Distribution of Two Strains of Leptoglossus zonatus (Dallas) (Hemiptera: Coreidae) in the Western Hemisphere: Is L. zonatus a Potential Invasive Species in California?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Sample Collection, DNA Extraction and Sequencing
2.2. Phylogenetic and Genetic Diversity Analyses
3. Results
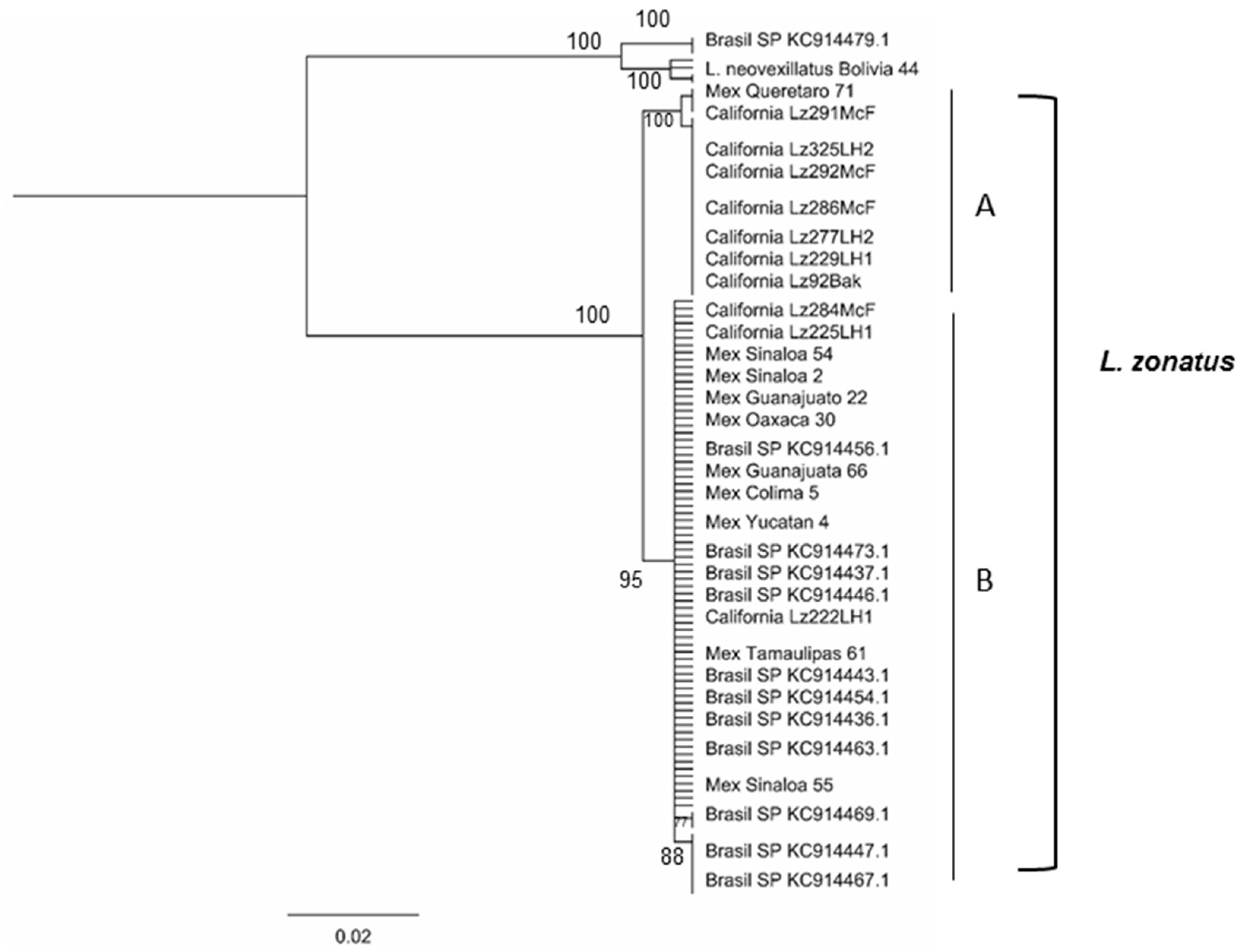
3.1. Phylogenetic Trees
3.2. Haplotype Determination, Haplotype and Nucleotide Diversity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brailovsky, H. Illustrated key for identification of the species included in the genus Leptoglossus (Hemiptera: Heteroptera: Coreidae: Coreinae: Anisoscelini), and descriptions of five new species and new synonyms. Zootaxa 2014, 3794, 143–178. [Google Scholar] [CrossRef] [Green Version]
- Allen, R.C. A revision of the genus Leptoglossus Guerin (Hemiptera: Coreidae). Entomol. Am. 1969, 45, 35–140. [Google Scholar]
- Essig, E.O. Insects and Mites of Western North America; McMillan Press: New York, NY, USA, 1958. [Google Scholar]
- Michailides, T.J. The Achilles heel of pistachio fruit. Calif. Agr. 1989, 43, 10–11. [Google Scholar]
- Panizzi, A.R. Desempenho de ninfas e adultos de Leptoglossus zonatus Dallas (Hemiptera: Coreidae) em diferentes alimentos. An. Soc. Entomol. Brasil 1989, 18, 375–389. [Google Scholar] [CrossRef]
- Matrangolo, W.J.R.; Waquil, J.M. Biologia de Leptoglossus zonatus (Dallas, 1852) (Hemiptera: Coreidae) alimentados com milho e sorgo. An. Soc. Entomol. Brasil 1994, 23, 419–423. [Google Scholar] [CrossRef]
- Mitchell, P.L. Leaf-footed bugs (Coreidae). In Heteroptera of Economic Importance; Schaefer, C.W., Panizzi, A.R., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 337–404. [Google Scholar]
