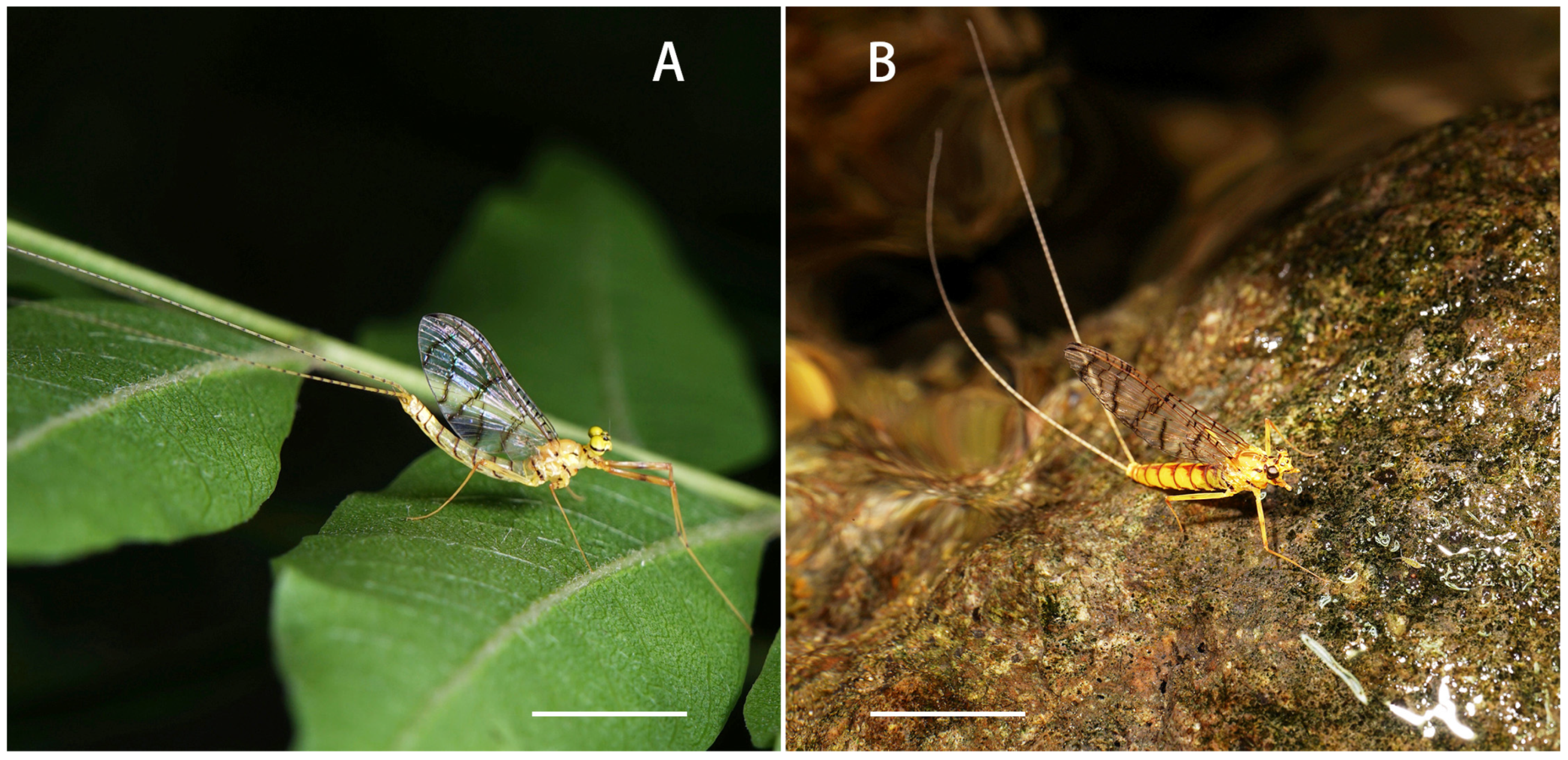
3.2. Genus Definition
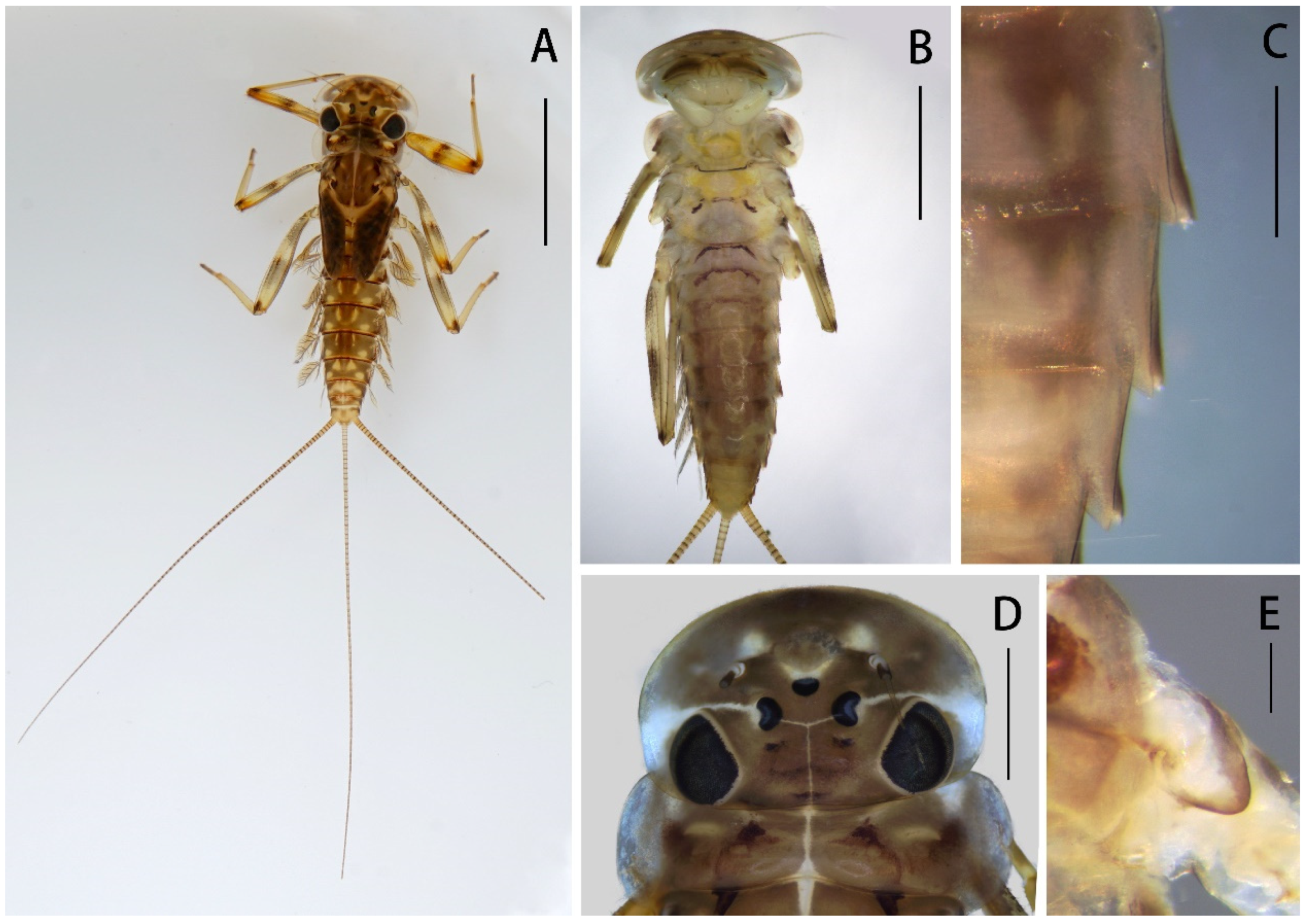
Nymph: pronotum expanded laterally, wider than head (
Figure 1D); labrum ca. half of head width, with round lateral apex (
Figure 2A); superlinguae of hypopharynx extended posterolaterally into bent blunt apex (
Figure 2B); maxillae with two distal dentisetae (second one bifurcated) and one proximal dentiseta (with two branches, second one fringed) (
Figure 3B,C); supracoxal spurs well-developed, round (
Figure 1E); lamellae of gills I–VI extended into projections (
Figure 4F–H), gills VII with lamellae only, near leaf-like in shape (
Figure 4I); caudal filaments with spines on articulations.
Imago: distance between two compound eyes of males 3× diameter of middle ocelli (
Figure 5A and
Figure 6A); numbers of crossveins on forewings reduced (less than 15 in C and Sc sections), all crossveins obviously pigmented in yellowish brown; except those in C and Sc fields and wingbase, all other crossveins of forewings arranged into five rows (
Figure 6D); length of hindwings 1.5× of width, with brownish pigmented margins (
Figure 6E); first tarsal segment of male forelegs 0.7× second one (
Figure 7A); tarsi of mid- and hindlegs subequal to tibiae in length (
Figure 7B,C); styliger plate convex, posterior margin with two remarkable lateral projections, penes entirely fused, inner median titillators present (
Figure 7D,E and
Figure 8B). Female subgenital and subanal plates greatly extended posteriorly (
Figure 7F and
Figure 8C,D).
Egg: Ovoid, chorion decorated randomly with irregularly shaped small KCTs on whole surface (
Figure 9A). Micropyle oval and located equatorially (
Figure 9B).
Etymology: The genus name Regulaneuria is feminine, from Latin word “Regula-” (regular) and “-neuria” (vein). It indicates the imagoes of this species having few regularly aligned crossveins on forewings.
Diagnosis: upon the anteriorly divergent medial depression of mesothoracic furcasternum in imagoes and scattered setae on the ventral surface of the maxillae, obviously this new genus is a member of the subfamily Ecdyonurinae according to the definition of Kluge [
10] and the key of Webb and McCafferty [
11].
