Reduction of Post-Harvest Injuries Caused by Drosophila suzukii in Some Cultivars of Sweet Cherries Using a High Carbon Dioxide Level and Cold Storage
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Emergence Rate
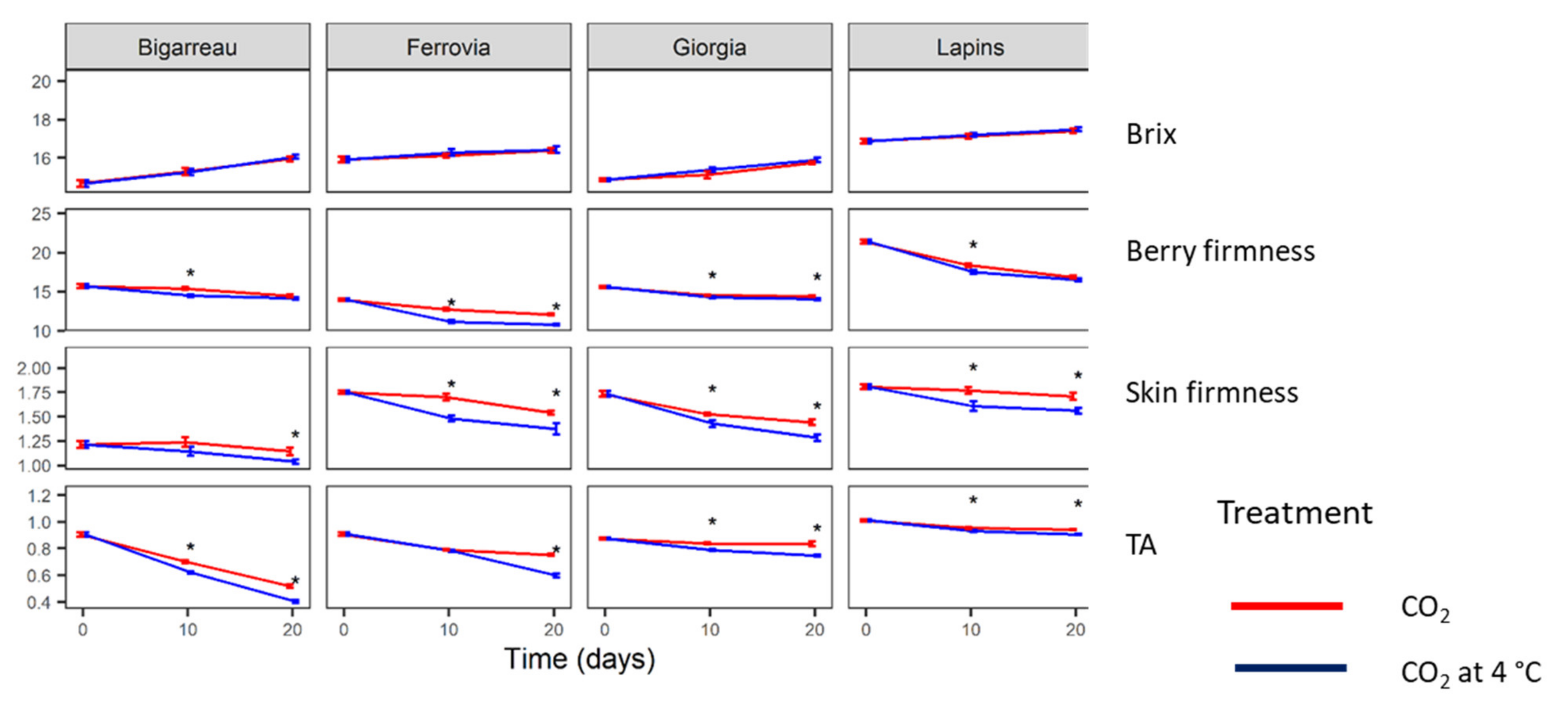
3.2. Quality Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavagnino, N.J.; Cichón, L.I.; De La Vega, G.J.; Garrido, S.A.; Fanara, J.J. New records of the invasive pest Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the South American continent. Soc. Entomológica Argent. 2018, 77, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M.T. Tethered-flight activity of drosophilids (Diptera: Drosophilidae) in relation to habitat and resource distribution. J. Insect Behav. 1992, 5, 395–401. [Google Scholar] [CrossRef]
- Calabria, G.; Máca, J.; Bächli, G.; Serra, L.; Pascual, M. First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J. Appl. Entomol. 2012, 136, 139–147. [Google Scholar] [CrossRef]
- Cini, A.; Anfora, G.; Escudero-Colomar, L.A.; Grassi, A.; Santosuosso, U.; Seljak, G.; Papini, A. Tracking the invasion of the alien fruit pest Drosophila suzukii in Europe. J. Pest Sci. 2014, 87, 559–566. [Google Scholar] [CrossRef]
- Tait, G.; Grassi, A.; Pfab, F.; Crava, C.; Dalton, D.; Magarey, R.; Ometto, L.; Vezzulli, S.; Rossi Stacconi, V.; Gottardello, A.; et al. Large-scale spatial dynamics of Drosophila suzukii in Trentino, Italy. J. Pest Sci. 2018, 91, 1213–1224. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Mendes, M.F.; Krüger, A.; Blauth, M.L.; Gottschalk, M.S.; Garcia, F.R.M. Global potential distribution of Drosophila suzukii (Diptera, Drosophilidae). PLoS ONE 2017, 12, e0174318. [Google Scholar] [CrossRef] [Green Version]
- Boughdad, A.; Haddi, K.; El Bouazzati, A.; Nassiri, A.; Tahiri, A.; El Anbri, C.; Eddaya, T.; Zaid, A.; Biondi, A. First record of the invasive spotted wing Drosophila infesting berry crops in Africa. J. Pest Sci. 2021, 94, 261–271. [Google Scholar] [CrossRef]
- EPPO. Drosophia Suzukii. 2020. Available online: https://gd.eppo.int/ (accessed on 6 June 2020).