- Daane, K.M.; Yokota, G.Y.; Krugner, R.; DaSilva, P.G.; Steffan, S.A.; Beede, R.H.; Bentley, W.J.; Weinberger, G.B. Large bug damage to San Joaquin Valley pistachios. Calif. Agr. 2005, 59, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.G.; Fadamiro, H.Y. Evaluation of damage to satsuma mandarin (Citrus unshiu) by the leaffooted bug, Leptoglossus zonatus (Hemiptera: Coreidae). J. Appl. Entomol. 2010, 134, 694–703. [Google Scholar] [CrossRef]
- Joyce, A.L.; Higbee, B.S.; Haviland, D.R.; Brailovsky, H. Genetic variability of two leaffooted bugs, Leptoglossus clypealis and Leptoglossus zonatus (Hemiptera: Coreidae), in the Central Valley of California. J. Econ. Entomol. 2017, 110, 2576–2589. [Google Scholar] [CrossRef]
- Esquivel, J.F.; JGlover, P.; Brewer, M.J.; Helms, A.M.; Ree, W.O.; Shirley, X.A.; Bell, A.A. Expansion of geographical range and plant associations of Leptoglossus clypealis: A potential invasive pest of sorghum along the Texas Gulf Coast. Southwest. Entomol. 2020, 45, 1–16. [Google Scholar] [CrossRef]
- Joyce, A.L.; Barman, A.K.; Doll, D.; Higbee, B.S. Assessing feeding damage from two leaffooted bugs Leptoglossus clypealis Heidemann and L. zonatus (Dallas) (Hemiptera: Coreidae), on four almond varieties. Insects 2019, 10, 333. [Google Scholar] [CrossRef] [Green Version]
- Rhijal, J.P.; Joyce, A.L.; Gyawaly, S. Biology, ecology and management of Hemiptera pests in almond orchards in the United States. J. Integr. Pest Manag. 2021, 12, 24. [Google Scholar] [CrossRef]
- California Department of Food and Agriculture (CDFA). 2018 California Almond Acreage Report; CDFA: Sacramento, CA, USA, 2019.
