Hornets and Honey Bees: A Coevolutionary Arms Race between Ancient Adaptations and New Invasive Threats
Simple Summary
Abstract
1. Introduction
2. Coevolving Prey and Predator
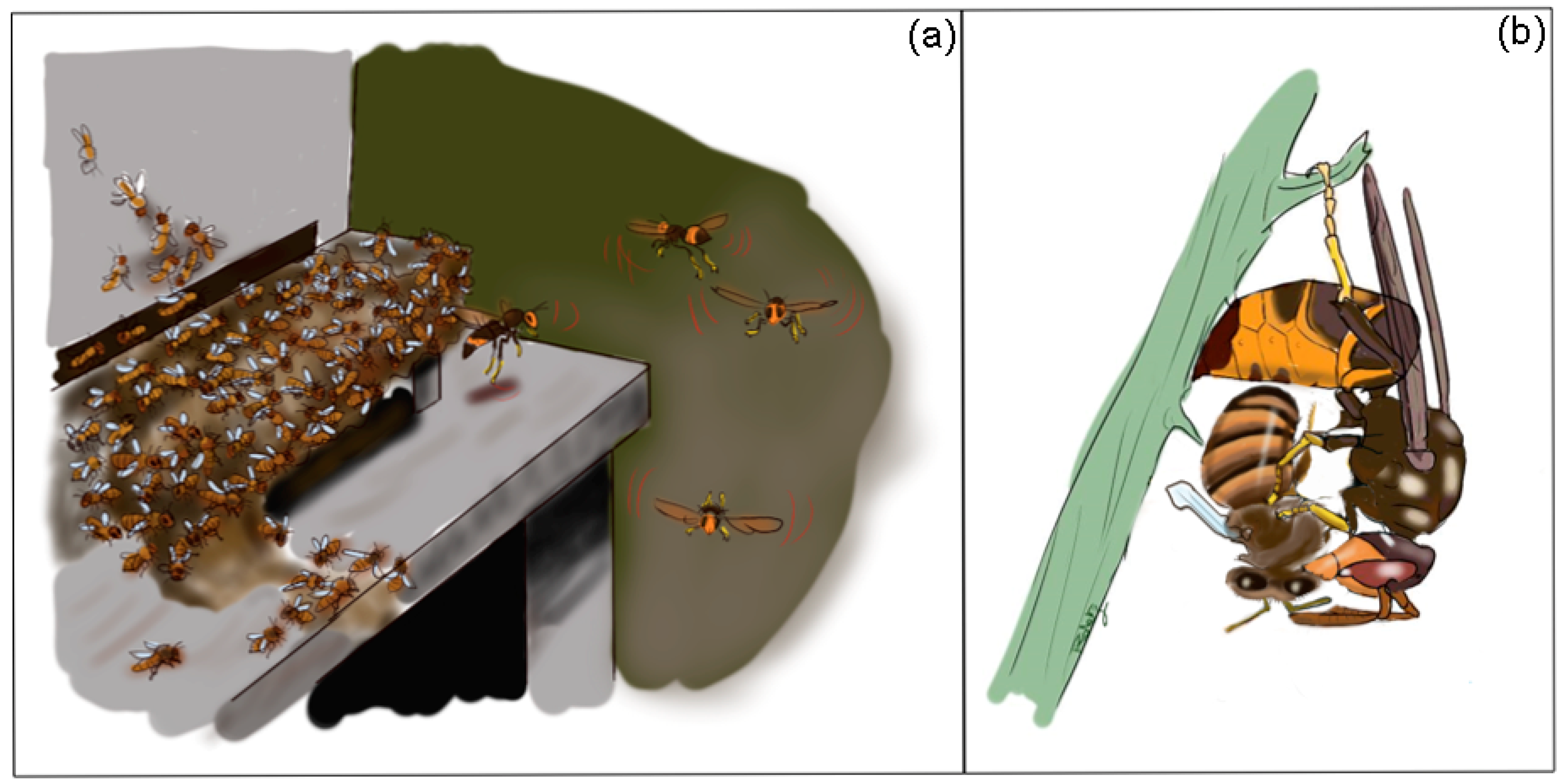
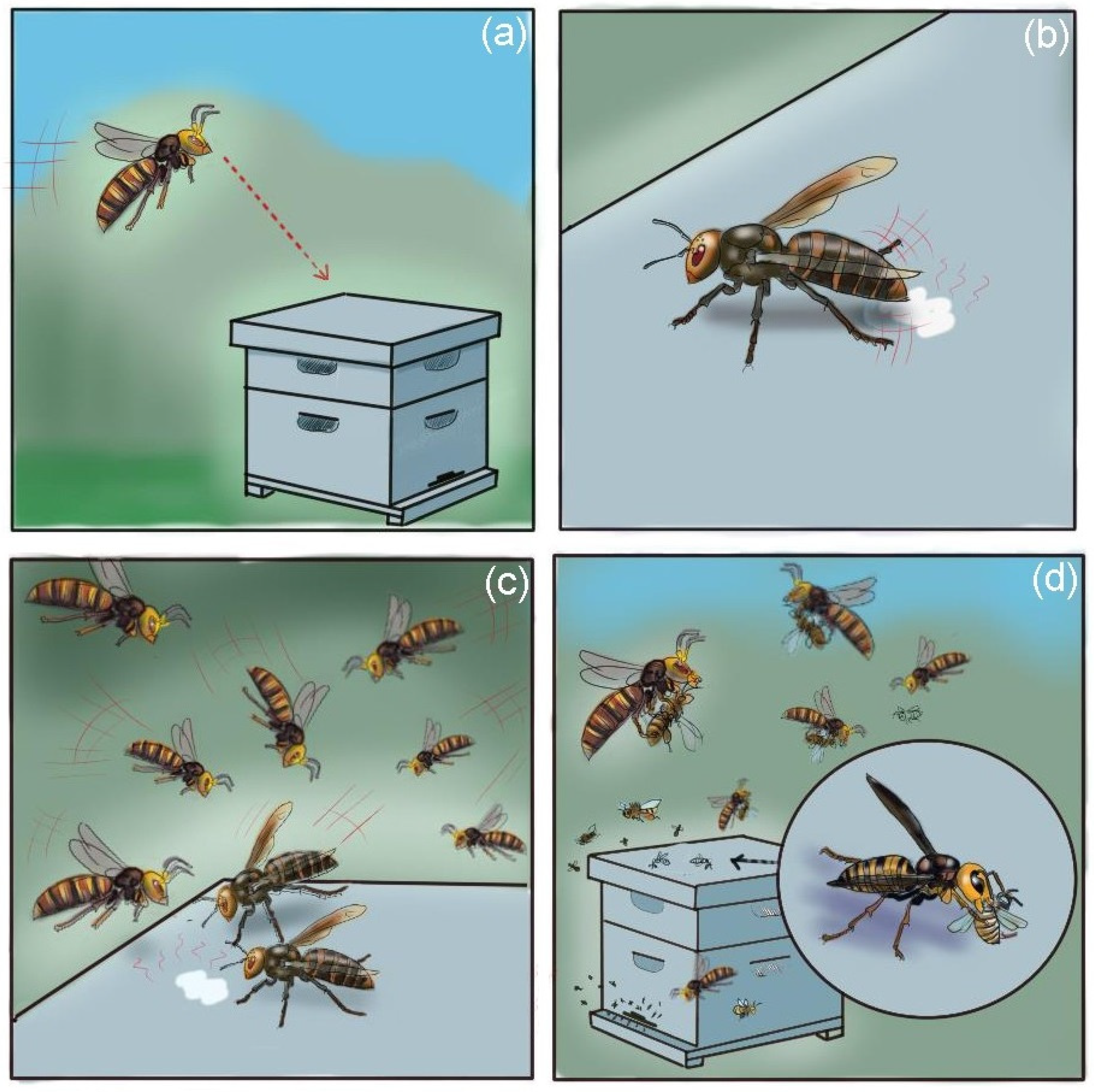
3. Hornet Predatory Strategies
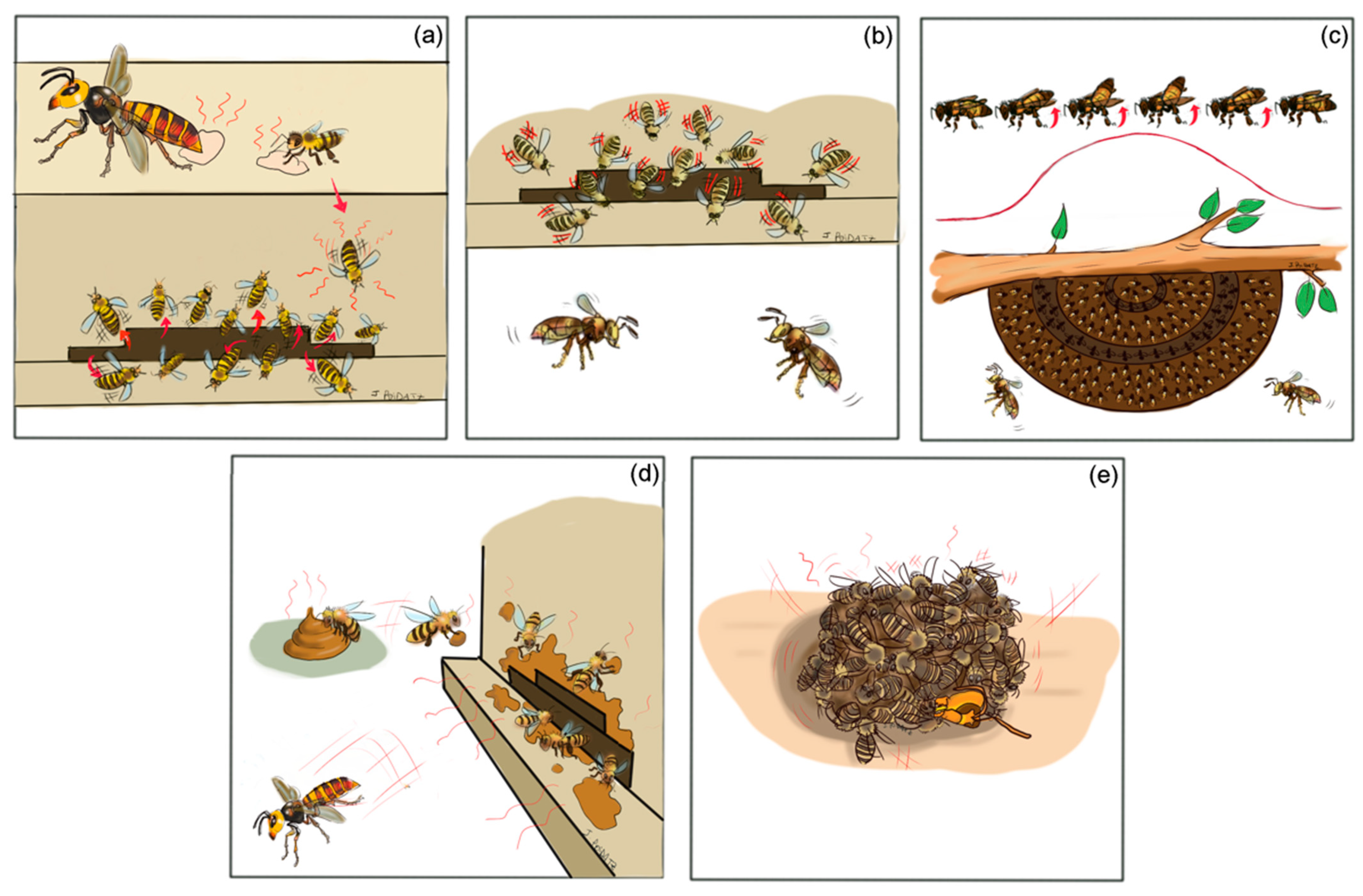
4. Honey Bee Defense Strategies
5. Less Effective Defense of A. mellifera against Hornets
6. Invasive Threats
7. Coevolution as a Tool to Develop Sustainable Management Strategies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsuura, M. Ecological study on vespine wasps (Hymenoptera: Vespidae) attacking honeybee colonies: I. seasonal changes in the frequency of visits to apiaries by vespine wasps and damage inflicted, especially in the absence of artificial protection. Appl. Èntomol. Zool. 1988, 23, 428–440. [Google Scholar] [CrossRef]
- Matsuura, M. Vespa and Provespa. In The Social Biology of Wasps; Ross, K.G., Matthews, R.W., Eds.; Cornell University Press: New York, NY, USA, 1991; pp. 232–262. [Google Scholar]
- Matsuura, M.; Yamane, S. Biology of Vespine Wasps; Springer: Berlin, Germany, 1990. [Google Scholar]
- Ono, M.; Okada, I.; Sasaki, M. Heat production by balling in the Japanese honeybee, Apis cerana japonica as a defensive behavior against the hornet, Vespa simillima xanthoptera (Hymenoptera: Vespidae). Experientia 1987, 43, 1031–1032. [Google Scholar] [CrossRef]
- Ono, M.; Igarashi, T.; Ohno, E.; Sasaki, M. Unusual thermal defence by a honeybee against mass attack by hornets. Nature 1995, 377, 334–336. [Google Scholar] [CrossRef]
- Tan, K.; Wang, Z.; Li, H.; Yang, S.; Hu, Z.; Kastberger, G.; Oldroyd, B.P. An ‘I see you’prey–predator signal between the Asian honeybee, Apis cerana, and the hornet, Vespa velutina. Anim. Behav. 2012, 83, 879–882. [Google Scholar] [CrossRef]
- Tan, K.; Dong, S.; Li, X.; Liu, X.; Wang, C.; Li, J.; Nieh, J.C. Honey bee inhibitory signaling is tuned to threat severity and can act as a colony alarm signal. PLoS Biol. 2016, 14, e1002423. [Google Scholar] [CrossRef] [PubMed]
- Archer, M.E. A phylogenetic study of the species of the genus Vespa (Hymenoptera: Vespinae). Insect Syst. Evol. 1994, 24, 469–478. [Google Scholar] [CrossRef]
- Perrard, A.; Pickett, K.; Villemant, C.; Kojima, J.I.; Carpenter, J. Phylogeny of hornets: A total evidence approach (Hymenoptera, Vespidae, Vespinae, Vespa). J. Hymenopt. Res. 2013, 32, 1–15. [Google Scholar] [CrossRef]
- Smith-Pardo, A.H.; Carpenter, J.M.; Kimsey, L. The diversity of hornets in the genus Vespa (Hymenoptera: Vespidae; Vespinae), their importance and interceptions in the United States. Insect Syst. Divers. 2020, 4, 1–27. [Google Scholar] [CrossRef]
- Carpenter, J.M.; Kojima, J.I. Checklist of the species in the subfamily Vespinae (Insecta: Hymenoptera: Vespidae). Nat. Hist. Bull. Ibaraki Univ. 1997, 1, 51–92. [Google Scholar]
- Shaw, F.R.; Weidhaas, J. Distribution and habits of the giant hornet in North America. J. Econ. Entomol. 1956, 49, 275. [Google Scholar] [CrossRef]
- de Saussure, H. Letter concerning insects introduced into the United States, incl. Vespa crabro. Entomol. News 1898, 9, 144–145. [Google Scholar]
- Kim, J.K.; Choi MBMoon, T.Y. Occurrence of Vespa velutina Lepeletier from Korea, and a revised key for Korean Vespa species (Hymenoptera: Vespidae). Entomol. Res. 2006, 36, 112–115. [Google Scholar] [CrossRef]
- Rortais, A.; Villemant, C.; Gargominy, O.; Rome, Q.; Haxaire, J.; Papachristoforou, A.; Arnold, G. A new enemy of honeybees in Europe: The Asian hornet Vespa velutina. In Atlas of Biodiversity Risks—From Europe to the Globe, From Stories to Maps; Settele, J., Ed.; Pensoft: Moscow, Russia, 2010; p. 11. [Google Scholar]
- Villemant, C.; Barbet-Massin, M.; Perrard, A.; Muller, F.; Gargominy, O.; Jiguet, F.; Rome, Q. Predicting the invasion risk by the alien bee-hawking Yellow-legged hornet Vespa velutina nigrithorax across Europe and other continents with niche models. Biol. Conserv. 2011, 144, 2142–2150. [Google Scholar] [CrossRef]