- De Ros, G.; Anfora, G.; Grassi, A.; Ioriatti, C. The potential economic impact of Drosophila suzukii on small fruits production in Trentino (Italy). J. IOBC-WPRS Bull. 2013, 91, 317–321. [Google Scholar]
- Mazzi, D.; Bravin, E.; Meraner, M.; Finger, R.; Kuske, S. Economic impact of the introduction and establishment of Drosophila suzukii on sweet cherry production in Switzerland. J. Insects 2017, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- De Ros, G.; Grassi, A.; Pantezzi, T. Recent Trends in the Economic Impact of Drosophila suzukii. In Drosophila suzukii Management; Garcia, F.R.M., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Farnsworth, D.; Hamby, K.A.; Bolda, M.; Goodhue, R.E.; Williams, J.C.; Zalom, F.G. Economic analysis of revenue losses and control costs associated with the spotted wing drosophila, Drosophila suzukii (Matsumura), in the California raspberry industry. J. Pest Manag. Sci. 2017, 73, 1083–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodhue, R.E.; Bolda, M.; Farnsworth, D.; Williams, J.C.; Zalom, F.G. Spotted wing drosophila infestation of California strawberries and raspberries: Economic analysis of potential revenue losses and control costs. J. Pest. Manag. Sci. 2011, 67, 1396–1402. [Google Scholar] [CrossRef]
- Walse, S.S.; Cha, D.H.; Lee, B.H.; Follett, P.A. Post-harvest Quarantine Treatments for Drosophila suzukii in Fresh Fruits. In Drosophila Suzukii Management; Garcia, F.R.M., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Follett, P.A.; Swedman, A.; Mackey, B. Effect of low-oxygen conditions created by modified atmosphere packaging on radiation tolerance in Drosophila suzukii(Diptera: Drosophilidae) in sweet cherries. J. Econ. Entomol. 2018, 111, 141–145. [Google Scholar]
- Dhouibi, M.; Hermi, N.; Hajjem, B.; Hammami, Y.; Arfa, M.B. Efficacy of CO and CO2 treatment to control the stored-date insects, Ephestia kuehniella and Ectomyelois ceratoniae for organic dates production. Int. J. Agric. Innov. Res. 2015, 3, 1737–1743. [Google Scholar]
- Iturralde-García, R.D.; Borboa-Flores, J.; Cinco-Moroyoqui, F.J.; Riudavets, J.; Del Toro-Sánchez, C.L.; Rueda-Puente, E.O.; Martínez-Cruz, O.; Wong-Corral, F.J. Effect of controlled atmospheres on the insect Callosobruchus maculatus Fab. in stored chickpea. J. Stored Pro. Res. 2016, 69, 78–85. [Google Scholar] [CrossRef]
