Shifts in Ecological Dominance between Two Lepidopteran Species in Refuge Areas of Bt Cotton
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
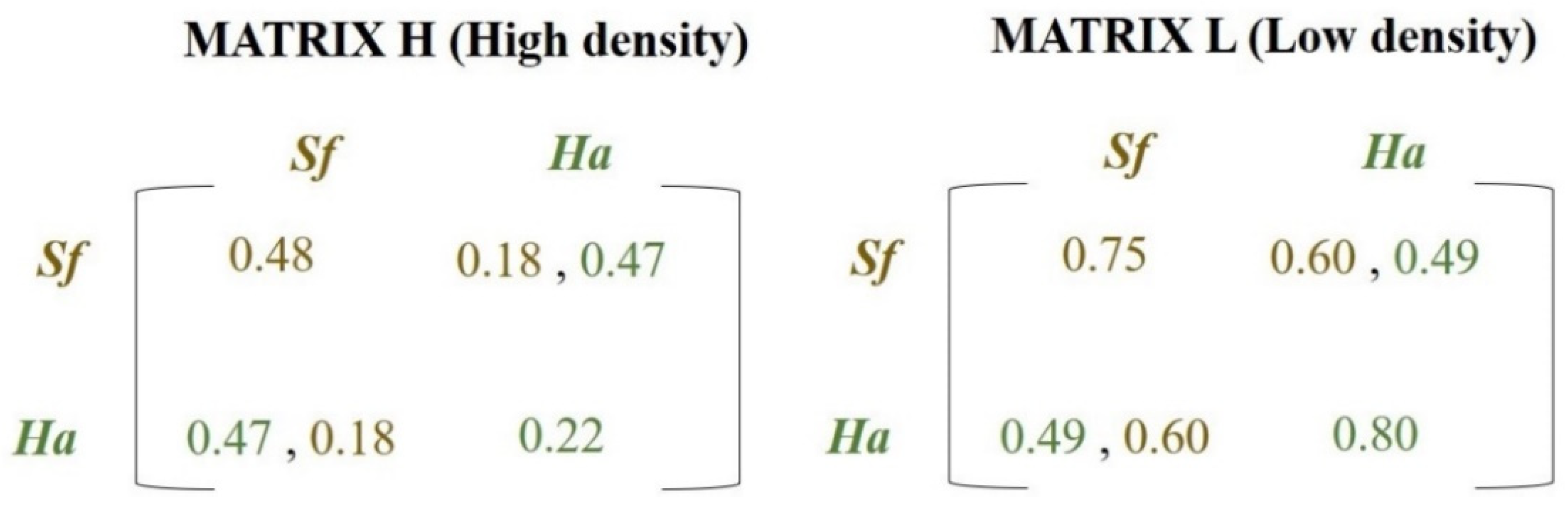
2.1. Intra- and Interspecific Competition in the Field
2.2. Computer Simulation
2.2.1. Description of the Proposed Model
- Non-adoption of insecticides.
- Adoption of insecticides without cases of resistance.
- Adoption of insecticides with population resistance of S. frugiperda to the used insecticide.
- Adoption of insecticides with population resistance of H. armigera to the used insecticide.
- Adoption of insecticides with population resistance of S. frugiperda and H. armigera to the used insecticide.
