Chemically Insignificant Social Parasites Exhibit More Anti-Dehydration Behaviors than Their Hosts
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System
2.1.1. The Host
2.1.2. The Parasite
2.2. Data Collection
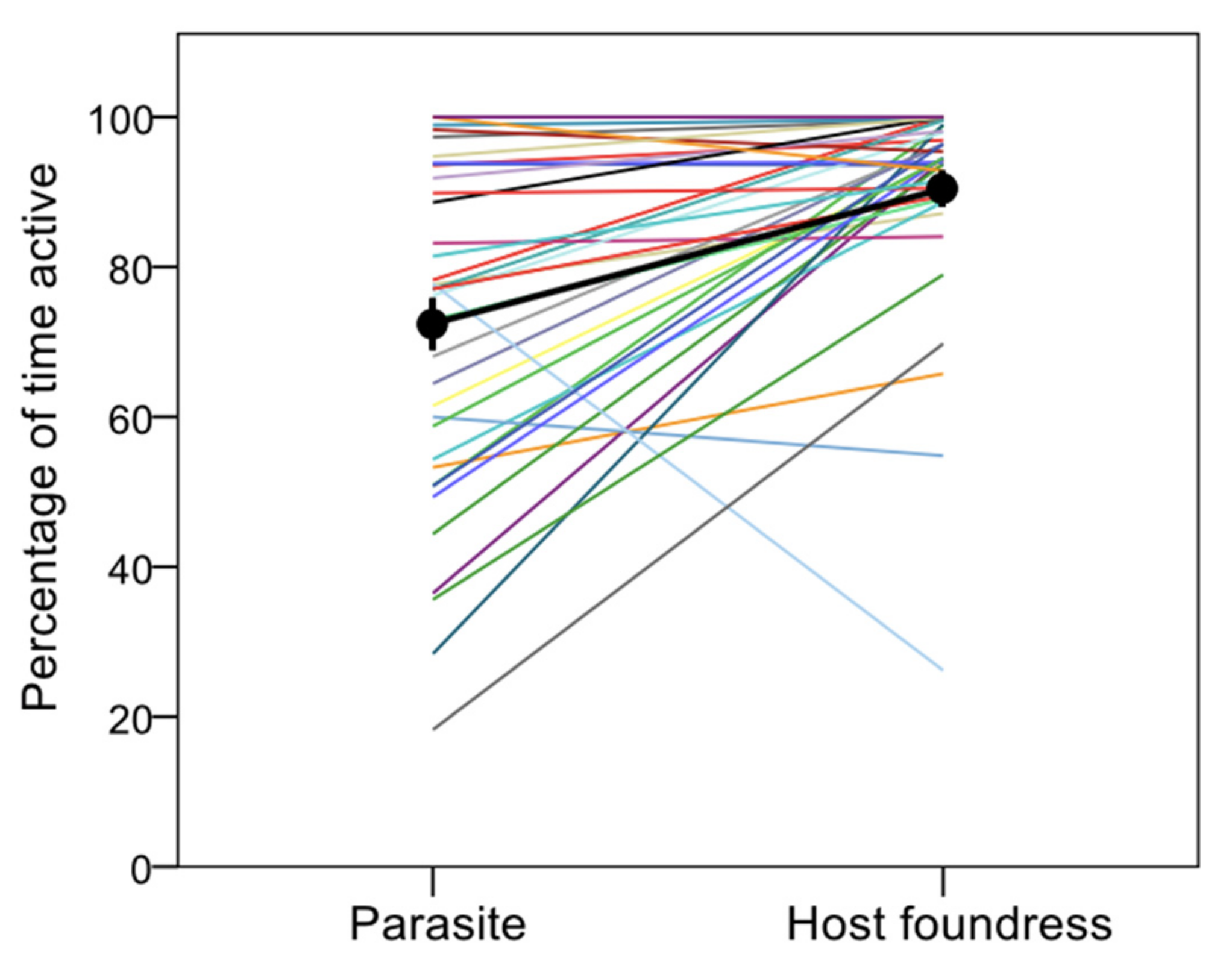
- Activity: any behavior (except resting);
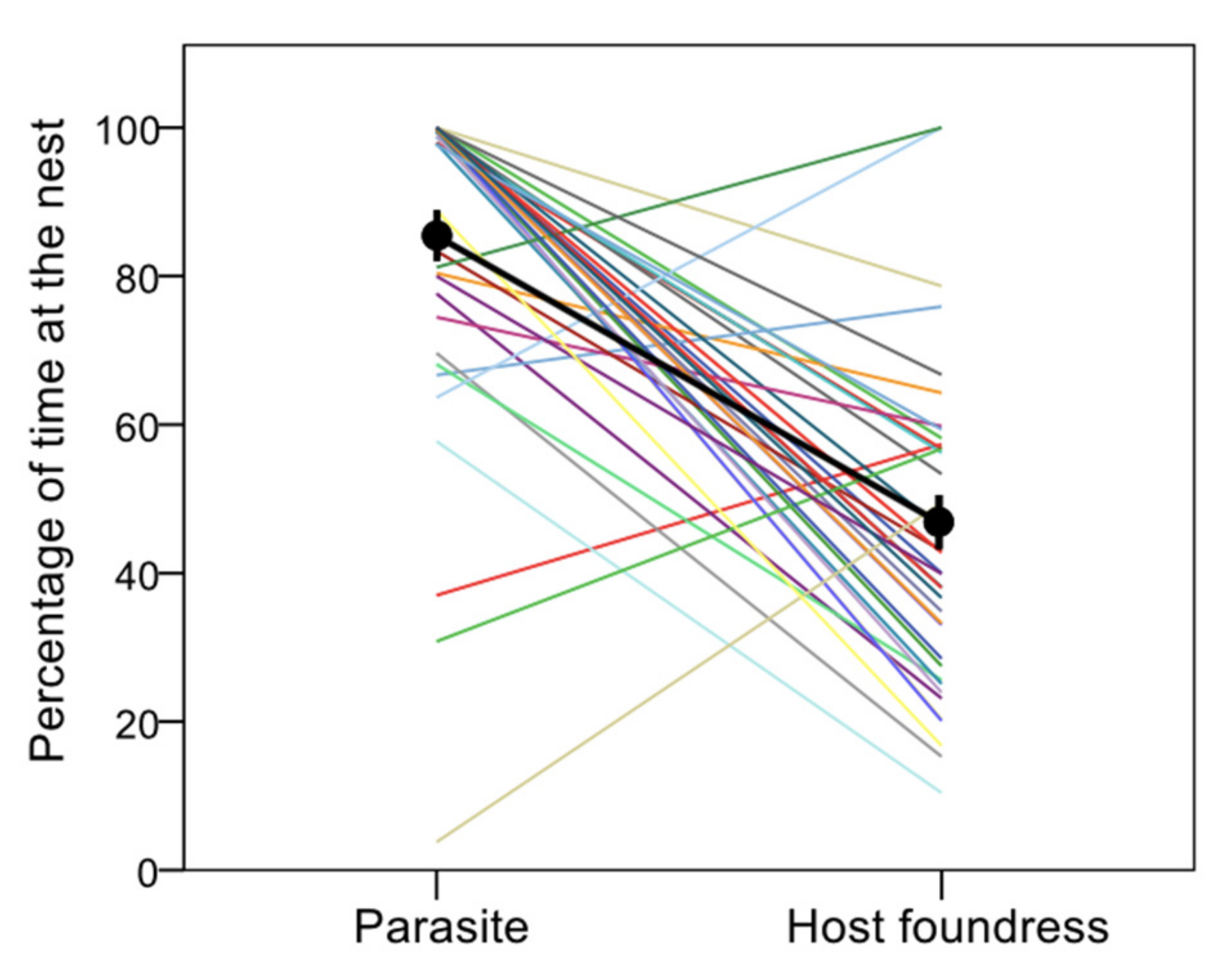
- Time at the nest (vs outside the nest, presumably foraging for nectar or preys);
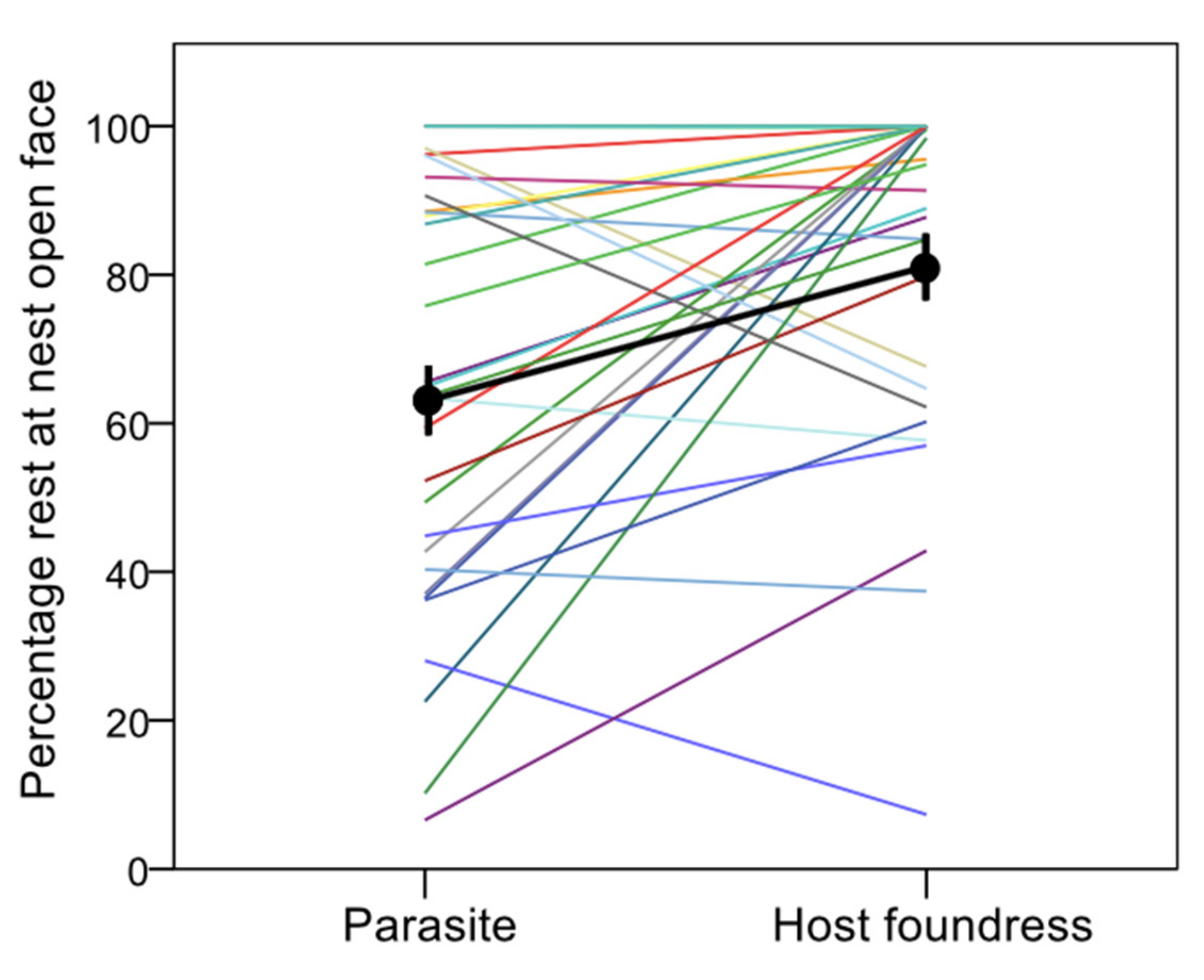
- Resting at the front face of the nest (where cells open) versus resting on the backside of the nest—typically within a wasp-body-size distance from the nest stalk (petiole) that suspends the comb to the substrate; the back face of the nest is shadowy, whereas the front face is sunny for a large part of the day;
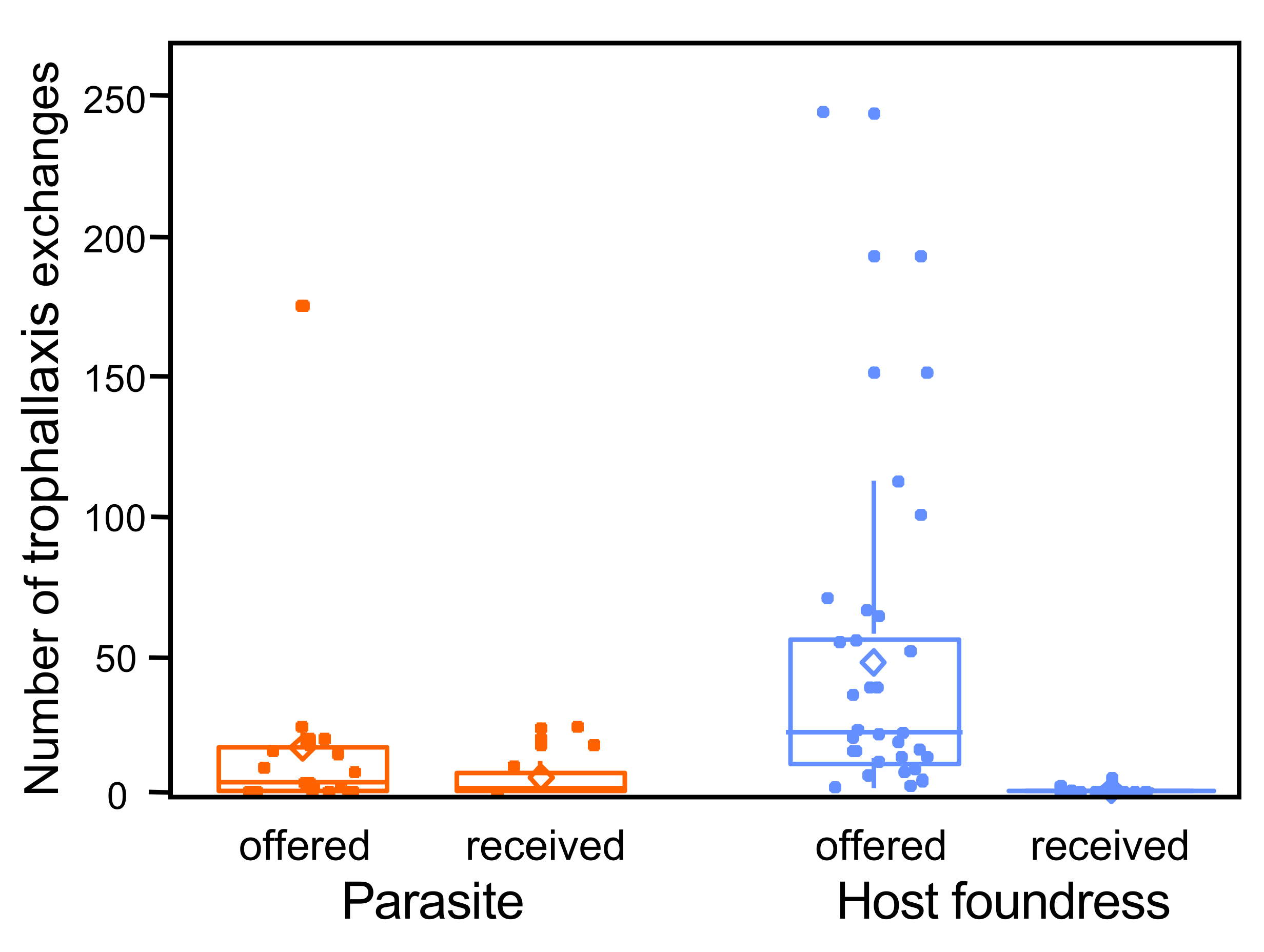
- Receiving versus offering liquids during trophallaxis (regurgitated liquid transfer between individuals) with any other adults or with larvae.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmid-Hempel, P. Parasites in Social Insects; Princeton University Press: Princeton, NJ, USA, 1998; p. 392. [Google Scholar]
- Parmentier, T. Guests of Social Insects. In Encyclopedia of Social Insects; Starr, C., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–15. [Google Scholar]