- Riudavets, J.; Alonso, M.; Gabarra, R.; Arnó, J.; Jaques, J.A.; Palou, L. The effects of postharvest carbon dioxide and a cold storage treatment on Tuta absoluta mortality and tomato fruit quality. J. Postharvest Biol. 2016, 120, 213–221. [Google Scholar] [CrossRef]
- Dalton, D.T.; Walton, V.M.; Shearer, P.W.; Walsh, D.B.; Caprile, J.; Isaacs, R. Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. J. Pest Manag. Sci. 2011, 67, 1368–1374. [Google Scholar] [CrossRef]
- Timm, E.; Brown, G.K.; Armstrong, P.R.; Beaudry, R.M.; Shirazi, A. Portable instrument for measuring firmness of cherries and berries. J. Appl. Eng. Agric. 1996, 12, 71–77. [Google Scholar] [CrossRef]
- Sadler, G.D.; Murphy, P.A. PH and Titratable Acidity in Food Anayisis; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 219–238. [Google Scholar]
- Sansavini, S.; Lugli, S. Sweet cherry breeding programs in Europe and Asia. Acta Hortic. 2008, 795, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Spornberger, A.; Ostojic, S.; Telfser, J.; Buvac, D.; Keppel, H. Suitability of early ripening sweet cherry (Prunus avium L.) cultivars for organic production-results of a long term trial in eastern Austria. in Ecofruit. In Proceedings of the 16th International Conference on Organic-Fruit Growing, Hohenheim, Germany, 17–19 February 2014. [Google Scholar]
- Dziadek, K.; Kopeć, A.; Czaplicki, S. The petioles and leaves of sweet cherry (Prunus avium L.) as a potential source of natural bioactive compounds. Eur. Food Res. Technol. 2018, 244, 1415–1426. [Google Scholar] [CrossRef]
- Rendon, D.; Buser, J.; Tait, G.; Lee, J.C.; Walton, V.M. Survival and fecundity parameters of two Drosophila suzukii (Diptera: Drosophilidae) morphs on variable diet under suboptimal temperatures. J. Insect Sci. 2018, 18, 8. [Google Scholar] [CrossRef] [Green Version]
- Young, Y.; Buckiewicz, N.; Long, T.A. Nutritional geometry and fitness consequences in Drosophila suzukii, the Spotted-Wing Drosophila. J. Ecol. Evol. 2018, 8, 2842–2851. [Google Scholar] [CrossRef] [Green Version]