- Ueno, T. Establishment of the invasive hornet Vespa velutina (Hymenoptera: Vespidae) in Japan. Int. J. Chem. Environ. Biol. Sci. 2014, 2, 220–222. [Google Scholar]
- Arca, M.; Mougel, F.; Guillemaud, T.; Dupas, S.; Rome, Q.; Perrard, A.; Silvain, J.F. Reconstructing the invasion and the demographic history of the yellow-legged hornet, Vespa velutina, in Europe. Biol. Invasions 2015, 17, 2357–2371. [Google Scholar] [CrossRef]
- Wilson, T.M.; Takahashi, J.; Spichiger, S.E.; Kim, I.; van Westendorp, P. First reports of Vespa mandarinia (Hymenoptera: Vespidae) in North America represent two separate maternal lineages in Washington State, United States, and British Columbia, Canada. Ann. Entomol. Soc. 2020, 113, 468–472. [Google Scholar] [CrossRef]
- Alaniz, A.J.; Carvajal, M.A.; Vergara, P.M. Giants are coming? Predicting the potential spread and impacts of the giant Asian hornet (Vespa mandarinia, Hymenoptera: Vespidae) in the USA. Pest Manag. Sci. 2021, 77, 104–112. [Google Scholar] [CrossRef]
- Vetter, R.S.; Visscher, P.K.; Camazine, S. Mass envenomations by honey bees and wasps. West. J. Med. 1999, 170, 223. [Google Scholar]
- Choi, M.B.; Martin, S.J.; Lee, J.W. Distribution, spread, and impact of the invasive hornet Vespa velutina in South Korea. J. Asia-Pac. Entomol. 2012, 15, 473–477. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.; Zhou, Y.; Xie, C.; Zhu, B.; Zhu, H.; Liu, S.; Wang, W.; Chen, H.; Ji, Y. Deciphering the Venomic Transcriptome of Killer-Wasp Vespa velutina. Sci. Rep. 2015, 5, srep09454. [Google Scholar] [CrossRef]
- Kim, W.M.; Kim, S.Y.; Song, W. Microhabitat characteristics affecting the occurrence and diversity of queen hornets (genus Vespa) in an urban green area. Landsc. Ecol. Eng. 2020, 16, 173–186. [Google Scholar] [CrossRef]
- Abrol, D.P. Ecology, behaviour and management of social wasp, Vespa velutina Smith (Hymenoptera: Vespidae), attacking honeybee colonies. Korean J. Apic. 1994, 9, 5–10. [Google Scholar]
- Ranabhat, N.B.; Tamrakar, A.S. Study on seasonal activity of predatory wasps attacking honeybee Apis cerana Fab. Colonies in Southern Belt of Kaski District, Nepal. J. Nat. Hist. Mus. 2009, 23, 125–128. [Google Scholar] [CrossRef][Green Version]
- Monceau, K.; Bonnard, O.; Thiéry, D. Vespa velutina: A new invasive predator of honeybees in Europe. J. Pest Sci. 2014, 87, 1–16. [Google Scholar]
- Baracchi, D.; Cusseau, G.; Pradella, D.; Turillazzi, S. Defence reactions of Apis mellifera ligustica against attacks from the European hornet Vespa crabro. Ethol. Ecol. Evol. 2010, 22, 281–294. [Google Scholar] [CrossRef]
- Ishay, J. Observations sur la biologie de la guêpe orientale Vespa orientalis F. Insect. Soc. 1964, 11, 193–206. [Google Scholar] [CrossRef]
- Papachristoforou, A.; Rortais, A.; Zafeiridou, G.; Theophilidis, G.; Garnery, L.; Thrasyvoulou, A.; Arnold, G. Smothered to death: Hornets asphyxiated by honeybees. Curr. Biol. 2007, 17, R795–R796. [Google Scholar] [CrossRef]
- Requier, F.; Rome, Q.; Chiron, G.; Decante, D.; Marion, S.; Menard, M.; Henry, M. Predation of the invasive Asian hornet affects foraging activity and survival probability of honey bees in Western Europe. J. Pest Sci. 2019, 92, 567–578. [Google Scholar]
- Tan, K.; Hu, Z.; Chen, W.; Wang, Z.; Wang, Y.; Nieh, J.C. Fearful foragers: Honey bees tune colony and individual foraging to multi-predator presence and food quality. PLoS ONE 2013, 8, e75841. [Google Scholar]
- Brodmann, J.; Twele, R.; Francke, W.; Yi-Bo, L.; Xi-qiang, S.; Ayasse, M. Orchid mimics honey bee alarm pheromone in order to attract hornets for pollination. Curr. Biol. 2009, 19, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M.; Sakagami, S.F. A bionomic sketch of the giant hornet, Vespa mandarinia, a serious pest for Japanese apiculture. J. Fac. Sci. Hokkaido Univ. 1973, 19, 125–162. [Google Scholar]
- Singh, S. Beekeeping in India; Council of Agricultural Research: New Delhi, India, 1962. [Google Scholar]
- Shah, F.A.; Shah, T.A. Vespa velutina, a serious pest of honey bees in Kashmir. Bee World 1991, 72, 161–164. [Google Scholar] [CrossRef]