- Boomsma, J.J.; Schmid-Hempel, P.; Hughes, W.O.H. Life Histories and Parasite Pressure Across the Major Groups of Social Insects. In Insect Evolutionary Ecology; Fellowes, M., Holloway, G., Rolff, J., Eds.; CABI Publishing: Wallingford Oxon, UK; Cambridge, MA, USA, 2005; pp. 139–175. [Google Scholar]
- Buschinger, A. Social parasitism among ants: A review (Hymenoptera: Formicidae). Myrmecol. News 2009, 12, 219–235. [Google Scholar]
- Dawkins, R.; Krebs, J.R. Arms races between and within species. Proc. R. Soc. London. Ser. B Biol. Sci. 1979, 205, 489–511. [Google Scholar] [CrossRef]
- Davies, N.; Bourke, A.F.; Brooke, M.D.L. Cuckoos and parasitic ants: Interspecific brood parasitism as an evolutionary arms race. Trends Ecol. Evol. 1989, 4, 274–278. [Google Scholar] [CrossRef]
- Kilner, R.M.; Langmore, N.E. Cuckoos versus hosts in insects and birds: Adaptations, counter-adaptations and outcomes. Biol. Rev. 2011, 86, 836–852. [Google Scholar] [CrossRef] [PubMed]
- Cini, A.; Sumner, S.; Cervo, R. Inquiline social parasites as tools to unlock the secrets of insect sociality. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180193. [Google Scholar] [CrossRef]
- Michener, C.D.; Smith, B.H. Kin Recognition in Primitively Eusocial Insects. In Kin Recognition in Animals; Fletcher, D.J.C., Michener, C.D., Eds.; John Wiley: New York, NY, USA, 1987; pp. 209–242. [Google Scholar]