2.2.2. Parameter Sensitivity Analysis
3. Results
3.1. Intra- and Interspecific Competition in the Field
3.2. Computer Simulation
3.3. Sensitivity Analysis
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coulson, T.; Godfray, H.C.J. Single-species dynamics. In Theoretical Ecology: Principles and Applications; Chapter 3; May, R.M., McLean, A.R., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 17–34. [Google Scholar]
- Lang, J.M.; Benbow, M.E. Species interactions and competition. Nat. Educ. Knowl. 2013, 4, 8. [Google Scholar]
- Carrara, F.; Giometto, A.; Seymour, M.; Rinaldo, A.; Altermatt, F. Inferring species interactions in ecological communities: A comparison of methods at different levels of complexity. Methods Ecol. Evol. 2015, 6, 895–906. [Google Scholar] [CrossRef]
- Chu, C.; Mu, C.; Liu, J.; Liu, C.; Liu, C.; Boccaletti, S.; Shi, L.; Wang, Z. Aspiration-based coevolution of node weights promotes cooperation in the spatial prisoner’s dilemma game. New J. Phys. 2019, 21, 063024. [Google Scholar] [CrossRef]
- Flores, J.C. Game theory approach to sterile release populations and replicator dynamics: Niche fragmentation and resilience. Physical A 2020, 124212. [Google Scholar] [CrossRef]
- Easley, D.; Kleinberg, J. “Games”. Networks, Crowds, and Markets: Reasoning about a Highly Connected World; Cambridge University Press: Cambridge, UK, 2010; pp. 155–208. [Google Scholar]
- Clements, K.C.; David, W.S. Testing models of non-kin cooperation: Mutualism and the prisoner’s dilemma. Anim. Behav. 1995, 50, 527–535. [Google Scholar] [CrossRef]
- Brown, J.S.; Staňková, K. Game theory as a conceptual framework for managing insect pests. Curr. Opin. Insect Sci. 2017, 21, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Bshary, R.; Oliveira, R.F. Cooperation in animals: Toward a game theory within the framework of social competence. Curr. Opin. Behav. Sci. 2015, 3, 31–37. [Google Scholar] [CrossRef]
- Dourado, P.M.; Bacalhau, F.B.; Amado, D.; Carvalho, R.A.; Martinelli, S.; Head, G.P.; Omoto, C. High susceptibility to Cry1Ac and low resistance allele frequency reduce the risk of resistance of Helicoverpa armigera to Bt soybean in Brazil. PLoS ONE 2016, 11, e0161388. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Gassmann, A.J.; Crowder, D.W.; Carrière, Y. Insect resistance to Bt crops: Evidence versus theory. Nat. Biotechnol. 2008, 26, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Carrière, Y.; Brown, Z.S.; Downes, S.J.; Gujar, G.; Epstein, G.; Omoto, C.; Storer, N.P.; Mota-Sanchez, D.; Søgaard Jørgensen, P.; Carroll, S.P. Governing evolution: A socio-ecological comparison of resistance management for insecticidal transgenic Bt crops among four countries. AMBIO 2020, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Head, G.; Dennehy, T. Insect resistance management for transgenic Bt cotton. In Cotton; Springer: Berlin/Heidelberg, Germany, 2010; pp. 113–125. [Google Scholar]
- Andow, D.A.; Hutchison, W.D. Bt corn resistance management. In Now or Never: Serious New Plans to Save a Natural Pest Control; Union of Concerned Scientists, Ed.; Two Brattle Square: Cambridge, MA, USA, 1998; pp. 19–66. [Google Scholar]
- Horikoshi, R.J.; Bernardi, D.; Bernardi, O.; Malaquias, J.B.; Okuma, D.M.; Miraldo, L.L.; Amaral, F.S.A.; Omoto, C. Effective dominance of resistance of Spodoptera frugiperda to Bt maize and cotton varieties: Implications for resistance management. Sci. Rep. 2016, 6, 34864. [Google Scholar] [CrossRef]
- Farias, J.R.; Andow, D.A.; Horikoshi, R.J.; Sorgatto, R.J.; Fresia, P.; dos Santos, A.C.; Omoto, C. Field-evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot. 2014, 64, 150–158. [Google Scholar] [CrossRef]
- Omoto, C.; Bernardi, O.; Salmeron, E.; Sorgatto, R.J.; Dourado, P.M.; Crivellari, A.; Carvalho, R.A.; Wilsse, A.; Martinelli, S.; Head, G.P. Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manag. Sci. 2016, 72, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, D.; Salmeron, E.; Horikoshi, R.J.; Bernardi, O.; Dourado, P.M.; Martinelli, S.; Head, G.; Omoto, C. Cross-resistance between Cryproteins in fall armyworm (Spodoptera frugiperda) may affect the durability of current pyramided Bt maize hybrids in Brazil. PLOS ONE 2015, 10, e0140130. [Google Scholar] [CrossRef] [PubMed]