- Koeniger, N.; Koeniger, G.; Mardan, M. Mimicking a honeybee queen? Vespa affinis indosinensis Perez 1910 hunts drones of Apis cerana F. 1973. Ethology 1994, 98, 149–153. [Google Scholar]
- Kastberger, G.; Weihmann, F.; Zierler, M.; Hötzl, T. Giant honeybees (Apis dorsata) mob wasps away from the nest by directed visual patterns. Naturwissenschaften 2014, 101, 861–873. [Google Scholar] [PubMed]
- Burgett, M.; Akratanakul, P. Predation on the Western honey bee, Apis mellifera L., by the hornet, Vespa tropica (L.). Psyche 1982, 89, 347–350. [Google Scholar] [CrossRef][Green Version]
- Koeniger, N.; Koeniger, G.; Gries, M.; Tingek, S.; Kelitu, A. Observations on colony defense of Apis nuluensis Tingek, Koeniger and Koeniger, 1996 and predatory behavior of the hornet, Vespa multimaculata Pérez, 1910. Apidologie 1996, 27, 341–352. [Google Scholar] [CrossRef]
- Mattila, H.R.; Otis, G.W.; Nguyen, L.T.; Pham, H.D.; Knight, O.M.; Phan, N.T. Honey bees (Apis cerana) use animal feces as a tool to defend colonies against group attack by giant hornets (Vespa soror). PLoS ONE 2020, 15, e0242668. [Google Scholar] [CrossRef]
- Kohsaka, R.; Park, M.S.; Uchiyama, Y. Beekeeping and honey production in Japan and South Korea: Past and present. J. Ethn. Foods 2017, 4, 72–79. [Google Scholar] [CrossRef]
- Kwon, O.; Choi, M.B. Interspecific hierarchies from aggressiveness and body size among the invasive alien hornet, Vespa velutina nigrithorax, and five native hornets in South Korea. PLoS ONE 2020, 15, e0226934. [Google Scholar] [CrossRef]
- Glaiim, M.K. Hunting behavior of the oriental hornet, Vespa orientalis L., and defense behavior of the honey bee, Apis mellifera L., in Iraq. Bull. Iraq Nat. Hist. Mus. 2009, 10, 17–30. [Google Scholar]
- Vaziritabar, S.; Esmaeilzade, S.M. Identification of differential defense reaction and tactic of Iranian honeybee Apis mellifera meda colonies under attack hornets Vespa orientalis and Vespa crabro in Iran. J. Entomol. Zool. Stud. 2018, 6, 102–111. [Google Scholar]
- Brodie, E.D., III; Brodie, E.D., Jr. Predator-prey arms races: Asymmetrical selection on predators and prey may be reduced when prey are dangerous. Bioscience 1999, 49, 557–568. [Google Scholar] [CrossRef]
- Tan, K.; Li, H.; Yang, M.X.; Hepburn, H.R.; Radloff, S.E. Wasp hawking induces endothermic heat production in guard bees. J. Insect Sci. 2010, 10, 142. [Google Scholar] [CrossRef]
- McClenaghan, B.; Schlaf, M.; Geddes, M.; Mazza, J.; Pitman, G.; McCallum, K.; Otis, G.W. Behavioral responses of honey bees, Apis cerana and Apis mellifera, to Vespa mandarinia marking and alarm pheromones. J. Apic. Res. 2019, 58, 141–148. [Google Scholar] [CrossRef]
- Oldroyd, B.P.; Wongsiri, S. Asian Honey Bees: Biology, Conservation, and Human Interactions; Harvard University Press: Boston, MA, USA, 2006. [Google Scholar]
- Koeniger, N.; Koeniger, G.; Smith, D. Phylogeny of the genus Apis. In Honeybees of Asia; Springer: Berlin/Heidelberg, Germany, 2011; pp. 23–50. [Google Scholar]
- Couto, A.; Monceau, K.; Bonnard, O.; Thiéry, D.; Sandoz, J.C. Olfactory attraction of the hornet Vespa velutina to honeybee colony odors and pheromones. PLoS ONE 2014, 9, e115943. [Google Scholar] [CrossRef]
- Cini, A.; Cappa, F.; Petrocelli, I.; Pepiciello, I.; Bortolotti, L.; Cervo, R. Competition between the native and the introduced hornets Vespa crabro and Vespa velutina: A comparison of potentially relevant life-history traits. Ecol. Entomol. 2018, 43, 351–362. [Google Scholar]
- Wang, Z.W.; Chen, G.; Tan, K. Both olfactory and visual cues promote the hornet Vespa velutina to locate its honeybee prey Apis cerana. Insect. Soc. 2014, 61, 67–70. [Google Scholar] [CrossRef]
- Balamurali, G.S.; Reshnuraj, R.S.; Johnson, J.; Kodandaramaiah, U.; Somanathan, H. Visual associative learning and olfactory preferences of the greater banded hornet, Vespa tropica. Insect. Soc. 2021, 68, 1–10. [Google Scholar]
- Tan, K.; Radloff, S.E.; Li, J.J.; Hepburn, H.R.; Yang, M.X.; Zhang, L.J.; Neumann, P. Bee-hawking by the wasp, Vespa velutina, on the honeybees Apis cerana and A. mellifera. Naturwissenschaften 2007, 94, 469–472. [Google Scholar] [CrossRef]