- Rhijal, J.; Gyawaly, S. Characterizing brown marmorated stink bug injury in almond, a new host crop in California. Insects 2018, 9, 126. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.S.; Ma, G.; Pinchebourde, S. Surviving a warming climate. Insect responses to extreme high temperatures. Ann. Rev. Entomol. 2021, 66, 163–184. [Google Scholar] [CrossRef]
- Guo, C.L.; Zhu, Y.Z.; Zhang, Y.J.; Keller, M.A.; Liu, T.X.; Chu, D. Invasion biology and management of sweet potato whitefly (Hemiptera: Aleyrodidae) in China. J. Integr. Pest Manag. 2021, 12, 2. [Google Scholar] [CrossRef]
- Heidemann, O. New species of Leptoglossus from North America. Proc. Entomol. Soc. Wash. 1910, 12, 191–197. [Google Scholar]
- Henne, D.C.; Johnson, S.J.; Bourgeois, W.J. Pest status of leaf-footed bugs (Heteroptera: Coreidae) on citrus in Louisiana. Proc. Annu. Meet. Fla. State Hortic. Society 2003, 116, 240–241. [Google Scholar]
- Buss, L.; Halbert, S.; Johnson, S. Leptoglossus zonatus—A New Leaf-Footed Bug in Florida (Hemiptera: Coreidae). FDACS-DPI Pest Alerts. Available online: http://www.doacs.state.fl.us/pi/enpp/ento/l.zonatus.html (accessed on 20 October 2021).
- Ramirez, M.M. Analisis Filogenetico y Panbiogeofraphico de los Grupos de Especies de Genero Leptoglossus Guerin (Hemiptera: Heteroptera: Coreidae Anisoscelini). Master’s Thesis, Universidad Autonoma de Mexico, Ciudad de Mexico, Mexico, 2012; 103p. [Google Scholar]
- Park, D.S.; Foottit, R.; Maw, E.; Hebert, P.D.N. Barcoding Bugs: DNA-based identification of the True bugs (Insecta: Hemiptera: Heteroptera). PLoS ONE 2011, 6, e18749. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.P.; Hardy, T.N.; Hammond, A.M.; Mihm, J.A. Genetic evidence for sibling species within the sugarcane borer (Lepidoptera: Pyralidae). Ann. Entomol. Soc. Amer. 1990, 83, 1048–1053. [Google Scholar] [CrossRef]
- Scheffer, S.J.; Lewis, M.L. Two nuclear genes confirm mitochondrial evidence of cryptic species within Liriomyza huidobrensis (Diptera: Agromyzidae). Ann. Entomol Soc. Am. 2001, 94, 648–653. [Google Scholar] [CrossRef] [Green Version]
- Hebert, P.D.N.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef] [Green Version]
- Groot, A.T.; Marr, M.; Schofl, G.; Lorenz, S.; Svatos, A.; Heckel, D.G. Host strain specific sex pheromone variation in Spodoptera frugiperda. Front. Zool. 2008, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Hartfield, E.A.; Harris, M.K.; Medina, R.F. Searching for pheromone strains in the pecan nut casebearer. Entomol. Exper. Appl. 2010, 137, 11–18. [Google Scholar] [CrossRef]
- Franco-Archundia, S.L.; Gonzaga-Segura, A.J.; Jiménez-Pérez, A.; Castrejón-Gómez, V.R. Behavioral Response of Leptoglossus zonatus (Heteroptera: Coreidae) to Stimuli Based on Colors and its Aggregation Pheromone. Insects 2018, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Medina, R.F.; Armstrong, S.J.; Harrison, K. Genetic population structure of sugarcane aphid, Melanaphis sacchari, in sorghum, sugarcane, and Johnsongrass in the continental USA. Entomol. Exp. Appl. 2017, 162, 358–365. [Google Scholar] [CrossRef]