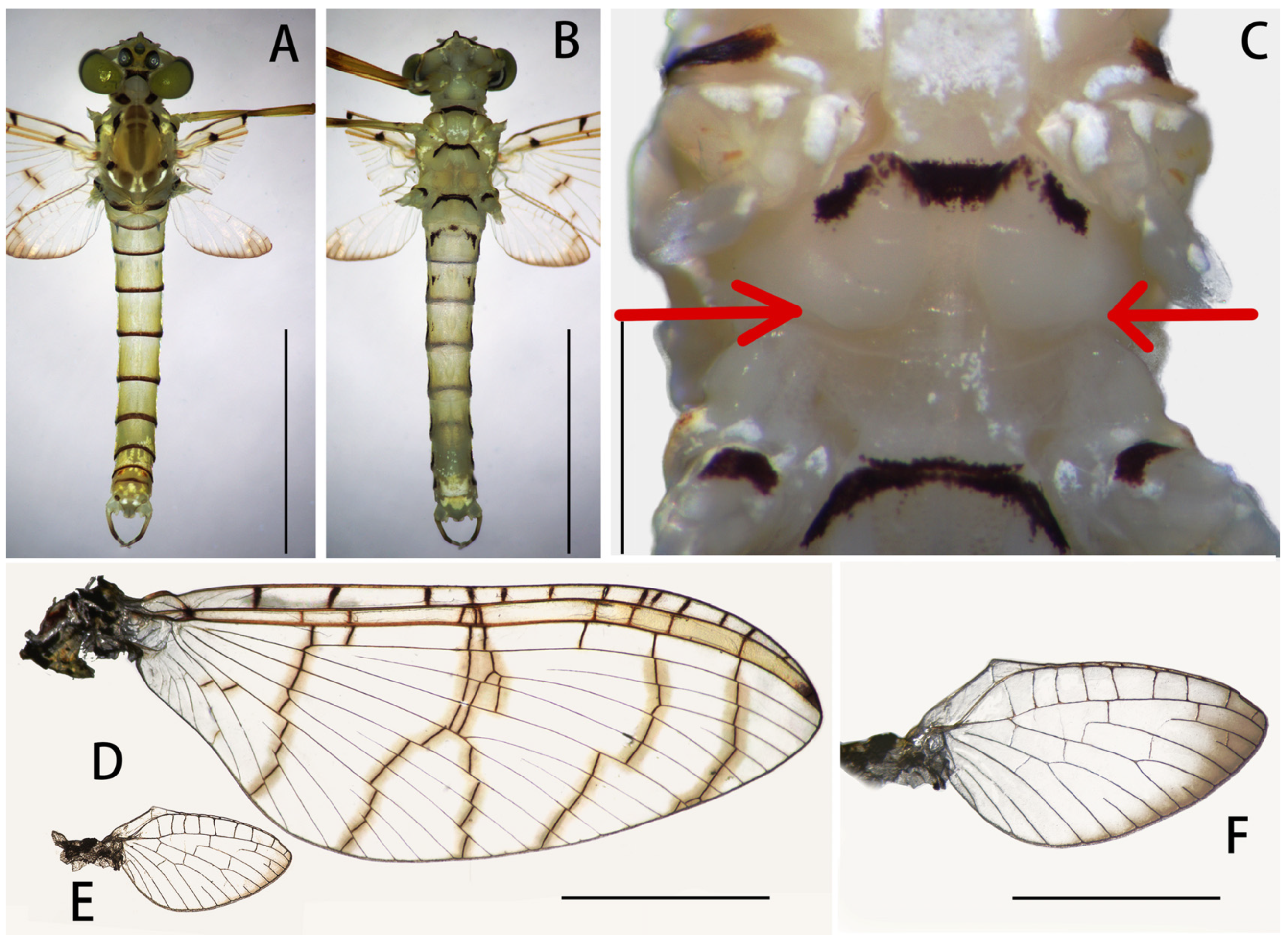
Figure 1.
Nymph of Regulaneuria cingulata: (A) Nymphal habitus; (B) Ventral surface of nymph; (C) Posterolateral projections of terga V–VII (ventral view); (D) Head capsule and pronotum; (E) Supracoxal spur (midleg). Scale bars: (A) = 5.0 mm; (B) = 2.0 mm; (C) = 0.5 mm; (D) = 1.0 mm; (E) = 0.1 mm.
Figure 1.
Nymph of Regulaneuria cingulata: (A) Nymphal habitus; (B) Ventral surface of nymph; (C) Posterolateral projections of terga V–VII (ventral view); (D) Head capsule and pronotum; (E) Supracoxal spur (midleg). Scale bars: (A) = 5.0 mm; (B) = 2.0 mm; (C) = 0.5 mm; (D) = 1.0 mm; (E) = 0.1 mm.
Figure 2.
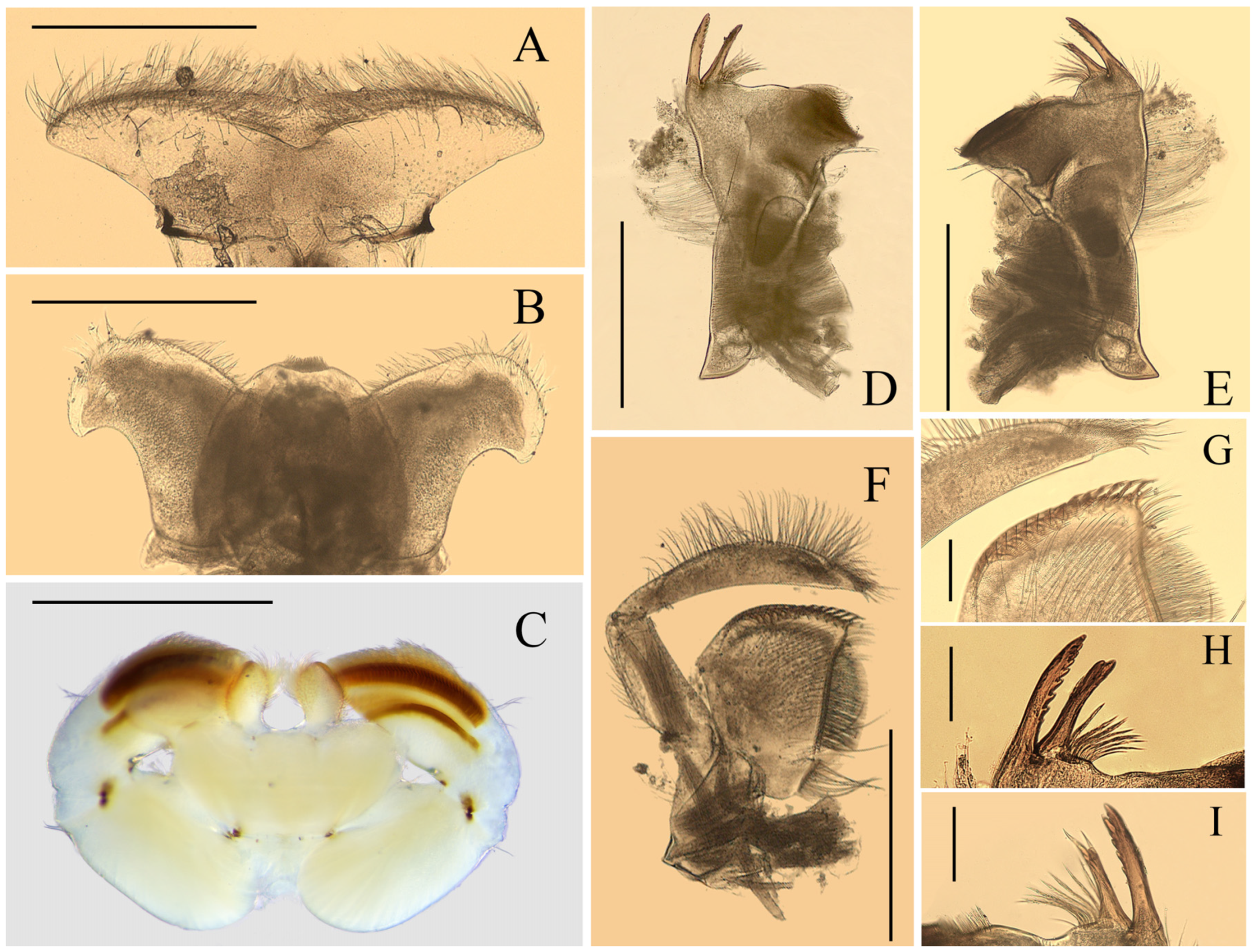
Mouthparts of Regulaneuria cingulata: (A) Labrum (ventral view); (B) Hypopharynx (ventral view); (C) Labium (ventral view); (D) Left mandible (ventral view); (E) Right mandible (ventral view); (F) Maxillae; (G) Apex of maxillae; (H) Incisors of left mandible; (I) Incisors of right mandible. Scale bars: (A,B,D–F) = 0.5 mm; (C) = 1.0 mm; (G) = 0.2 mm; (H,I) = 0.1 mm.