- Sakagami, S.; Akahira, Y. Studies on the Japanese honeybee, Apis cerana cerana Fabricius. VIII. Two opposing adaptations in the post-stinging behavior of honeybees. Evolution 1960, 14, 29–40. [Google Scholar] [CrossRef]
- Singh, G. Defensive behaviour of Apis cerana F. (Hill strain) against predatory hornets in Kashmir. Indian Bee J. 1972, 34, 65–69. [Google Scholar]
- Sharma, O.P.; Raj, D. Ecological studies on predatory wasps attacking Italian honey bee, Apis mellifera L., in Kangra Shivaliks. Indian J. Ecol. 1988, 15, 168–171. [Google Scholar]
- Ken, T.; Radloff, H.R.H.S.E.; Hepburn, H.R.; Radloff, S.E.; Yusheng, Y.; Yiqiu, L.; Danyin, Z.; Neumann, P. Heat-balling wasps by honeybees. Naturwissenschaften 2005, 92, 492–495. [Google Scholar] [CrossRef]
- Abrol, D.P. Defensive behaviour of Apis cerana F. against predatory wasps. J. Apic. Sci. 2006, 50, 39. [Google Scholar]
- Ono, M.; Terabe, H.; Hori, H.; Sasaki, M. Components of giant hornet alarm pheromone. Nature 2003, 424, 637–638. [Google Scholar] [CrossRef]
- Abrol, D.P. Asiatic Honey Bee Apis Cerana; Springer: London, UK, 2013. [Google Scholar]
- Breed, M.D.; Guzmán-Novoa, E.; Hunt, G.J. Defensive behavior of honey bees: Organization, genetics, and comparisons with other bees. Ann. Rev. Entomol. 2004, 49, 271–298. [Google Scholar] [CrossRef]
- Dong, S.; Wen, P.; Zhang, Q.; Wang, Y.; Cheng, Y.; Tan, K.; Nieh, J.C. Olfactory eavesdropping of predator alarm pheromone by sympatric but not allopatric prey. Anim. Behav. 2018, 141, 115–125. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, Y.; Dong, S.; Wen, P.; Li, J.; Tan, K.; Menzel, R. Honey bees modulate their olfactory learning in the presence of hornet predators and alarm component. PLoS ONE 2016, 11, e0150399. [Google Scholar] [CrossRef] [PubMed]
- Woyke, J.; Wilde, J.; Wilde, M.; Sivaram, V.; Cervancia, C.; Nagaraja, N.; Reddy, M. Comparison of defense body movements of Apis laboriosa, Apis dorsata dorsata and Apis dorsata breviligula honey bees. J. Insect Behav. 2008, 21, 481–494. [Google Scholar] [CrossRef]
- Fuchs, S.; Tautz, J. Colony defence and natural enemies. In Honeybees of Asia; Springer: Berlin/Heidelberg, Germany, 2011; pp. 369–395. [Google Scholar]
- Tan, K.; Wang, Z.; Chen, W.; Hu, Z.; Oldroyd, B.P. The ‘I see you’prey–predator signal of Apis cerana is innate. Naturwissenschaften 2013, 100, 245–248. [Google Scholar]
- Seeley, T.D.; Seeley, R.H.; Akratanakul, P. Defense strategies of the honeybees of Thailand. Ecol. Monogr. 1982, 52, 43–63. [Google Scholar] [CrossRef]
- Sarma, M.S.; Fuchs, S.; Werber, C.; Tautz, J. Worker piping triggers hissing for coordinated colony defence in the dwarf honeybee Apis florea. Zoology 2002, 105, 215–223. [Google Scholar] [CrossRef]
- Papachristoforou, A.; Sueur, J.; Rortais, A.; Angelopoulos, S.; Thrasyvoulou, A.; Arnold, G. High frequency sounds produced by Cyprian honeybees Apis mellifera cypria when confronting their predator, the Oriental hornet Vespa orientalis. Apidologie 2008, 39, 468–474. [Google Scholar] [CrossRef]
- Dong, S.; Tan, K.; Nieh, J.C. Visual contagion in prey defence signals can enhance honest defence. J. Anim. Ecol. 2021, 90, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Ken, T.; Wang, J.M. Reduction of foraging activity by A. cerana colonies attacked by Vespa velutina. J. Bee 2004, 2, 7–9. [Google Scholar]
- Dong, S.; Tan, K.; Zhang, Q.; Nieh, J.C. Playbacks of Asian honey bee stop signals demonstrate referential inhibitory communication. Anim. Behav. 2019, 148, 29–37. [Google Scholar] [CrossRef]
- Pirk, C.W.; Hepburn, R.; Radloff, S.E.; Erlandsson, J. Defense posture in the dwarf honeybee, Apis Florea. Apidologie 2002, 33, 289–294. [Google Scholar] [CrossRef][Green Version]
- Duangphakdee, O.; Koeniger, N.; Koeniger, G.; Wongsiri, S.; Deowanish, S. Reinforcing a barrier–a specific social defense of the dwarf honeybee (Apis florea) released by the weaver ant (Oecophylla smaragdina). Apidologie 2005, 36, 505–511. [Google Scholar] [CrossRef]
