The First Record of Teredidae (Coleoptera, Coccinelloidea) from China, with Description of a New Species of Teredus Dejean, 1835 †
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- FSCA—Florida State Collection of Arthropods, Gainesville, Florida, USA;
- GDZI—Institute of Zoology, Guangdong Academy of Sciences, Guangzhou, China;
- IZAS—Insitute of Zoology, Chinese Academy of Sciences, Beijing, China;
- IOZ—Chinese National Zoological Museum of China, Academy of Sciences, Institute of Zoology, Beijing, China;
- KIZ—Kunming Institute of Zoology. CAS, Kunming, China;
- NHMUK—The Natural History Museum, London, UK;
- SYSBM—The Museum of Biology, Sun Yat-sen University, Guangzhou, China;
- ZIN—Russian Academy of Sciences, Zoological Institute, St. Petersburg, Russia.
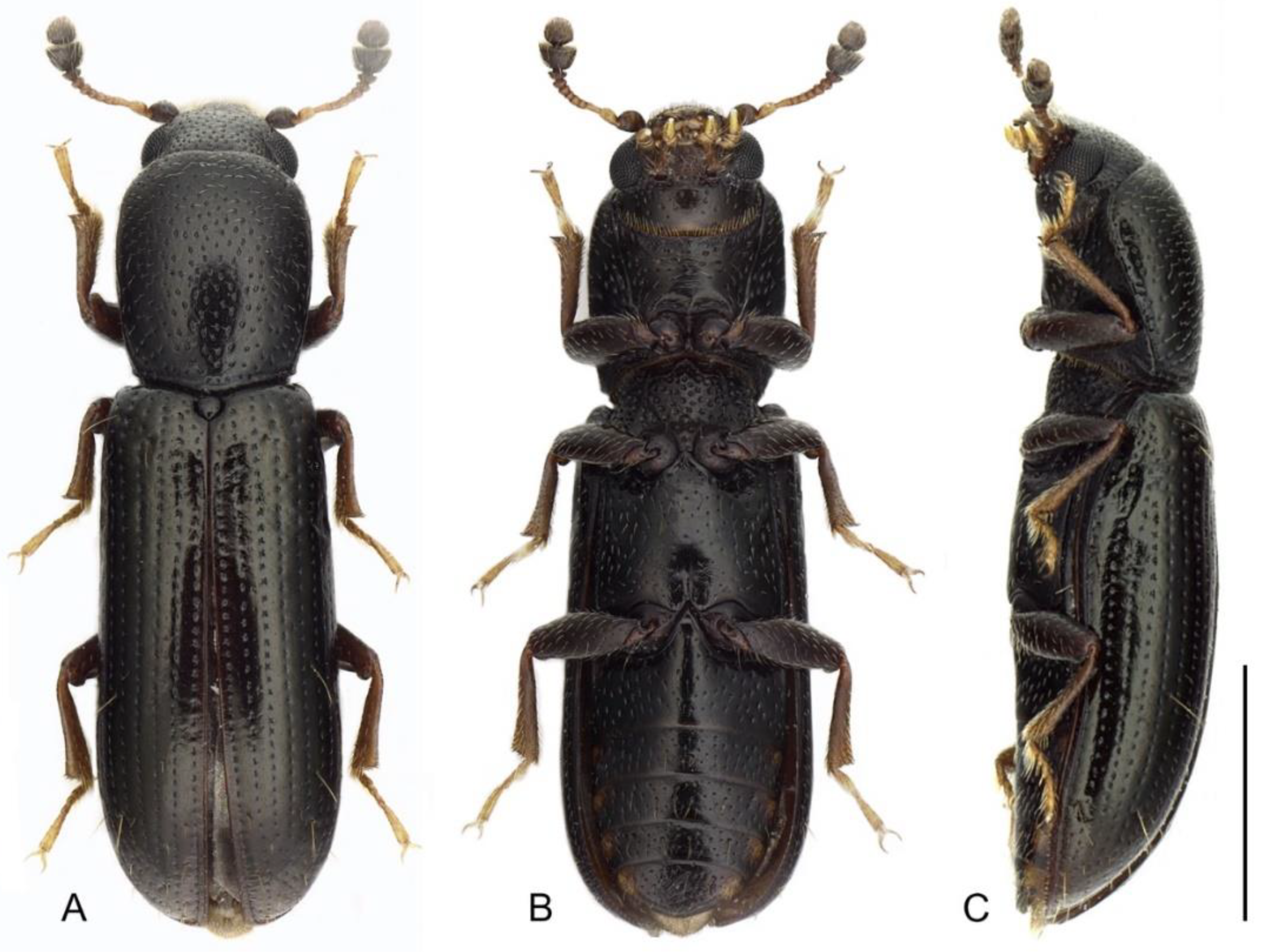
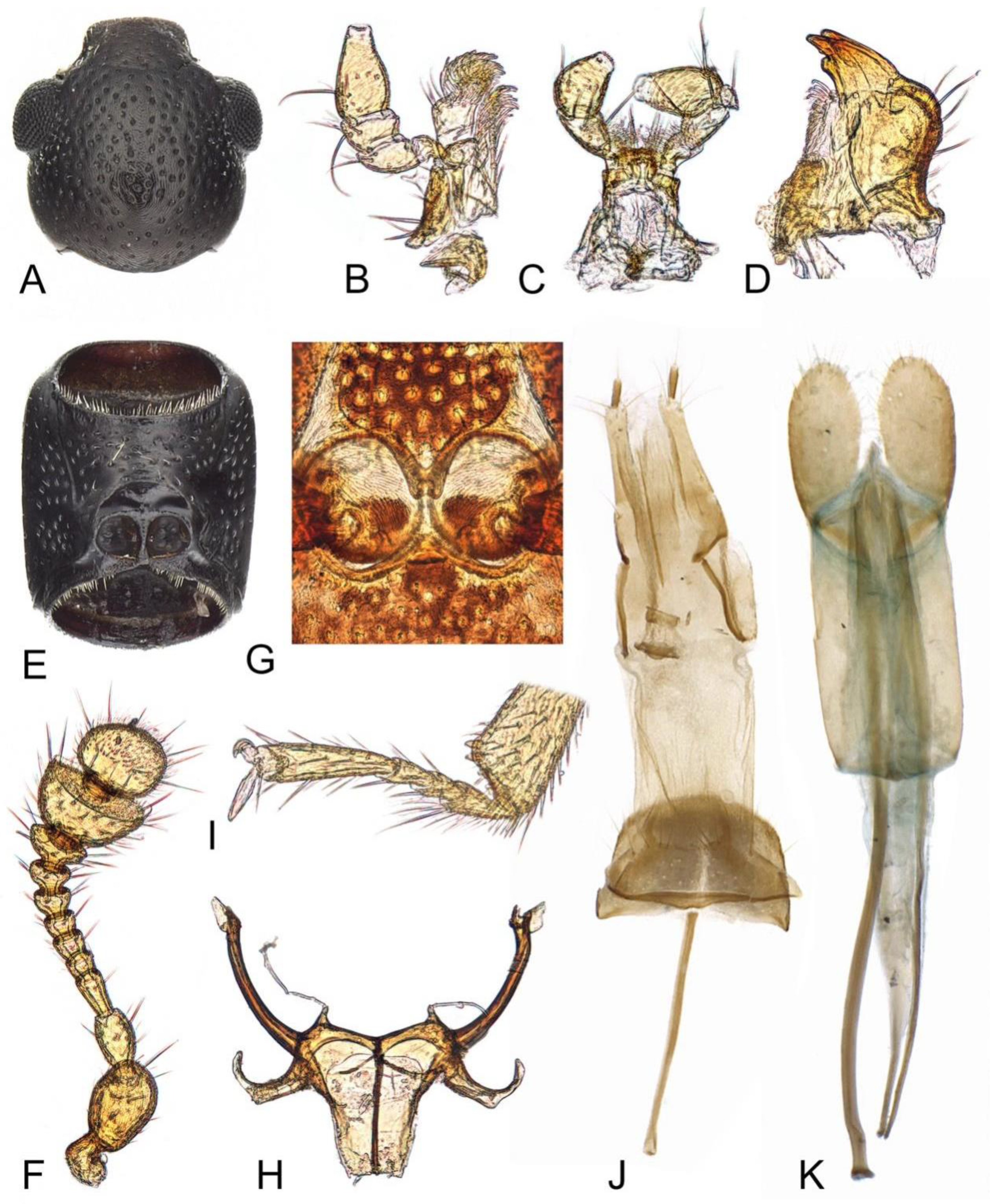
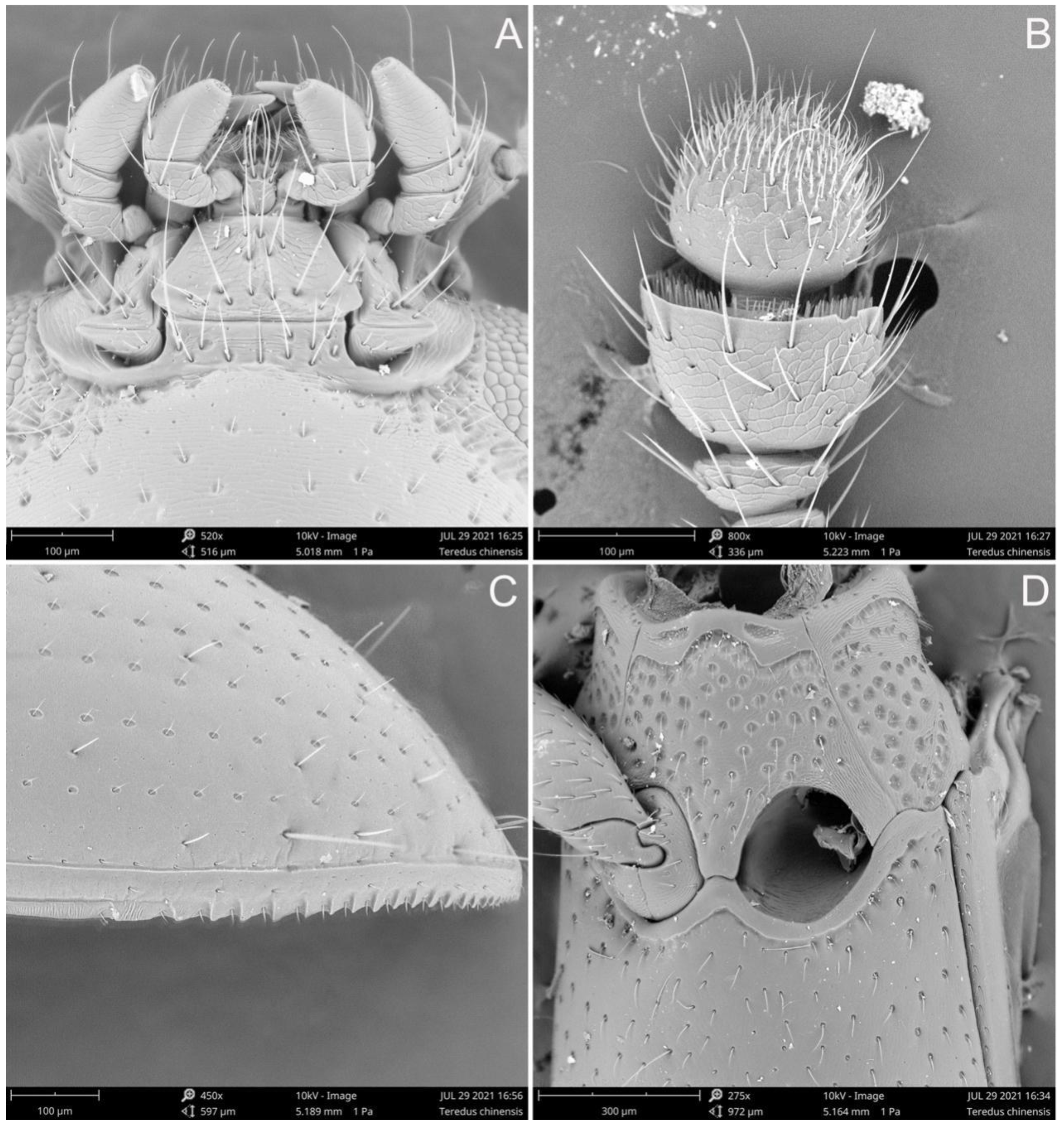
3. Results
- The elytra present with several pairs of long setae, apical fifth of lateral margins serrate; the punctation on pronotum and elytra are relatively coarser···················Teredus chinensis sp. nov.