Figure 2.
Mouthparts of Regulaneuria cingulata: (A) Labrum (ventral view); (B) Hypopharynx (ventral view); (C) Labium (ventral view); (D) Left mandible (ventral view); (E) Right mandible (ventral view); (F) Maxillae; (G) Apex of maxillae; (H) Incisors of left mandible; (I) Incisors of right mandible. Scale bars: (A,B,D–F) = 0.5 mm; (C) = 1.0 mm; (G) = 0.2 mm; (H,I) = 0.1 mm.
Figure 3.
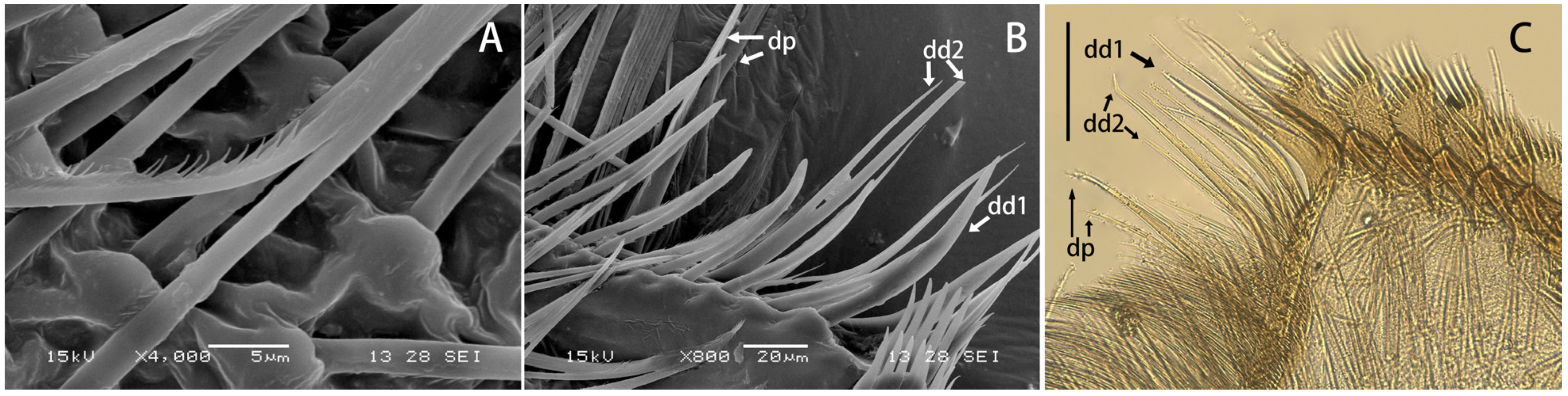
Maxillae of Regulaneuria cingulata (A) SEM view of fimbriate scattered setae on the ventral face of the galea; (B) SEM view of the distal dentisetae and proximal dentiseta; (C) Same view in optical microscope. Scale bars: (C) = 0.5 mm.
Figure 3.
Maxillae of Regulaneuria cingulata (A) SEM view of fimbriate scattered setae on the ventral face of the galea; (B) SEM view of the distal dentisetae and proximal dentiseta; (C) Same view in optical microscope. Scale bars: (C) = 0.5 mm.
Figure 4.