- Nibouche, S.; Costet, L.; Holt, J.R.; Jacobson, A.; Pekarcik, A.; Sadeyen, J.; Armstrong, J.S.; Peterson, G.C.; McLaren, N.; Medina, R.F. Invasion of sorghum in the Americas by a new sugarcane aphid (Melanaphis sacchari) superclone. PLoS ONE 2018, 13, e0196124. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Kandemir, I.; Walsh, D.B.; Zalom, F.G.; Lavine, L.C. Identification of Lygus hesperus by DNA Barcoding Reveals Insignificant Levels of Genetic Structure among Distant and Habitat Diverse Populations. PLoS ONE 2012, 7, e34528. [Google Scholar] [CrossRef] [Green Version]
- Gariepy, T.D.; Bruin, A.; Haye, T.; Milonas, P.; Vetek, G. Occurrence and genetic diversity of new populations of Halyomorpha halys in Europe. J. Pest. Sci. 2015, 88, 451–460. [Google Scholar] [CrossRef]
- Lesieur, V.; Lombaert, E.; Guillemaud, T.; Courtial, B.; Strong, W.; Roques, A.; Auger-Rozenberg, M.-A. The rapid spread of Leptoglossus occidentalis in Europe: A bridgehead invasion. J. Pest Sci. 2019, 92, 189–200. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, S.; Stones-Havas, M.; Cheung, S.; Sturrock, S.; Buxton, A.; Cooper, S.; Markowitz, C.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Joyce, A.L.; White, W.H.; Nuessly, G.S.; Solis, M.A.; Scheffer, S.J.; Lewis, M.L.; Medina, R.F. Geographic population structure of the sugarcane borer, Diatraea saccharalis (F.) (Lepidoptera: Crambidae), in the southern United States. PLoS ONE 2014, 9, e110036. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Librado, P.; Rozas, J. Dnasp v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2005, 25, 1451–1452. [Google Scholar] [CrossRef] [Green Version]
- Leigh, J.W.; Bryant, D. POPART: Full feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Wheeler, A.G. Leptoglossus clypealis Heidemann (Hemiptera: Coreidae): Eastward spread in North America, new host records, and evaluation of host range. Proc. Entomol. Soc. Wash. 2018, 120, 196–210. [Google Scholar] [CrossRef] [Green Version]
- McPherson, J.E.; Packauskas, R.J.; Taylor, S.J.; O’Brien, M.F. Eastern range extension of Leptoglossus occidentalis with a key to Leptoglossus species of America north of Mexico (Heteroptera: Coreidae). Great Lakes Entomol. 1990, 23, 99–104. [Google Scholar]
- Fent, M.; Kment, P. First record of the invasive western conifer seed bug Leptoglossus occidentalis (Heteroptera: Coreidae) in Turkey. N. W. J. Zool. 2011, 7, 72–80. [Google Scholar]
- Yan, J.; Pal, C.; Anderson, D.; Vetek, G.; Farkas, P.; Burne, A.; Fan, Q.H.; Zhang, J.; Gunawardana, D.N.; Balan, R.K.; et al. Genetic diversity analysis of brown marmorated stink bug, Halyomorpha halys based on mitochondrial COI and COII haplotypes. BMC Genom. Data 2015, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Barman, A.K.; Joyce, A.L.; Torres, R.; Higbee, B.S. Assessing genetic diversity in four stink bug species, Chinavia hilaris, Chlorochroa uhleri, Chlorochroa sayi and Thyanta pallidovirens (Hemiptera: Pentatomidae), using DNA barcodes. J. Econ. Entomol. 2017, 110, 2590–2598. [Google Scholar] [CrossRef] [Green Version]
- Francischini, F.J.B.; Cordeiro, E.M.G.; de Campos, J.B.; Alves-Pereira, A.; Viana, J.P.G.; Wu, X.; Wei, W.; Brown, P.; Joyce, A.L.; Murua, G.; et al. Diatraea saccharalis history of colonization in the Americas. The case for human mediated dispersal. PLoS ONE 2019, 14, e0220031. [Google Scholar] [CrossRef] [PubMed]
- National Invasive Species Information Center (NISIC). What are Invasive Species? 2021. Available online: www.invasivespeciesinfo.gov/what-are-invasive-species (accessed on 25 October 2021).