- Kastberger, G.; Schmelzer, E.; Kranner, I. Social waves in giant honeybees repel hornets. PLoS ONE 2008, 3, e3141. [Google Scholar] [CrossRef]
- Kastberger, G.; Weihmann, F.; Hoetzl, T. Self-assembly processes in honeybees: The phenomenon of shimmering. In Honeybees of Asia; Springer: Berlin/Heidelberg, Germany, 2011; pp. 397–443. [Google Scholar]
- Kastberger, G.; Weihmann, F.; Hoetzl, T.; Weiss, S.E.; Maurer, M.; Kranner, I. How to join a wave: Decision-making processes in shimmering behavior of giant honeybees (Apis dorsata). PLoS ONE 2012, 7, e36736. [Google Scholar] [CrossRef]
- Lindauer, M. Über die Verständigung bei indischen Bienen. Z. Vergl. Physiol. 1956, 38, 521–557. [Google Scholar] [CrossRef]
- Sakagami, S. Preliminary report on the specific deference of behavior and other ecological characters between European and Japanese honeybees. Acta Hymenopterologica 1960, 1, 172–198. [Google Scholar]
- Schmelzer, E.; Kastberger, G. ‘Special agents’ trigger social waves in giant honeybees (Apis dorsata). Naturwissenschaften 2009, 96, 1431–1441. [Google Scholar] [CrossRef]
- Farkas, I.; Helbing, D.; Vicsek, T. Social behaviour: Mexican waves in an excitable medium. Nature 2002, 419, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Kastberger, G.; Raspotnig, G.; Biswas, S.; Winder, O. Evidence of Nasonov scenting in colony defence of the Giant honeybee Apis dorsata. Ethology 1998, 104, 27–37. [Google Scholar] [CrossRef]
- Kastberger, G.; Sharma, D.K. The predator-prey interaction between blue-bearded bee eaters (Nyctyornis athertoni) and Giant honeybees (Apis dorsata). Apidologie 2000, 31, 727–736. [Google Scholar] [CrossRef]
- Kastberger, G.; Winder, O.; Steindl, K. Defence strategies in the Giant honeybee Apis dorsata. Proc. Dtsch. Zool. Ges. 2001, 94, 7. [Google Scholar]
- Tan, K.; Yang, M.X.; Wang, Z.W.; Li, H.; Zhang, Z.Y.; Radloff, S.E.; Hepburn, R. Cooperative wasp-killing by mixed-species colonies of honeybees, Apis cerana and Apis mellifera. Apidologie 2012, 43, 195–200. [Google Scholar] [CrossRef][Green Version]
- Sugahara, M.; Sakamoto, F. Heat and carbon dioxide generated by honeybees jointly act to kill hornets. Naturwissenschaften 2009, 96, 1133–1136. [Google Scholar] [CrossRef]
- Gu, G.; Meng, Y.; Tan, K.; Dong, S.; Nieh, J.C. Lethality of Honey Bee Stings to Heavy Armored Hornets. Biology 2021, 10, 484. [Google Scholar] [CrossRef]
- Sugahara, M.; Nishimura, Y.; Sakamoto, F. Differences in heat sensitivity between Japanese honeybees and hornets under high carbon dioxide and humidity conditions inside bee balls. Zool. Sci. 2012, 29, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Garnery, L.; Cornuet, J.M.; Solignac, M. Evolutionary history of the honey bee Apis mellifera inferred from mitochondrial DNA analysis. Mol. Ecol. 1992, 1, 145–154. [Google Scholar] [CrossRef]
- Monceau, K.; Maher, N.; Bonnard, O.; Thiéry, D. Predation pressure dynamics study of the recently introduced honeybee killer Vespa velutina: Learning from the enemy. Apidologie 2013, 44, 209–221. [Google Scholar] [CrossRef]
- Arca, M.; Papachristoforou, A.; Mougel, F.; Rortais, A.; Monceau, K.; Bonnard, O.; Arnold, G. Defensive behaviour of Apis mellifera against Vespa velutina in France: Testing whether European honeybees can develop an effective collective defence against a new predator. Behav. Process. 2014, 106, 122–129. [Google Scholar] [CrossRef]
- Moritz, R.F.; Härtel, S.; Neumann, P. Global invasions of the western honeybee (Apis mellifera) and the consequences for biodiversity. Ecoscience 2005, 12, 289–301. [Google Scholar] [CrossRef]
- Yang, G.H. Harm of introducing the western honeybee Apis mellifera L. to the Chinese honeybee Apis cerana F. and its ecological impact. Acta Entomol. Sin. 2005, 48, 401–406. [Google Scholar]
- Hosono, S.; Nakamura, J.; Ono, M. European honeybee defense against Japanese yellow hornet using heat generation by bee-balling behavior. Entomol. Sci. 2017, 20, 163–167. [Google Scholar] [CrossRef]