- -
- The elytra are glabrous, without long setae; the lateral margins are smooth to the apex; the punctuation on pronotum and elytra are fine································· ····························2.
- The body presents with a rufous dorsum, similar to the colour of antennae and legs; the pronotum is slightly constricted near the base···················································Teredus opacus Habelmann.
- -
- The body presents with a nearly black dorsum, distinctly different from the brownish antennae and legs; the pronotum is not constricted near the base················Teredus cylindricus (Oliver).
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ślipiński, A.; Lord, N.; Lawrence, J.F. 10.28. Bothrideridae Erichson, 1845. In Handbook of Zoology. Arthropoda: Insecta Coleoptera; Beetles Morphology and Systematics (Elateroidea, Bostriformia, Cucujiformia Partim); Leschen, R.A.B., Beutel, R.G., Lawrence, J.F., Eds.; De Gruyter: Berlin, Gemany, 2010; Volume 2, pp. 411–422. [Google Scholar]
- Robertson, J.A.; Ślipiński, A.D.; Moulton, M.; Shockley, F.W.; Giorgi, A.; Lord, N.P.; Mckenna, D.D.; Tomaszewska, W.; Forrester, J.; Miller, K.B.; et al. Phylogeny and classification of Cucujoidea and the recognition of a new superfamily Coccinelloidea (Coleoptera: Cucujiformia). Syst. Entomol. 2015, 40, 745–778. [Google Scholar] [CrossRef]
- Blackwelder, R.E. (Ceryloninae) Checklist of the coleopterous insects of México, Central America, The West Indies, and South America. Bull. U.S. Natl. Mus. 1945, 185, 472–473. [Google Scholar]
- Horion, A. Faunistik der Mitteleuropäischen Käfer. Band VIII: Clavicornia.2 Teil (Thorictidae bis Ciidae), Teredilia, Coccinellidae; A. Feyel: Überlingen, Germany, 1961; Volume 8, pp. 1–375. [Google Scholar]
- Lawrence, J.F. The genus Teredolaemus Sharp (Coleoptera: Bothrideridae) in Australia. J. Aust. Entomol. Soc. 1985, 24, 205–206. [Google Scholar] [CrossRef]
- Pal, T.K.; Lawrence, J.F. A new genus and subfamily of mycophagous Bothrideridae (Coleoptera: Cucujoidea) from the Indo-Australian region, with notes on related families. Aust. J. Entomol. Soc. 1986, 25, 185–210. [Google Scholar] [CrossRef]
- Dejean, P.F.M.A. Catalogus des Coleopteres de la Collection de M. le Comte Dejean; Deuxieme Edition (Livraison 4); Mequignon-Marvis Pere et Filsm: Paris, France, 1835; pp. 257–360. [Google Scholar]
- Ganlbauer, L. Die Käfer von Mitteleuropa: Käfer der Österreichisch-Ungarischen Monarchie, Deutschlands, der Schweiz, Sowie des Französischen und Italienischen Alpengebietes; Carl Gerold’s Sohn: Wien, Austria, 1899; p. 1048. [Google Scholar]
- Crowson, R.A. The Natural Classification of the Families of Coleoptera; The Holywell Press Ltd.: Oxford, UK, 1995; p. 187. [Google Scholar]
- Craighead, F.C. Biology of some Coleoptera of the families Colydiidae and Bothrideridae. Proc. Entomol. Soc. Wash. 1920, 22, 1–13. [Google Scholar]