- Leite, N.A.; Pereira, R.M.; Durigan, M.R.; Amado, D.; Fatoretto, J.; Medeiros, F.C.L.; Omoto, C. Susceptibility of Brazilian populations of Helicoverpa armigera and Helicoverpa zea (Lepidoptera: Noctuidae) to Vip3Aa20. J. Econ. Entomol. 2018, 111, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Malaquias, J.B.; Ramalho, F.S.; Omoto, C.; Godoy, W.A.C.; Silveira, R.F. Imidacloprid affects the functional response of predator Podisus nigrispinus (Dallas) (Heteroptera: Pentatomidae) to strains of Spodoptera frugiperda (JE Smith) on Bt cotton. Ecotoxicology 2014, 23, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Malaquias, J.B.; Omoto, C.; Ramalho, F.D.S.; Wesley, W.A.C.; Silveira, R.F. Bt cotton and the predator Podisus nigrispinus (Dallas) (Heteroptera: Pentatomidae) in the management of Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) resistance to lambda-cyhalothrin. J. Pest Sci. 2015, 88, 57–63. [Google Scholar] [CrossRef]
- Pereira, R.M.; Neto, D.A.; Amado, D.; Durigan, M.R.; Franciscatti, R.A.; Mocheti, M.; Omoto, C. Baseline susceptibility and frequency of resistance to diamide insecticides in Helicoverpa armigera (Lepidoptera: Noctuidae) populations in Brazil. Crop Prot. 2020, 137, 105266. [Google Scholar] [CrossRef]
- Bolzan, A.; Padovez, F.E.; Nascimento, A.R.; Kaiser, I.S.; Lira, E.C.; Amaral, F.S.; Kanno, R.H.; Malaquias, J.B.; Omoto, C. Selection and characterization of the inheritance of resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to chlorantraniliprole and cross-resistance to other diamide insecticides. Pest Manag. Sci. 2019, 75, 2682–2689. [Google Scholar] [CrossRef]
- Bentivenha, J.P.F.; Baldin, E.L.L.; Montezano, D.G.; Hunt, T.E.; Paula-Moraes, S.V. Attack and defense movements involved in the interaction of Spodoptera frugiperda and Helicoverpa zea (Lepidoptera: Noctuidae). J. Pest Sci. 2016, 90, 433–445. [Google Scholar] [CrossRef]
- Wise, D.H. Cannibalism, food limitation, intraspecific competition, and the regulation of spider populations. Annu. Rev. Entomol. 2006, 51, 441–465. [Google Scholar] [CrossRef]
- Malaquias, J.B.; Caprio, M.A.; Godoy, W.A.C.; Omoto, C.; Ramalho, F.S.; Pachú, J.K.S. Experimental and theoretical landscape influences on Spodoptera frugiperda movement and resistance evolution in contaminated refuge areas of Bt cotton. J. Pest Sci. 2020, 93, 329–340. [Google Scholar] [CrossRef]
- Turchin, P. Quantitative Analysis of Movement: Measuring and Modeling Population Redistribution in Animals and Plants; Sinauer Associates: Sunderland, MA, USA, 1998; 396p. [Google Scholar]
- Ferreira, I.E.P.; Moral, R.A.; Ferreira, C.P.; Godoy, W.A.C. Modelling fungus dispersal scenarios using cellular automata. Ecol. Inform. 2013, 14, 53–58. [Google Scholar] [CrossRef]
- Burtet, L.M.; Bernardi, O.; Melo, A.A.; Pes, M.P.; Strahl, T.T.; Guedes, J.V.C. Managing fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), with Bt maize and insecticides in southern Brazil. Pest Manag. Sci. 2017, 73, 2569–2577. [Google Scholar] [CrossRef]
- Kasten, P., Jr.; Precetti, A.A.C.M.; Parra, J.R.P. Dados biológicos comparativos de Spodoptera frugiperda (J.E. Smith) em duas dietas artificiais e substrato natural. Rev. Agric. 1797, 53, 68–78. [Google Scholar]
- Embrapa-Empresa Brasileira de Pesquisa Agropecuária. Sistema Brasileiro de Classificação de Solos; Embrapa: Rio de Janeiro, Brazil, 2006; 306p. [Google Scholar]
- Moral, R.A.; Hinde, J.; Demétrio, C.G.B. Half-normal plots and overdispersed models. In R: The hnp Package. J. Stat. Softw. 2017, 81, 1–23. [Google Scholar] [CrossRef]
- Gomes, E.S.; Santos, V.; Ávila, C.J. Biology and fertility life table of Helicoverpa armigera (Lepidoptera: Noctuidae) in different hosts. Entomol. Sci. 2017, 20, 419–426. [Google Scholar] [CrossRef]
- Barros, E.M.; Torres, J.B.; Bueno, A.F. Oviposição, desenvolvimento e reprodução de Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) em diferentes hospedeiros de importância econômica. Neotrop. Entomol. 2010, 39, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Varella, A.C.; Menezes-Netto, A.C.; Alonson, J.D.S.; Caixeta, D.F.; Peterson, R.K.D.; Fernandes, O.A. Mortality dynamics of Spodoptera frugiperda (Lepidoptera: Noctuidae) immatures in maize. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Lamboni, M.; Makowski, D.; Lehuger, S.; Gabrielle, B.; Monod, H. Multivariate global sensitivity analysis for dynamic crop models. Field Crop. Res. 2009, 113, 312–320. [Google Scholar] [CrossRef]
- Bidot, C.; Lamboni, M.; Monod, H. Multisensi: Multivariate Sensitivity Analysis. R Package Version 2.1-1. Available online: https://CRAN.R-project.org/package=multisensi (accessed on 1 July 2018).