- Baser, N.; Broutou, O.; Verrastro, V.; Porcelli, F.; Ioriatti, C.; Anfora, G.; Mazzoni, V.; Rossi Stacconi, M.V. Susceptibility of table grape varieties grown in south-eastern Italy to Drosophila suzukii. J. Appl. Entomol. 2018, 142, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Simoni, S.; Anfora, G.; Ioratti, C.; Frontuto, A.; Grassi, A. Drosophila suzukii, Matsumura, una Nuova Specie Invasivadannosa Alle Colture di Piccoli Frutti; Fondazione Edmund Mach—San Michele all’Adige: Trento, Italy, 2011; pp. 69–80. [Google Scholar]
- Lee, J.C.; Bruck, D.J.; Curry, H.; Edwards, D.; Haviland, D.R.; Van Steenwyk, R.A.; Yorgey, B.M. The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. J. Pest Manag. Sci. 2011, 67, 1358–1367. [Google Scholar] [CrossRef]
- Aly, M.F.; Kraus, D.A.; Burrack, H.J. Effects of postharvest cold storage on the development and survival of immature Drosophila suzukii (Diptera: Drosophilidae) in artificial diet and fruit. J. Econ. Entomol. 2017, 110, 87–93. [Google Scholar]
- Kim, M.J.; Kim, J.S.; Jeong, J.S.; Choi, D.S.; Park, J.; Kim, I. Phytosanitary Cold Treatment of Spotted-Wing Drosophila, Drosophila suzukii (Diptera: Drosophilidae) in ‘Campbell Early’ Grape. J. Econ. Entomol. 2018, 111, 1638–1643. [Google Scholar] [CrossRef]
- Shi, B.; Huang, J.L.; Hu, C.X.; Hou, M.L. Interactive effects of elevated CO2 and temperature on rice planthopper, Nila parvatalugens. J. Integr. Agric. 2014, 13, 1520–1529. [Google Scholar] [CrossRef]
- Wong-Corral, F.J.; Castañé, C.; Riudavets, J. Lethal effects of CO2-modified atmospheres for the control of three Bruchidaespecies. J. Stored Prod. Res. 2013, 55, 62–67. [Google Scholar] [CrossRef]
- Clymans, R.; Van Kerckvoorde, V.; Bangels, E.; Akkermans, W.; Alhmedi, A.; De Clercq, P.; Beliën, T.; Bylemans, D. Olfactory preference of Drosophila suzukii shifts between fruit and fermentation cues over the season: Effects of physiological status. J. Insects 2019, 10, 200. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Kaçar, G.; Daane, K.M. Temporal dynamics of host use by Drosophila suzukii in California’s San Joaquin Valley: Implications for area-wide pest management. J. Insects 2019, 10, 206. [Google Scholar] [CrossRef] [Green Version]