- Beck, K.G.; Zimmerman, K.; Schardt, J.D.; Stone, J.; Lukens, R.R.; Reichard, S.; Randall, J.; Cangelosi, A.A.; Cooper, D.; Thompson, J.P. Invasive Species Defined in a Policy Context: Recommendations from the Federal Invasive Species Advisory Committee. Invasive Plant Sci. Manag. 2008, 1, 414–421. [Google Scholar] [CrossRef]


| L.z. # | Collection Locality Country, State/City, Other | Latitude, Longitude | Collection Date | Strain |
|---|---|---|---|---|
| 1 | Mex, Queretaro-Santa Rosa Jauregui | 20°42′22″ N, 100°26′34″ W | 27 September 2006 | 2 |
| 2 | Mex, Sinaloa, Concordia | 23°19′47″ N, 105°58′40″ W | 26 September 2010 | 2 |
| 3 | Mex, Nayarit, Altavista, Compostela | 21°4′27.08″ N, 105°8′56.49″ W * | 5 June 2012 | 2 |
| 4 | Mex, Yucatan, Yaxcopoil | 20°44′26.20″ N, 89°43′22.07″ W * | 5 August 2002 | 2 |
| 5 | Mex, Colima | 18°59′43.9″ N, 103°45′23.9′ W | 25 November 2006 | 2 |
| 7 | Bolivia, Chapare | 16°44′6.38″ S, 65°37′0.29″ W * | 31 March 2000 | 2 |
| 8 | Mex, Nayarit, Ahuacatlan | 20°59′ 06″ N, 104°27′08″ W | 13 June 2009 | 2 |
| 11 | Mex, Yucatan, Kinchil | 20°54′39.96″ N, 89°56′59.15″ W * | 16 August 2002 | 2 |
| 14 | Mex, Guanajato, San Juan La Lagunita | 21°34′22.56″ N, 101°32′2.71″ W * | 9 November 2006 | 2 |
| 15 | Mexico, Sonora | 28°32′21.7″ N, 109°41′31.5″ W | 15 September 2004 | 1 |
| 18 | Mex, Chiapas, Tuxla Gutierrez | 16°46′05.6″ N, 93°08′39.2″ W | 3 July 2003 | 2 |
| 19 | Mex, Oaxaca | 17°3′41.30″ N, 96°43′17.08″ W * | 15 July 2000 | 2 |
| 21 | Mex, Oaxaca, El Charquito | 17°5′2.16″ N, 96°40′5.41″ W * | 19 June 1982 | 2 |
| 22 | Mex, Guanajuato, San Juan La Lagunita | 21°29′54.83″ N, 101°25′25.60″ W * | 9 November 2006 | 2 |
| 28 | Mex, Chiapas, Cintalapa Ejido Tehuacan | 16°35′41.3″ N, 93°08′43.6″ W | 25 June 2003 | 2 |
| 30 | Mex, Oaxaca, Dominguillo | 17°38′907″ N, 96°54′703″ W | 18 October 1998 | 2 |
| 33 | Nicaragua, Masaya | 11°58′2.94″ N, 86°5′18.29″ W * | 26 November 1991 | 2 |
| 48 | L. vexillatus, Ecuador, Imbabura | 0°20′22.98″ N, 78°7′42.09″ W * | 12 December 1993 | 1 |
| 53 | Mexico, Sonora, Tecoripa | 28°37′19.5″ N, 109°57′0″ W | 16 December 2004 | 2 |
| 54 | Mex, Sinaloa, Concordia km32, VUD 1 | 23°19′47″ N, 105°58′40″ W | 26 September 2010 | 2 |
| 55 | Mex, Sinaloa, Concordia km32 VUD 1 | 23°19′47″ N, 105°58′40″ W | 26 September 2010 | 2 |
| 56 | Mex, Sinaloa, Concordia km72 VUD 1 | 23°27′29″ N, 105°49′51″ W | 26 September 2010 | 2 |
| 57 | Mex, Sinaloa, Concordia km32 VUD 1 | 23°19′47″ N, 105°58′40″ W | 26 September 2010 | 1 |
| 60 | Mex, Tamaulipas, Altamira km49 TCV | 22°31′49″ N, 98°07′49″ W | 9 May 2007 | 2 |
| 61 | Mex, Tamaulipas, Gomez Farias, EEA 1 | 25°04′55″ N, 99°09′41″ W | 12 May 2007 | 2 |
| 63 | Mex, San Luis Potosí, 3 km N SMA 1 | 22°03′52″ N, 100°31′00″ W | 20 August 2008 | 2 |
| 66 | Mex, Guanajuato, San Juan Lagunita | 21°33′38.37″ N, 101°28′55.63″ W * | 9 November 2006 | 2 |