- Raffa, K.F. Effect of host plant on cannibalism rates by fall armyworm (Lepidoptera: Noctuidae) larvae. Environ. Entomol. 1987, 16, 672–675. [Google Scholar] [CrossRef]
- Chapman, J.W.; Williams, T.; Escribano, A.; Caballero, P.; Cave, R.D.; Goulson, D. Fitness consequences of cannibalism in the fall armyworm. Spodoptera Frugiperda Behav. Ecol. 1999, 10, 298–303. [Google Scholar] [CrossRef]
- Horner, T.A.; Dively, G.P.; Herbert, D.A. Development, survival and fitness performance of Helicoverpa zea (Lepidoptera: Noctuidae) in MON-Bt field corn. J. Econ. Entomol. 2003, 96, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Chilcutt, C.F. Cannibalism of Helicoverpa zea (Lepidoptera: Noctuidae) from Bacillus thuringiensis (Bt) transgenic corn versus non-Bt corn. J. Econ. Entomol. 2006, 99, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Dively, G.P.; Huang, F.; Oyediran, I.; Burd, T.; Morsello, S. Evaluation of gene fow in structured and seed blend refuge systems of non-Bt and Bt corn. J. Pest Sci. 2020, 93, 439–447. [Google Scholar] [CrossRef]
- SAP (FIFRA Scientific Advisory Panel). Final Report of the FIFRA Scientific Advisory Panel Subpanel on Bacillus thuringiensis (Bt) Plant-Pesticides and Resistance Management. Docket No. OPPTS-00231; 1998; Volume 59, pp. 1–59. Available online: https://archive.epa.gov/scipoly/sap/meetings/web/pdf/finalfeb.pdf (accessed on 2 December 2020).
- EPA (U.S. Environmental Protection Agency). Bt Plant-Pesticides Biopesticides Registration Action Document; EPA: Washington, DC, USA, 2000; 106p. Available online: http://www.epa.gov/aphome/lawregs.htm (accessed on 2 December 2020).