- Karacali, I. Storage and Marketing of Horticultural Crops; No: 494; Ege University Press: Bornova, İzmir, 2004; ISBN 9754830487. [Google Scholar]
- Yan, X.; Jun Yan, Y.; Pan, S.; Yuan, F. Changes of the aroma composition and other quality traits of blueberry ‘garden blue’ during the cold storage and subsequent shelf life. Foods 2020, 9, 1223. [Google Scholar] [CrossRef]
- Rojas-Argudo, C.; Pérez-Gago, M.; Del Río, M. Postharvest quality of coated cherries cv. ‘Burlat’as affected by coating composition and solids content. J. Food Sci. Technol. 2005, 11, 417–424. [Google Scholar] [CrossRef]
- Padilla-Zakour, O.; Tandon, K.; Wargo, J. Quality of modified atmosphere packaged Hedelfingen and Lapins sweet cherries. HortTechnology 2004, 14, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Remón, S.; Ferrer, A.; Marquina, P.; Burgos, J.; Oria, R. Use of modified atmospheres to prolong the postharvest life of Burlat cherries at two different degrees of ripeness. J. Sci. Food Agric. 2000, 80, 1545–1552. [Google Scholar] [CrossRef]
- Meheriuk, M.; McKenzie, D.-L.; Girard, B.; Moyls, A.; Weintraub, S.; Hocking, R.; Kopp, T. Storage of ‘sweetheart’ cherries in sealed plastic film. J. Food Qual. 1997, 20, 189–198. [Google Scholar] [CrossRef]
- Esti, M.; Cinquanta, L.; Sinesio, F.; Moneta, E.; Di Matteo, M. Physicochemical and sensory fruit characteristics of two sweet cherry cultivars after cool storage. J. Food Chem. 2002, 76, 399–405. [Google Scholar] [CrossRef]
- Usenik, V.; Kastelec, D.; Štampar, F. Physicochemical changes of sweet cherry fruits related to application of gibberellic acid. J. Food Chem. 2005, 90, 663–671. [Google Scholar] [CrossRef]
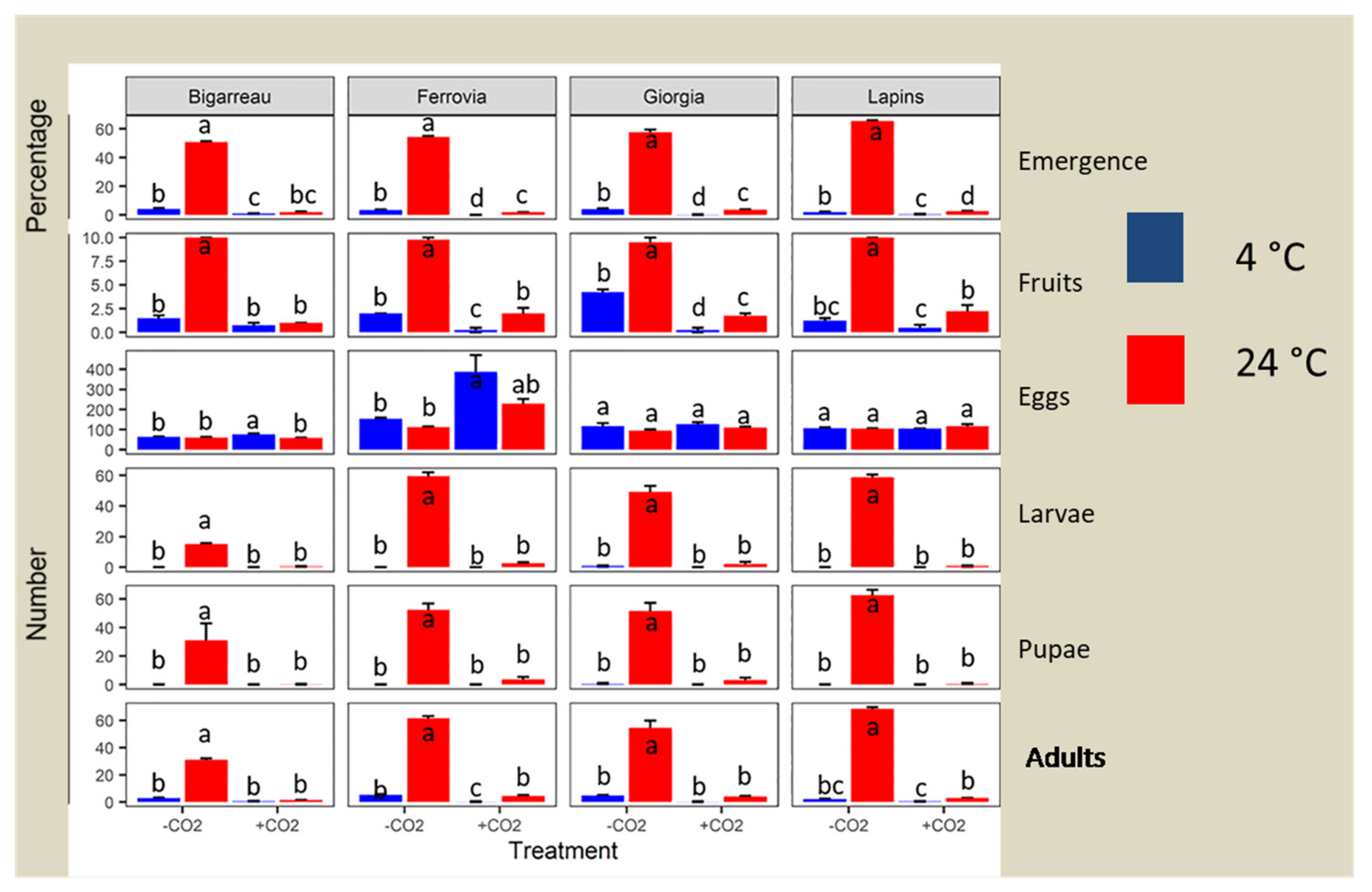

| Source of Variability | P > LR | |||||
|---|---|---|---|---|---|---|
| E | F | Eg | L | P | I | |
| Cultivar | 0.012 | 0.691 | <0.001 | <0.001 | <0.001 | <0.001 |
| CO2 Treatment | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Temperature | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Cultivar × CO2 Treatment | 0.05 | 0.226 | <0.001 | 0.172 | 0.01 | <0.001 |
| Cultivar × Temperature | <0.001 | 0.417 | <0.001 | 0.051 | 0.057 | <0.001 |