- Gamboa, G.J. Kin recognition in eusocial wasps. Ann. Zool. Fenn. 2004, 41, 789–808. [Google Scholar]
- Singer, T.L. Roles of Hydrocarbons in the Recognition Systems of Insects. Am. Zool. 1998, 38, 394–405. [Google Scholar] [CrossRef]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [CrossRef] [PubMed]
- d’Ettorre, P.; Moore, A.J. Chemical communication and the coordination of social interactions in insects. In Sociobiology of Communication: An Interdisciplinary Perspective; d’Ettorre, P., Hughes, D.P., Eds.; Oxford University Press: Oxford, UK, 2008; pp. 81–96. [Google Scholar]
- Martin, S.; Drijfhout, F. A review of ant cuticular hydrocarbons. J. Chem. Ecol. 2009, 35, 1151–1161. [Google Scholar] [CrossRef]
- Blomquist, G.; Bagnères, A.-G. (Eds.) Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Cambridge University Press: New York, NY, USA, 2010. [Google Scholar]
- Sprenger, P.P.; Menzel, F. Cuticular hydrocarbons in ants (Hymenoptera: Formicidae) and other insects: How and why they differ among individuals, colonies and species. Myrmecol. News 2020, 30, 1–26. [Google Scholar]
- Bonavita-Cougourdan, A.; Theraulaz, G.; Bagnères, A.-G.; Roux, M.; Pratte, M.; Provost, E.; Clément, J.-L. Cuticular hydrocarbons, social organization and ovarian development in a polistine wasp: Polistes dominulus Christ. Comp. Biochem. Physiol. Part B Comp. Biochem. 1991, 100, 667–680. [Google Scholar] [CrossRef]
- Nehring, V.; Evison, S.E.F.; Santorelli, L.A.; d’Ettorre, P.; Hughes, W.O.H. Kin-informative recognition cues in ants. Proc. R. Soc. B Biol. Sci. 2010, 278, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- van Zweden, J.S.; d’Ettorre, P. Nestmate recognition in social insects and the role of hydrocarbons. In Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology; Blomquist, G.J., Bagnères, A.-G., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 222–243. [Google Scholar]
- Lorenzi, M.C.; Bagnères, A.-G.; Clément, J.-L.; Turillazzi, S. Polistes biglumis bimaculatus epicuticular hydrocarbons and nest-mate recognition (Hymenoptera: Vespidae). Insectes Soc. 1997, 44, 123–138. [Google Scholar] [CrossRef]
- Martin, S.J.; Vitikainen, E.; Helanterä, H.; Drijfhout, F. Chemical basis of nest-mate discrimination in the ant Formica exsecta. Proc. R. Soc. B Biol. Sci. 2008, 275, 1271–1278. [Google Scholar] [CrossRef]
- Villalta, I.; Rami, L.; Alvarez-Blanco, P.; Angulo, E.; Cerdá, X.; Boulay, R. Environmental and genetic constraints on cuticular hydrocarbon composition and nestmate recognition in ants. Anim. Behav. 2019, 159, 105–119. [Google Scholar] [CrossRef]