- Boving, A.G.; Craighead, F.C. An Illustrated Synopsis of the Principal Larval Forms of the Order Coleoptera; Brooklyn Entomological Society: New York, NY, USA, 1931; p. 351. [Google Scholar]
- Lawrence, J.F. A new genus of Indo-Australian Gempylodini with notes on the constitution of the Colydidae (Coleoptera). Aust. J. Entomol. 1980, 19, 293–310. [Google Scholar] [CrossRef]
- Lawrence, J.F. Bothrideridae (Cucujoidea). In Immature Insects; Stehr, F.W., Ed.; Kendall Hunt Publishing Company: Dubuque, IA, USA, 1991; Volume 2, pp. 477–479. [Google Scholar]
- Lord, N.P.; McHugh, J.V. A Taxonomic Revision of the Genus Deretaphrus Newman, 1842 (Coleoptera: Cucujoidea: Bothrideridae). Coleopt. Bull. 2013, 12, 1–107. [Google Scholar] [CrossRef]
- Mckenna, D.D.; Wild, A.L.; Kanda, K.; Bellamy, C.L.; Beutel, R.G.; Caterino, M.S.; Farnum, C.W.; Hawks, D.C.; Ivie, M.A.; Jameson, M.L.; et al. The beetle tree of life reveals that Coleoptera survived end-Permian mass extinction to diversify during the Cretaceous terrestrial revolution. Syst. Entomol. 2015, 40, 835–880. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.Q.; Che, L.H.; Li, Y.; Liang, D.; Pang, H.; Ślipiński, A.; Zhang, P. Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenna, D.D.; Shin, S.; Ahrens, D.; Balke, M.; Beza-Beza, C.; Clarke, D.J.; Donath, A.; Escalona, H.E.; Friedrich, F.; Letsch, H.; et al. The evolution and genomic basis of beetle diversity. Proc. Natl. Acad. Sci. USA 2019, 116, 24729–24737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, J.; Slipinski, A. Australian Beetles Volume 1: Morphology, Classification and Keys; Csiro Publishing: Clayton, Australia, 2018; p. 561. [Google Scholar]
- Zhou, Y.L.; Lord, N.P.; Ślipiński, A. Review of the Australian Teredolaemus Sharp, 1885 (Coleoptera: Teredidae), with descriptions of five new species. Austral Entomol. 2017, 56, 439–450. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Lin, W.; Li, Z. The First Record of Teredidae (Coleoptera, Coccinelloidea) from China, with Description of a New Species of Teredus Dejean, 1835. Insects 2021, 12, 1028. https://doi.org/10.3390/insects12111028
Liu Z, Lin W, Li Z. The First Record of Teredidae (Coleoptera, Coccinelloidea) from China, with Description of a New Species of Teredus Dejean, 1835. Insects. 2021; 12(11):1028. https://doi.org/10.3390/insects12111028
Chicago/Turabian StyleLiu, Zhenhua, Wei Lin, and Zhiqiang Li. 2021. "The First Record of Teredidae (Coleoptera, Coccinelloidea) from China, with Description of a New Species of Teredus Dejean, 1835" Insects 12, no. 11: 1028. https://doi.org/10.3390/insects12111028
APA StyleLiu, Z., Lin, W., & Li, Z. (2021). The First Record of Teredidae (Coleoptera, Coccinelloidea) from China, with Description of a New Species of Teredus Dejean, 1835. Insects, 12(11), 1028. https://doi.org/10.3390/insects12111028