| CO2 Treatment × Temperature | <0.001 | <0.001 | 0.489 | 0.606 | 0.563 | <0.001 |
| Cultivar × CO2 Treatment × Temperature | <0.001 | 0.019 | 0.190 | 1 | 1 | <0.001 |
| Source of Variability | P > F | |||
|---|---|---|---|---|
| Brix | Berry Firmness | Skin Firmness | TA | |
| Between subjects | ||||
| Cultivar | <0.001 | <0.001 | <0.001 | <0.001 |
| Treatment | <0.001 | 0.044 | 0.041 | <0.001 |
| Cultivar × Treatment | 0.943 | 0.015 | 0.752 | <0.001 |
| Within subjects | ||||
| Time | 0.006 | <0.001 | <0.001 | <0.001 |
| Time × Cultivar | <0.001 | <0.001 | <0.001 | <0.001 |
| Time × Treatment | <0.001 | <0.001 | <0.001 | <0.001 |
| Time × Cultivar × Treatment | 0.894 | 0.76 | 0.713 | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, M.; Ibn Amor, A.; Admane, N.; Anfora, G.; Bubici, G.; Verrastro, V.; Scarano, L.; El Moujabber, M.; Baser, N. Reduction of Post-Harvest Injuries Caused by Drosophila suzukii in Some Cultivars of Sweet Cherries Using a High Carbon Dioxide Level and Cold Storage. Insects 2021, 12, 1009. https://doi.org/10.3390/insects12111009
Mostafa M, Ibn Amor A, Admane N, Anfora G, Bubici G, Verrastro V, Scarano L, El Moujabber M, Baser N. Reduction of Post-Harvest Injuries Caused by Drosophila suzukii in Some Cultivars of Sweet Cherries Using a High Carbon Dioxide Level and Cold Storage. Insects. 2021; 12(11):1009. https://doi.org/10.3390/insects12111009
Chicago/Turabian StyleMostafa, Manal, Abir Ibn Amor, Naouel Admane, Gianfranco Anfora, Giovanni Bubici, Vincenzo Verrastro, Luciano Scarano, Maroun El Moujabber, and Nuray Baser. 2021. "Reduction of Post-Harvest Injuries Caused by Drosophila suzukii in Some Cultivars of Sweet Cherries Using a High Carbon Dioxide Level and Cold Storage" Insects 12, no. 11: 1009. https://doi.org/10.3390/insects12111009
APA StyleMostafa, M., Ibn Amor, A., Admane, N., Anfora, G., Bubici, G., Verrastro, V., Scarano, L., El Moujabber, M., & Baser, N. (2021). Reduction of Post-Harvest Injuries Caused by Drosophila suzukii in Some Cultivars of Sweet Cherries Using a High Carbon Dioxide Level and Cold Storage. Insects, 12(11), 1009. https://doi.org/10.3390/insects12111009