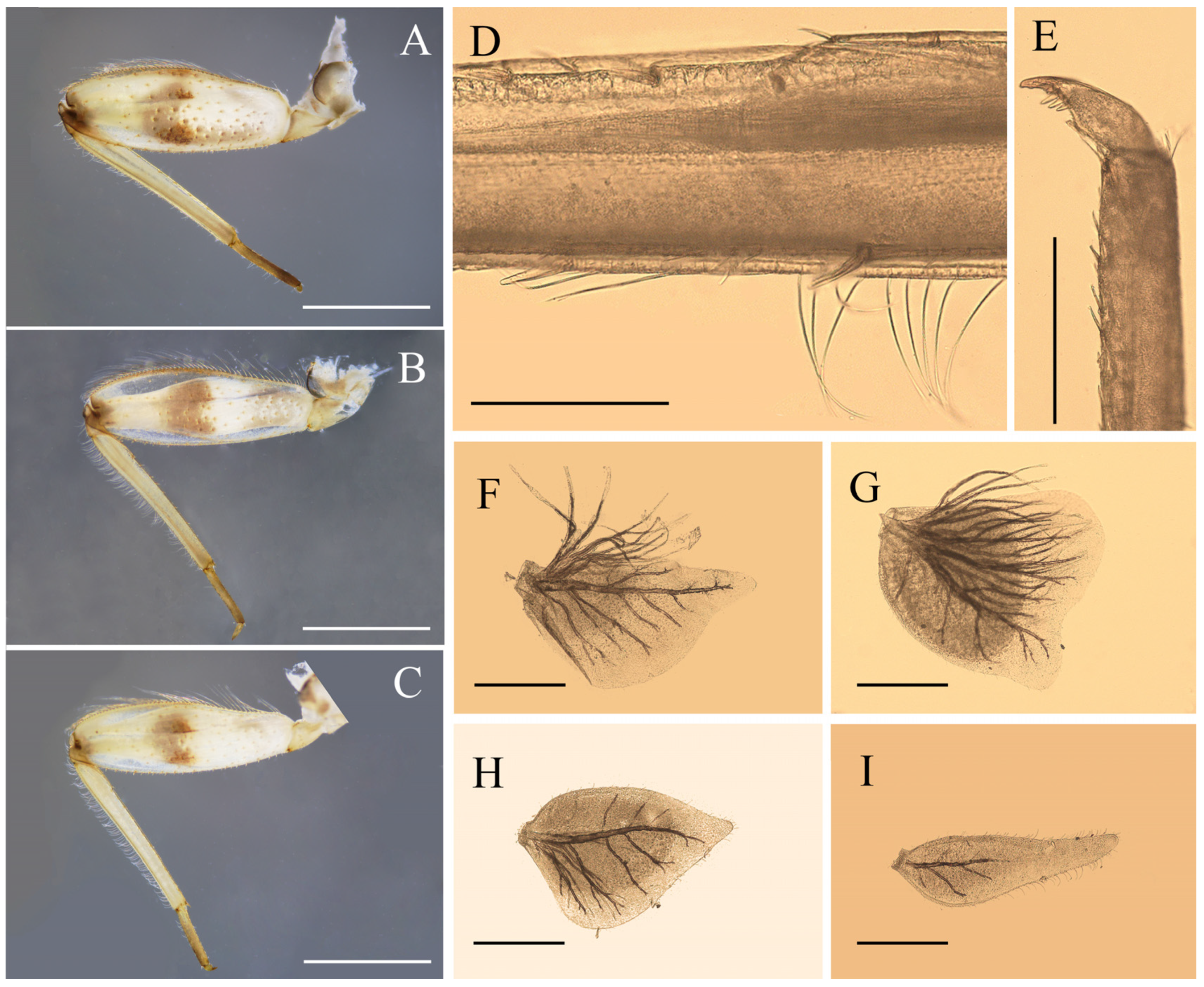
Nymphal structures of Regulaneuria cingulata: (A) Foreleg; (B) Midleg; (C) Hindleg; (D) Hindleg tibia; (E) Foreleg claw; (F) Gill I; (G) Gill II; (H) Gill VI; (I) Gill VII. Scale bars: (A–C) = 1.0 mm; (D) = 0.2 mm; (E) = 0.5 mm; (F–I) = 0.2 mm.
Figure 4.
Nymphal structures of Regulaneuria cingulata: (A) Foreleg; (B) Midleg; (C) Hindleg; (D) Hindleg tibia; (E) Foreleg claw; (F) Gill I; (G) Gill II; (H) Gill VI; (I) Gill VII. Scale bars: (A–C) = 1.0 mm; (D) = 0.2 mm; (E) = 0.5 mm; (F–I) = 0.2 mm.
Figure 5.
Adults of Regulaneuria cingulata: (A) Male imago habitus; (B) Female imago habitus. Scale bars: (A,B) = 5.0 mm.
Figure 5.
Adults of Regulaneuria cingulata: (A) Male imago habitus; (B) Female imago habitus. Scale bars: (A,B) = 5.0 mm.
Figure 6.
Male structures of Regulaneuria cingulata: (A) Male imago (dorsal view); (B) Male imago (ventral view); (C) Medial depression of furcasternum (shown in red arrow); (D) Forewing; (E) Hindwing; (F) Hindwing enlarged. Scale bars: (A,B) = 3.0 mm; (C) = 1.0 mm; (D,E) = 2.0 mm; (F) = 1.0 mm.
Figure 6.
Male structures of Regulaneuria cingulata: (A) Male imago (dorsal view); (B) Male imago (ventral view); (C) Medial depression of furcasternum (shown in red arrow); (D) Forewing; (E) Hindwing; (F) Hindwing enlarged. Scale bars: (A,B) = 3.0 mm; (C) = 1.0 mm; (D,E) = 2.0 mm; (F) = 1.0 mm.