- Nansen, C.; Ridsdill-Smith, T.J. The performance of insecticides—A critical review. In Insecticides; Trdan, S., Ed.; InTech Europe: Rijeka, Croatia, 2013; pp. 195–232. [Google Scholar] [CrossRef]
- Courchamp, F.; Clutton-Brock, T.; Grenfell, B. Inverse density dependence and the Allee effect. TREE 1999, 14, 405–410. [Google Scholar] [CrossRef]
- Téllez-Rodríguez, P.; Raymond, B.; Morán-Bertot, I.; Rodríguez-Cabrera, L.; Wright, D.J.; Borroto, C.G.; Ayra-Pardo, C. Strong oviposition preference for Bt over non-Bt maize in Spodoptera frugiperda and its implications for the evolution of resistance. BMC Biol. 2014, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Pachú, J.K.; Macedo, F.C.; da Silva, F.B.; Malaquias, J.B.; Ramalho, F.S.; Oliveira, R.F.; Godoy, W.A. Imidacloprid-mediated stress on non-Bt and Bt cotton, aphid and ladybug interaction: Approaches based on insect behaviour, fluorescence, dark respiration and plant electrophysiology. Chemosphere 2021, 263, 127561. [Google Scholar]




| Parameter | Description | Value-S 1 (Value-AS) 2 | Reference |
|---|---|---|---|
| AE | Adult emergency | 0.86 (0.65–1.00) | |
| Ʊ | Encounter rate between species 1 and 2 | * | - |
| T | Threshold for defining the state (high or low) of density | 0.50 (***) | ** |
| CL | Threshold for decision making for insecticide control | 0.2000 (0.15–0.25) | - |
| RoHa | Reproductive capacity (neonates/female) of Helicoverpa armigera | 725.06 (543.80–965.33) | Gomes et al. [33] |
| RoSf | Reproductive capacity (neonates/female) of Spodoptera frugiperda | 400.00 (300.00–500.00) | Barros et al. [34] |
| WHa | Relative fitness of H. armigera | * | - |
| NM | Probability associated with mortality factors # | 0.25 (0.19–0.31) | Varella et al. [35] |
| WSf | Relative fitness of S. frugiperda | * | - |
| IF | Initial number (absolute frequency) of H. armigera and S. frugiperda | 500 (375–625) | |
| α11 | Survival of S. frugiperda in intraspecific competition at low densities | 0.63 (0.47–0.79) | **** |
| α12 | Survival of S. frugiperda in intra- and interspecific competition at low densities | 0.58 (0.44–0.73) | **** |
| α21 | Survival of H. armigera in intraspecific competition at low densities | 0.46 (0.35–0.58) | **** |
| α22 | Survival of H. armigera in intra- and interspecific competition at low densities | 0.90 (0.68–1.00) | **** |
| β11 | Survival of S. frugiperda in intraspecific competition at high densities | 0.31 (0.23–0.39) | **** |
| β12 | Survival of S. frugiperda in intra- and interspecific competition at high densities | 0.11 (0.08–0.14) | **** |
| β21 | Survival of H. armigera in intraspecific competition at high densities | 0.38 (0.29–0.48) | **** |
| β22 | Survival of H. armigera in intra- and interspecific competition at high densities | 0.16 (0.12–0.18) | **** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaquias, J.B.; Santana, D.R.S.; Degrande, P.E.; Ferreira, C.P.; de Melo, E.P.; Godoy, W.A.C.; Pachú, J.K.d.S.; de Sousa Ramalho, F.; Omoto, C.; de Azevedo Pereira, A.I.; et al. Shifts in Ecological Dominance between Two Lepidopteran Species in Refuge Areas of Bt Cotton. Insects 2021, 12, 157. https://doi.org/10.3390/insects12020157
Malaquias JB, Santana DRS, Degrande PE, Ferreira CP, de Melo EP, Godoy WAC, Pachú JKdS, de Sousa Ramalho F, Omoto C, de Azevedo Pereira AI, et al. Shifts in Ecological Dominance between Two Lepidopteran Species in Refuge Areas of Bt Cotton. Insects. 2021; 12(2):157. https://doi.org/10.3390/insects12020157
Chicago/Turabian StyleMalaquias, José Bruno, Danilo Renato Santiago Santana, Paulo Eduardo Degrande, Claudia Pio Ferreira, Elmo Pontes de Melo, Wesley Augusto Conde Godoy, Jéssica Karina da Silva Pachú, Francisco de Sousa Ramalho, Celso Omoto, Alexandre Igor de Azevedo Pereira, and et al. 2021. "Shifts in Ecological Dominance between Two Lepidopteran Species in Refuge Areas of Bt Cotton" Insects 12, no. 2: 157. https://doi.org/10.3390/insects12020157
APA StyleMalaquias, J. B., Santana, D. R. S., Degrande, P. E., Ferreira, C. P., de Melo, E. P., Godoy, W. A. C., Pachú, J. K. d. S., de Sousa Ramalho, F., Omoto, C., de Azevedo Pereira, A. I., & Guazina, R. A. (2021). Shifts in Ecological Dominance between Two Lepidopteran Species in Refuge Areas of Bt Cotton. Insects, 12(2), 157. https://doi.org/10.3390/insects12020157