- Monceau, K.; Arca, M.; Leprêtre, L.; Bonnard, O.; Arnold, G.; Thiéry, D. How Apis mellifera behaves with its invasive hornet predator Vespa velutina? J. Insect Behav. 2018, 31, 1–11. [Google Scholar] [CrossRef]
- Papachristoforou, A.; Rortais, A.; Sueur, J.; Arnold, G. Attack or retreat: Contrasted defensive tactics used by Cyprian honeybee colonies under attack from hornets. Behav. Process. 2011, 86, 236–241. [Google Scholar] [CrossRef]
- Kandemir, I.; Cakmak, I.; Abramson, C.I.; Cakmak, S.S.; Serrano, E.; Song, D.; Wells, H. A colony defence difference between two honey bee subspecies (Apis mellifera cypria and Apis mellifera caucasica). J. Apic. Res. 2012, 51, 169–173. [Google Scholar] [CrossRef]
- Rankin, E.E.W. Emerging patterns in social wasp invasions. Curr. Opin. Insect Sci. 2021, 46, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, E. Le frelon asiatique, déjà là. ActuApi 2011, 55, 1–6. [Google Scholar]
- López, S.; González, M.; Goldarazena, A. Vespa velutina lepeletier, 1836 (Hymenoptera: Vespidae): First records in Iberian Peninsula. Bull. OEPP 2011, 41, 439–441. [Google Scholar] [CrossRef]
- Grosso-Silva, J.M.; Maia, M. Vespa velutina Lepeletier, 1836 (Hymenoptera, Vespidae), new species for Portugal. Arq. Entomolóxicos 2012, 6, 53–54. [Google Scholar]
- Rome, Q.; Dambrine, L.; Onate, C.; Muller, F.; Villemant, C.; García-Pérez, A.; Bruneau, E. Spread of the invasive hornet Vespa velutina Lepeletier, 1836, in Europe in 2012 (Hym., Vespidae). Bull. Soc. Entomol. Fr. 2013, 118, 21–22. [Google Scholar] [CrossRef]
- Witt, R. Erstfund eines Nestes der Asiatischen Hornisse Vespa velutina Lepeletier, 1838 in Deutschland und Details zum Nestbau (Hymenoptera, Vespinae). Ampulex 2015, 7, 42–53. [Google Scholar]
- Budge, G.E.; Hodgetts, J.; Jones, E.P.; Ostojá-Starzewski, J.C.; Jall, J.; Tomkies, V.; Semmence, N.; Brown, M.; Wakefield, M.; Stainton, K. The invasion, provenance and diversity of Vespa velutina Lepeltier (Hymenoptera: Vespidae) in Great Britain. PLoS ONE 2017, 12, e0185172. [Google Scholar] [CrossRef]
- Granato, A.; Negrisolo, E.; Bonomi, J.; Zulian, L.; Cappa, F.; Bortolotti, L.; Mutinelli, F. Recent confirmation of a single haplotype in the Italian population of Vespa velutina. Biol. Invasions 2019, 21, 2811–2817. [Google Scholar] [CrossRef]
- Rodríguez-Flores, M.S.; Seijo-Rodríguez, A.; Escuredo, O.; del Carmen Seijo-Coello, M. Spreading of Vespa velutina in northwestern Spain: Influence of elevation and meteorological factors and effect of bait trapping on target and non-target living organisms. J. Pest Sci. 2019, 92, 557–565. [Google Scholar] [CrossRef]
- Slikboer, L.; Zeegers, T. Der aziatische hoornaar in Nederland in 2019. EIS Rep. 2019, 28, 1–14. [Google Scholar]
- Husemann, M.; Sterr, A.; Maack, S.; Abraham, R. The northernmost record of the Asian hornet Vespa velutina nigrithorax (Hymenoptera, Vespidae). Evol. Syst. 2020, 4, 1. [Google Scholar] [CrossRef]
- REGULATION, C. Commission Implementing Regulation (EU) 2016/1141 of 13 July 2016 adopting a list of invasive alien species of Union concern pursuant to Regulation (EU) No 1143/2014 of the European Parliament and of the Council. Off. J. Eur. Union L 2016, 189, 4–8. [Google Scholar]
- de Haro, L.; Labadie, M.; Chanseau, P.; Cabot, C.; Blanc-Brisset, I.; Penouil, F.; Toxicovigilance, N.C.C. Medical consequences of the Asian black hornet (Vespa velutina) invasion in Southwestern France. Toxicon 2010, 55, 650–652. [Google Scholar] [CrossRef]
- Laborde-Castérot, H.; Darrouzet, E.; Le Roux, G.; Labadie, M.; Delcourt, N.; de Haro, L.; French Poison Control Centers Research Group. Ocular lesions other than stings following yellow-legged hornet (Vespa velutina nigrithorax) projections, as reported to French Poison control centers. JAMA Ophthalmol. 2021, 139, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Villemant, C.; Muller, F.; Haubois, S.; Perrard, A.; Darrouzet, E.; Rome, Q. Bilan des Travaux (MNHN et IRBI) sur L’invasion en France de Vespa velutina, le Frelon Asiatique Prédateur D’abeilles. Available online: https://inpn.mnhn.fr/fichesEspece/Vespa_velutina_fichiers/2011_02_11_Bilan_Invasion_Vespa_velutina_JSA.pdf (accessed on 15 November 2021).