Figure 7.
Adult structures of Regulaneuria cingulata: (A) Foreleg of male; (B) Midleg of male; (C) Hindleg of male; (D) Genitalia; (E) Penes enlarged (ventral view); (F) Subgenital and subanal plates of female (ventral view). Scale bars: (A–C) = 2.0 mm; (D) = 0.5 mm; (E) = 0.2 mm; (F) = 2.0 mm.
Figure 7.
Adult structures of Regulaneuria cingulata: (A) Foreleg of male; (B) Midleg of male; (C) Hindleg of male; (D) Genitalia; (E) Penes enlarged (ventral view); (F) Subgenital and subanal plates of female (ventral view). Scale bars: (A–C) = 2.0 mm; (D) = 0.5 mm; (E) = 0.2 mm; (F) = 2.0 mm.
Figure 8.
Nymphal and imaginal structures of Regulaneuria cingulata: (A) Whorls of spines of caudal filaments; (B) Genitalia (ventral view); (C) Subgenital and subanal plates of female imago (ventral view); (D) Subanal plate of female imago (dorsal view). Scale bars: (A,B) = 0.2 mm; (C,D) = 0.5 mm.
Figure 8.
Nymphal and imaginal structures of Regulaneuria cingulata: (A) Whorls of spines of caudal filaments; (B) Genitalia (ventral view); (C) Subgenital and subanal plates of female imago (ventral view); (D) Subanal plate of female imago (dorsal view). Scale bars: (A,B) = 0.2 mm; (C,D) = 0.5 mm.
Figure 9.
Egg of Regulaneuria cingulata: (A) Whole picture; (B) Micropyle and small KCTs enlarged.
Figure 9.
Egg of Regulaneuria cingulata: (A) Whole picture; (B) Micropyle and small KCTs enlarged.
Regarding imaginal reduced crossveins of C and Sc sections on forewings and nymphal two independent distal dentisetae and similar proximal dentiseta (with a fringed branch), our new genus is related to the three genera (
Compsoneuria Eaton, 1881,
Compsoneuriella Ulmer, 1939 and
Notonurus Crass, 1947) of Compsoneuriini
sensu Wang and McCafferty (2004) and Sartori (2014) [
8,
12,
13,
14,
15]. However, in contrast to the two Oriental genera
Compsoneuria and
Compsoneuriella, our new genus can be differentiated from the former one by longer first segment of foretarsi (ca. 0.7× of second one), colorful wings and regularly aligned crossveins; in nymph, by narrower labrum, acute tips of hypopharynx superlinguae, extended gill lamellae and pronotum, forked second distal dentisetae of maxillae. In contrast with the genus
Compsoneuriella, our new genus has less crossveins of forewings and fused penes. The nymphal characters mentioned above are also good features to separate these two genera and their supracoxal spurs are also different (acute in
Compsoneuriella but blunt in
Regulaneuria). The Afrotropical genus
Notonurus is different to our new genus
Regulaneuria in multi-branched distal dentisetae and simple scattered setae on the maxillae in nymphal stage. In imaginal stage, their penes are divergent apically and crossveins are randomly distributed [
8].
Generally, both imagoes and nymphs of our new genus
Regulaneuria gen. nov. are similar to those of
Asionurus Braasch and Soldán, 1986a in some way, such as imaginal genitalia (almost totally fused penes with tiny titillators) and nymphal elongated gills VII, bent tips of hypopharynx superlinguae, expanded laterally pronotum [
7]. However, the imagoes of the latter genus have more randomly arranged crossveins on forewings and shorter tarsi of mid-and hindlegs than the former one. In the nymphal stages, two genera can be separated by the tips of hypopharynx superlinguae (acute in
Asionurus but blunt in
Regulaneuria gen. nov.), labrum (greatly extended in
Asionurus while only slightly extended in
Regulaneuria) and gills I–V (with smooth outlines in
Asionurus but with waved posterior margins in
Regulaneuria).
Regulaneuria gen. nov. has several common characters of different related genera: the widely separated compound eyes (such as
Leucrocuta Flowers, 1980 and
Stenacron Jensen, 1972 [
16,
17], for example,
Leucrocuta hebe (McDunnough, 1924) and
Stenacron carolina (Banks, 1914) [
18,
19]); remarkably reduced crossveins of forewings (as in some species in the genera
Compsoneuria and
Compsoneuriella, e.g.,
Compsoneuria spectabilis Eaton, 1881 and
Compsoneuriella braaschi Boonsoong and Sartori, 2015) [
12,
20]; hindwings with pigmented margins (similar to
Atopopus Eaton, 1881 [
12], such as
Atopopus edmundsi Wang and McCafferty, 1995) [
21]; subequal tarsi and tibiae of hindlegs (similar to Compsoneuriini,
Thalerosphyrus Eaton, 1881 and some species of
Epeorus Eaton, 1881, such as
E.
melli Ulmer, 1925) [
12]. Male genitalia, female subanal plate, nymphal gills VII and hypopharynx of this new genus is close to
Asionurus Braasch and Soldán, 1986 (e.g.,
A. primus Braasch and Soldán, 1986b) [
22]. The morphology of proximal dentiseta of our new genus such as those of the genera
Compsoneuria and
Compsoneuriella [
8].
