann. Rev. Entomol. 1995, 40, 297–331. [Google Scholar] [CrossRef]
- Sampson, C.; Kirk, W. Flower stage and position affect population estimates of the Western Flower Thrips, Frankliniella occidentalis (Pergande), in strawberry. Acta Phytopathol. Entomol. Hung. 2012, 47, 133–139. [Google Scholar] [CrossRef]
- Leis, M.; Martinelli, A. Plant Patent on the Strawberry Cultivar Murano. U.S. Patent US PP25,070 P3, 1–4 November 2014. [Google Scholar]
- Rahman, T.; Spafford, H.; Broughton, S. Variation in preference and performance of Frankliniella occidentalis (Thysanoptera: Thripidae) on three strawberry cultivars. J. Econ. Entomol. 2010, 103, 1744–1753. [Google Scholar] [CrossRef]
- Rahman, T.; Broughton, S.; Spafford, H. Effect of spinosad and predatory mites on control of Frankliniella occidentalis in three strawberry cultivars. Entomol. Exp. Appl. 2011, 138, 154–161. [Google Scholar] [CrossRef]
- Ayalew, G.; Sciarretta, A.; Baumgärtner, J.; Ogol, C.; Löhr, B. Spatial distribution of Diamondback Moth, Plutella xylostella L. (Lepidoptera: Plutellidae), at the field and the regional level in Ethiopia. Int. J. Pest Manag. 2008, 54, 31–38. [Google Scholar] [CrossRef]
- Labou, B.; Brévault, T.; Sylla, S.; DIatte, M.; Bordat, D.; DIarra, K. Spatial and temporal incidence of insect pests in farmers’ cabbage fields in Senegal. Int. J. Trop. Insect Sci. 2017, 37, 225–233. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).