- Rome, Q.; Perrard, A.; Muller, F.; Fontaine, C.; Quilès, A.; Zuccon, D.; Villemant, C. Not just honeybees: Predatory habits of Vespa velutina (Hymenoptera: Vespidae) in France. Ann. Soc. Entomol. Fr. 2021, 57, 1–11. [Google Scholar]
- Rome, Q.; Perrard, A.; Muller, F.; Villemant, C. Monitoring and control modalities of a honeybee predator, the yellow-legged hornet Vespa velutina nigrithorax (Hymenoptera: Vespidae). Aliens 2011, 31, 7–15. [Google Scholar]
- Monceau, K.; Arca, M.; Leprêtre, L.; Mougel, F.; Bonnard, O.; Silvain, J.F.; Thiery, D. Native prey and invasive predator patterns of foraging activity: The case of the yellow-legged hornet predation at European honeybee hives. PLoS ONE 2013, 8, e66492. [Google Scholar]
- Perrard, A.; Haxaire, J.; Rortais, A.; Villemant, C. Observations on the colony activity of the Asian hornet Vespa velutina Lepeletier 1836 (Hymenoptera: Vespidae: Vespinae) in France. Ann. Soc. Entomol. Fr. 2009, 45, 119–127. [Google Scholar] [CrossRef]
- Verdasca, M.J.; Godinho, R.; Rocha, R.G.; Portocarrero, M.; Carvalheiro, L.G.; Rebelo, R.; Rebelo, H. A metabarcoding tool to detect predation of the honeybee Apis mellifera and other wild insects by the invasive Vespa velutina. J. Pest Sci. 2021, 1–11. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Ford, S.M.; Poidatz, J.; Thiéry, D.; Osborne, J. Searching for nests of the invasive Asian hornet (Vespa velutina) using radio-telemetry. Commun. Biol. 2018, 1, 1–8. [Google Scholar] [CrossRef]
- Jones, E.P.; Conyers, C.; Tomkies, V.; Semmence, N.; Fouracre, D.; Wakefield, M.; Stainton, K. Managing incursions of Vespa velutina nigrithorax in the UK: An emerging threat to apiculture. Sci. Rep. 2020, 10, 1–8. [Google Scholar]
- Nuñez-Penichet, C.; Osorio-Olvera, L.; Gonzalez, V.H.; Cobos, M.E.; Jiménez, L.; DeRaad, D.A.; Soberon, J. Geographic potential of the world’s largest hornet, Vespa mandarinia Smith (Hymenoptera: Vespidae), worldwide and particularly in North America. PeerJ 2021, 9, e10690. [Google Scholar] [CrossRef]
- Norderud, E.D.; Powell, S.L.; Peterson, R.K. Risk assessment for the establishment of the Asian giant hornet (Vespa mandarinia) in the Pacific Northwest. bioRxiv 2021. [Google Scholar] [CrossRef]
- Dalmon, A.; Gayral, P.; Decante, D.; Klopp, C.; Bigot, D.; Thomasson, M.; Le Conte, Y. Viruses in the invasive hornet Vespa velutina. Viruses 2019, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, M.; Forzan, M.; Cilia, G.; Sagona, S.; Bortolotti, L.; Felicioli, A. First detection of replicative de-formed wing virus (DWV) in Vespa velutina nigrithorax. Bull. Insectol. 2018, 71, 211–216. [Google Scholar]
- Mazzei, M.; Cilia, G.; Forzan, M.; Lavazza, A.; Mutinelli, F.; Felicioli, A. Detection of replicative Kashmir bee virus and Black queen cell virus in Asian hornet Vespa velutina (Lepelieter 1836) in Italy. Sci. Rep. 2019, 9, 1–9. [Google Scholar]
- Yang, S.; Gayral, P.; Zhao, H.; Wu, Y.; Jiang, X.; Wu, Y.; Hou, C. Occurrence and molecular phylogeny of honey bee viruses in Vespids. Viruses 2020, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Marzoli, F.; Forzan, M.; Bortolotti, L.; Pacini, M.I.; Rodríguez-Flores, M.S.; Felicioli, A.; Mazzei, M. Next generation sequencing study on RNA viruses of Vespa velutina and Apis mellifera sharing the same foraging area. Transbound. Emerg. Dis. 2021, 68, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Cappa, F.; Cini, A.; Pepiciello, I.; Petrocelli, I.; Cervo, R. Female body size, weight and fat storage rather than nestmateship determine male attraction in the invasive yellow-legged hornet Vespa velutina nigrithorax. Ethol. Ecol. Evol. 2019, 31, 73–85. [Google Scholar] [CrossRef]
- Rome, Q.; Muller, F.J.; Touret-Alby, A.; Darrouzet, E.; Perrard, A.; Villemant, C. Caste differentiation and seasonal changes in Vespa velutina (Hym.: Vespidae) colonies in its introduced range. J. Appl. Entomol. 2015, 139, 771–782. [Google Scholar] [CrossRef]