In recent years, more heptageniid eggs are scanned [
23,
24,
25]. Although without any recent comprehensive review on heptageniid eggs, we can compare them preliminarily among related genera. Due to being without any polar cap and similar knob-terminated coiled threads in size and position, the eggs of our new genus
Regulaneuria is alike those of
Ecdyonurus in some level [
25]. However, the knob-terminated coiled threads of the former are less and bigger than the latter. The threads on eggs of the
Asionurus,
Notacanthurus and
Thalerosphyrus are further smaller than those of
Ecdyonurus [
26]. In contrast, the eggs of
Compsoneuria and
Compsoneuriella have much larger and denser coiled threads on surface than those of
Regulaneuria. The eggs of
Afronurus have large coiled threads on equatorial portion.
Among above combinations, three characters are regarded as the key identification ones of our new genus: greatly reduced, regularly aligned and pigmented crossveins of forewings, midlegs with subequal tibiae and tarsi, nymphal lamellae of gills I–VII with expanded apexes.
Distribution: China (Zhejiang Province).
3.3. Regulaneuria Cingulata (Navás, 1933) Comb. Nov.
Thalerosphyrus cingulatus Navás, 1933: 18. Types: adult, from Chusan, Zhejiang, China.
Thalerosphyrus cingulatus: Wu, 1935: 252; Ulmer, 1935–1936: 215 [
27]; Gui, 1985: 86; Zhou, 2013: 195; Zhou et al., 2015: 244; Sartori, 2014: 12.
Compsoneuria cingulata: Braasch and Soldán, 1986a: 46 (generic transfer); Webb, Braasch and McCafferty, 2006: 58.
Materials examined. Male imago (designed herein as neotype), Jiu-Feng Mountain (29°51′19.28″ N, 121°50′24.48″ E; alt. 62 m), Ningbo city, Zhejiang Province, 2021-VI-8-10, leg. Peng-Xu MU, De-Wen GONG; 15 male imagoes, 28 female imagoes and 10 larvae, same as neotype; 20 male imagoes, 4 male subimagoes, 15 female imagoes, 2 female subimagoes and 18 larvae, Jing-Tou village (27°30′44.11″ N, 120°29′37.51″ E; alt. 80 m), Wenzhou city, Zhejiang Province, 2021-VII-2-3, leg. De-Wen GONG.
Description:
Nymph: (in alcohol,
Figure 1,
Figure 2 and
Figure 3 and
Figure 8A): body length 6.0–8.0 mm, caudal filaments 7.0 mm, body yellowish-brown to brown (
Figure 1A). Head capsule near sub-oval but posterior margin nearly straight, 2–3 pale dots located near base of antennae (
Figure 1A,D).
Labrum ca. 0.5× width of head, with round apexes; anterior margin with shallow median groove, both surfaces with dense and long setae but those on dorsal surface much longer and denser (
Figure 2A). Both mandibles covered with numerous setae on outer margins; prostheca with 8–12 fimbriate bristles (
Figure 2D,E,H,I); outer incisor of left mandible with serrated margin and one larger terminal denticle; inner incisor subequal to outer one in length and with bifurcated apex (
Figure 2D,H); outer incisor of right mandible serrated and with two apical terminal denticles; inner incisor divided into two sharp denticles too (
Figure 2E,I). Hypopharynx: superlinguae curved and extended into blunt apex, row of hair-like setae on lateral margins from base to sub-apex; lingua bell-like, shorter than superlinguae in length and with tuft of short setae at apex (
Figure 2B). Maxillae with scattered setae on ventral surface (
Figure 2F,G), row of 13 comb-shaped setae on crown of galea-lacinia; two distal dentisetae arise from galea-lacinia independently; first one longer than others, second one divided into two branches; proximal dentiseta bifurcated, smaller one fringed; scattered setae on ventral surface of maxillae fimbriate (
Figure 3A−C); first segment of maxillary palpi with sparse setae on outer margin; second segment obviously longer than basal one, outer margin with long setae, terminal segment with dense setaceous brush (
Figure 2F). Labium: glossae slightly rhomboid, inner margin with tuft of long setae; paraglossae with dense hair-like setae and bristle-like setae on dorsal and anterior margins; labial palpi broad, ventral surface with setae brushes and scattered setae, similar setae on margins of labial palpi (
Figure 2C).
Nota of thorax yellowish brown with pale markings, posterior tips of wingpads dark; sterna of thorax pale (sub-imaginal brown stripes visible in some mature nymphs) (
Figure 1A,B); pronotum extended laterally, obviously wider than head (
Figure 1A,D). Supracoxal spurs rounded (
Figure 1E). Color pattern of all femora similar: three yellowish brown stripes washed at base, middle and apex, respectively, two additional dark brown dots at middle and apex; tibiae yellowish, tarsi of all legs slightly browner than tibiae; femora of all legs with long setae on outer margins, dorsal surfaces and inner margins with spatulate setae (
Figure 4A–C). Foretibiae length 0.85× of femora, outer margin with short hair-like setae at base (
Figure 4A), inner margin with row of bristle-like setae only; foretarsi length ca. 1/3 of tibiae, outer and inner margins with tiny hair-like setae. Midlegs similar to forelegs, except tibiae 0.79× length of femora (
Figure 4B). Hindleg tibiae 0.82× of femora, outer margin with rows of dense hair-like setae and bristles, dorsal surface with row of bristle-like setae (
Figure 4C,D). Claws of all legs with 3–4 subapical denticles (
Figure 4E).