| 68 | Mex, Guanajuato, San Juan Lagunita | 21°33′49.97″ N, 101°34′53.30″ W * | 9 November 2006 | 2 |
| 69 | Mex, Queretaro, San Juan de Rio | 20°22′02″ N, 100°01′13″ W | 24 October 2007 | 2 |
| 70 | Mex, Queretaro, Carretera La Venta-Lira | 20°30′51.64″ N, 100°9′39.31″ W * | 12 September 2007 | 2 |
| 71 | Mex, Queretaro, Galindo, SMG 1 | 20°22′57.92″ N, 100°5′14.84″ W * | 22 November 2011 | 1 |
| 73 | Mex, Queretaro, Carretera La Venta-Lira | 20°30′55.72″ N, 100°9′44.06″ W * | 12 September 2007 | 1 |
| 75 | Mex, Morelos, Tepalcingo, El Limon | 18°32′34″ N, 98°56′104″ W | 23 October 2006 | 2 |
| L. neovexillatus * | ||||
| 43 | Bolivia, Andres Ibanez, Canton Terebinto | 17°46′15.46″ S, 63°21′40.52″ W * | 18 March 2005 | n/a |
| 44 | Bolivia, Santa Cruz, Potrerillo de Guenda | 17°45′16.39″ S, 63°14′53.63″ W * | 13 October 2011 | n/a |
| Year Collected | Sequence Obtained | Not Obtained |
|---|---|---|
| 2000–2012 | 31 | 13 |
| Before 1999 | 4 | 27 |
| Lz Collection (n) | Number of Haplotypes | Haplotype Diversity | Nucleotide Diversity |
|---|---|---|---|
| California (41) | 3 | 0.526 | 0.008 |
| Cal. lineage 1 (24) | 2 | 0.083 | 0.008 |
| Cal. lineage 2 (17) | 1 | 0.000 | 0.000 |
| Mexico (30) | 3 | 0.248 | 0.002 |
| Mex. lineage 1 (4) | 2 | 0.667 | 0.002 |
| Mex. lineage 2 (26) | 1 | 0.000 | 0.000 |
| Brazil (37) (all lineage 2) | 3 | 0.505 | 0.001 |
| Combined lineage 1 and 2 (111) | 5 | 0.538 | 0.006 |
| Lz lineage 1 Cal./Mex. (28) | 2 | 0.198 | 0.001 |
| Lz lineage 2 Cal./Mex. (43) | 1 | 0.000 | 0.000 |
| Lz lineage 2 All (82) * | 3 | 0.245 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joyce, A.L.; Parolini, H.; Brailovsky, H. Distribution of Two Strains of Leptoglossus zonatus (Dallas) (Hemiptera: Coreidae) in the Western Hemisphere: Is L. zonatus a Potential Invasive Species in California? Insects 2021, 12, 1094. https://doi.org/10.3390/insects12121094
Joyce AL, Parolini H, Brailovsky H. Distribution of Two Strains of Leptoglossus zonatus (Dallas) (Hemiptera: Coreidae) in the Western Hemisphere: Is L. zonatus a Potential Invasive Species in California? Insects. 2021; 12(12):1094. https://doi.org/10.3390/insects12121094
Chicago/Turabian StyleJoyce, Andrea L., Hannah Parolini, and Harry Brailovsky. 2021. "Distribution of Two Strains of Leptoglossus zonatus (Dallas) (Hemiptera: Coreidae) in the Western Hemisphere: Is L. zonatus a Potential Invasive Species in California?" Insects 12, no. 12: 1094. https://doi.org/10.3390/insects12121094
APA StyleJoyce, A. L., Parolini, H., & Brailovsky, H. (2021). Distribution of Two Strains of Leptoglossus zonatus (Dallas) (Hemiptera: Coreidae) in the Western Hemisphere: Is L. zonatus a Potential Invasive Species in California? Insects, 12(12), 1094. https://doi.org/10.3390/insects12121094