- Kishi, S.; Goka, K. Review of the invasive yellow-legged hornet, Vespa velutina nigrithorax (Hymenoptera: Vespidae), in Japan and its possible chemical control. Appl. Entomol. Zool. 2017, 52, 361–368. [Google Scholar] [CrossRef]
- Beggs, J.R.; Brockerhoff, E.G.; Corley, J.C.; Kenis, M.; Masciocchi, M.; Muller, F.; Villemant, C. Ecological effects and management of invasive alien Vespidae. BioControl 2011, 56, 505–526. [Google Scholar] [CrossRef]
- Turchi, L.; Derijard, B. Options for the biological and physical control of Vespa velutina nigrithorax (Hym.: Vespidae) in Europe: A review. J. Appl. Entomol. 2018, 142, 553–562. [Google Scholar] [CrossRef]
- Requier, F.; Rome, Q.; Villemant, C.; Henry, M. A biodiversity-friendly method to mitigate the invasive Asian hornet’s impact on European honey bees. J. Pest Sci. 2020, 93, 1–9. [Google Scholar] [CrossRef]
- Wen, P.; Cheng, Y.N.; Dong, S.H.; Wang, Z.W.; Tan, K.; Nieh, J.C. The sex pheromone of a globally invasive honey bee predator, the Asian eusocial hornet, Vespa velutina. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Cappa, F.; Cini, A.; Pepiciello, I.; Petrocelli, I.; Inghilesi, A.F.; Anfora, G.; Cervo, R. Female volatiles as sex attractants in the invasive population of Vespa velutina nigrithorax. J. Insect Physiol. 2019, 119, 103952. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.J.; Lee, J.E.; Lee, J.S.; Kim, I.; Kim, I.S. Attraction of the Invasive Hornet, Vespa velutina nigrithorax, by using Bacillus sp. BV-1 Cultures. Korean J. Environ. Agric. 2019, 38, 104–109. [Google Scholar] [CrossRef]
- Becher, M.A.; Osborne, J.L.; Thorbek, P.; Kennedy, P.J.; Grimm, V. Towards a systems approach for understanding honeybee decline: A stocktaking and synthesis of existing models. J. Appl. Ecol. 2013, 50, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Tatem, A.J. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef]
| Geographic Area | Native Hornet Predator | Introduced Hornet Predator | Native Honey Bee Prey | Introduced Honey Bee Prey | References |
|---|---|---|---|---|---|
| China | Vespa velutina, V. bicolor, V. mandarinia, V. tropica | / | Apis cerana | A. mellifera | [6,7,32,33] |
| Japan | V. mandarinia, V. simillima | V. velutina | A. cerana | A. mellifera | [4,5,17,34] |
| India | V. velutina, V. magnifica, V. orientalis, V. basalis, V. tropica, V. affinis | / | A. cerana, A. dorsata | A. mellifera | [35,36,37,38] |
| Thailand | V. tropica | / | A. cerana | A. mellifera | [39] |
| Borneo | V. multimaculata | / | A. nuluensis | A. mellifera | [40] |
| Vietnam | V. soror | / | A. cerana | A. mellifera | [41] |
| Korea | V. analis, V. crabro, V. dybowskii, V. mandarinia, V. simillima | V. velutina | A. cerana | A. mellifera | [14,42,43] |
| Middle East | V. orientalis, V. crabro | / | A. mellifera | / | [44,45] |
| Europe | V. orientalis, V. crabro | V. velutina | A. mellifera | / | [15,16,27,28,29,30,31] |
| North America | / | V. crabro, V. mandarinia | / | A. mellifera | [12,13,19,20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappa, F.; Cini, A.; Bortolotti, L.; Poidatz, J.; Cervo, R. Hornets and Honey Bees: A Coevolutionary Arms Race between Ancient Adaptations and New Invasive Threats. Insects 2021, 12, 1037. https://doi.org/10.3390/insects12111037
Cappa F, Cini A, Bortolotti L, Poidatz J, Cervo R. Hornets and Honey Bees: A Coevolutionary Arms Race between Ancient Adaptations and New Invasive Threats. Insects. 2021; 12(11):1037. https://doi.org/10.3390/insects12111037
Chicago/Turabian StyleCappa, Federico, Alessandro Cini, Laura Bortolotti, Juliette Poidatz, and Rita Cervo. 2021. "Hornets and Honey Bees: A Coevolutionary Arms Race between Ancient Adaptations and New Invasive Threats" Insects 12, no. 11: 1037. https://doi.org/10.3390/insects12111037
APA StyleCappa, F., Cini, A., Bortolotti, L., Poidatz, J., & Cervo, R. (2021). Hornets and Honey Bees: A Coevolutionary Arms Race between Ancient Adaptations and New Invasive Threats. Insects, 12(11), 1037. https://doi.org/10.3390/insects12111037