Abdominal terga I–X yellowish brown, with two median and two sub-lateral pale dots, but those dots on terga VIII–X fused together forming a larger one (
Figure 1A). Sterna I–II with brown stripes on anterior margins (
Figure 1B). Posterolateral angles of terga V–VII extended into small acute projections (
Figure 1B,C). Lamellae of gills I broad with blunt apexes (
Figure 4F); gills II–IV lamellae greatly expanded, posterior apexes rounded, with more fibrilliform than gills I (
Figure 4G), lamellae of gills V–VI heart-like, with small blunt apexes (
Figure 4H); gills VII near leaf-like in shape, with fine marginal setae while without fibrillae (
Figure 4I). Caudal filaments pale to yellowish, with whorled spines on articulations (
Figure 1A and
Figure 8A).
Male imago: (in alcohol,
Figure 5A,
Figure 6, 7Figure A–E and
Figure 8B): Body length 6.0–8.0 mm, forewings 7.0–8.0 mm, hindwings 1.5–2.0 mm, cerci 16.0–19.0 mm. Compound eyes clearly separated, distance between them 3× width of median ocellus (
Figure 6A).
Mesonotum with apparent transverse suture, medial depression of furcasternum parallel (
Figure 6B,C); pronota, mesonota and metanota with dark brown posterior margins, respectively, those stripes extended to coxa of all legs (
Figure 6B,C); mesosternum and metasternum with dark brown posterior margins (
Figure 6B). All legs with similar color pattern: femora reddish yellow to reddish brown, with three dark brown dots at base, middle and apex; tibiae yellowish brown with dark brown apex; tarsi yellowish (
Figure 7A–C). Forelegs: length ratio of femora:tibiae:tarsi = 2.3:2.2:3.9, tarsal segments from basal to apical = 0.7:1.0:1.1:0.8:0.3 (
Figure 7A). Midlegs: length ratio of femora:tibiae:tarsi = 1.9:1.4:1.7, tarsal segments arranged in decreasing order as 1, 2, 3, 5, 4 (
Figure 7B). Hindlegs: length ratio of femora:tibiae:tarsi = 2.1:1.6:1.5, tarsal segments arranged in decreasing order as 1, 2, 3, 5, 4 (
Figure 7C). All claws of legs similar, one blunt and one hooked.
Forewings hyaline but with dark tips, C, Sc and R1 fields with slightly thickened and brown crossveins; all crossveins washed with yellowish brown pigments, especially those of C and Sc fields; 13 crossveins in C section, those of Sc Sections 9–10, those of other regions arranged into five regular rows; Rs and MP forked at same level at wing base, MA forked over 1/2 distance from base of wing to margin (
Figure 6D). Hindwings transparent but with remarkable brown stain on outer margins; costal margins slightly waved, blunt costal projection near base, MP forked in middle, MA forked much apically than MP (
Figure 6E,F).
Abdominal terga I–IX yellowish, with dark brown stripes on posterior and lateral margins (
Figure 6A). Sterna I–III with dark brown stripes (
Figure 6B). Genitalia: styliger plate convex and with two distinct finger-like projections laterally (
Figure 7D,E and
Figure 8B). Forceps segment III subequal to segment IV in length (length ratio of segment III:segment IV = 1.1:1), and combination segments III–IV slightly shorter than half segment II. Penes almost totally fused totally but with apical incision, penial lobe round. Titillators present in subapical and medial position on penis lobes (
Figure 7E and
Figure 8B). Cerci yellowish, with brown dots on articulations.
Female imago: (in alcohol,
Figure 5B,
Figure 7F and
Figure 8C,D): Body length 7.0–10.0 mm, forewings 8.0–10.0 mm, hindwings 2.0–3.0 mm, cerci 19.0–25.0 mm. Similar to male imago except as follows: length of femora:tibiae:tarsi of forelegs = 2.6:2.2:2.6, length order of tarsal segments arranged in decreasing order as 2, 1, 3, 4, 5; length of femora:tibiae:tarsi of midlegs = 2.6:2.0:1.7, tarsal segments arranged in decreasing order as 1, 2, 3, 5, 4; length of femora:tibiae:tarsi of hindlegs = 2.8:2.2:1.6, tarsal segments order similar to midlegs. Abdominal terga I–IX with reddish brown longitudinal stripes medially (
Figure 5B). Subgenital plate reaching posterior margin of sternum VIII (
Figure 7F and
Figure 8C), posterior margin of subanal plate extended remarkably into a straight lobe, lateral margins of it thickened into ridges dorsally (
Figure 7F and
Figure 8C,D).
Male subimago: (in alcohol): body length 6.0–7.0 mm, forewings 6.0–7.5 mm, hindwings 1.5–2.0 mm, cerci 16.0–18.0 mm. Femora:tibiae:tarsi of forelegs = 1.8:1.2:2.0, tarsal segments arranged in decreasing order as 3, 4, 2, 1, 5; length of femora:tibiae:tarsi of midlegs = 1.9:1.2:1.3, length order of tarsal segments in decreasing order as 1, 2, 3, 5, 4; length of femora:tibiae:tarsi of hindlegs = 2.0:1.4:1.2, tarsal segments similar to midlegs. Color pattern similar to male except wings semi-hyaline and body paler.
Female subimago: (in alcohol): body length 10.0 mm, forewings 11.0 mm, hindwings 3.0 mm, cerci 19.0 mm. Body dull, color pattern similar to female imago. Femora:tibiae:tarsi of forelegs = 2.5:2.0:2.0, tarsal segments arranged in decreasing order as 2, 1, 3, 5, 4; length of femora:tibiae:tarsi of midlegs = 2.2:2.0:1.5, length order of tarsal segments in decreasing order as 1, 5, 2, 3, 4; length of femora:tibiae:tarsi of hindlegs = 2.3:2.0:1.4, tarsal segments similar to midlegs.