- Ichinose, K.; Lenoir, A. Hydrocarbons detection levels in ants. Insectes Sociaux 2010, 57, 453–455. [Google Scholar] [CrossRef]
- Di Mauro, G.; Perez, M.; Lorenzi, M.C.; Guerrieri, F.; Millar, J.G.; d’Ettorre, P. Ants discriminate between different hydrocarbon concentrations. Front. Ecol. Evol. 2015, 3, 133. [Google Scholar] [CrossRef]
- Cini, A.; Gioli, L.; Cervo, R. A quantitative threshold for nest-mate recognition in a paper social wasp. Biol. Lett. 2009, 5, 459–461. [Google Scholar] [CrossRef]
- Cappa, F.; Bruschini, C.; Cipollini, M.; Pieraccini, G.; Cervo, R. Sensing the intruder: A quantitative threshold for recognition cues perception in honeybees. Naturwissenschaften 2014, 101, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, M.C.; Cometto, I.; Marchisio, G. Species and colony components in the recognition odor of young wasps: Their expression and learning (Polistes biglumis and P. atrimandibularis, Hymenoptera Vespidae). J. Insect Behav. 1999, 12, 147–158. [Google Scholar] [CrossRef]
- Lenoir, A.; d’Ettorre, P.; Errard, C.; Hefetz, A. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 2001, 46, 573–599. [Google Scholar] [CrossRef] [PubMed]
- Dani, F.R.; Jones, G.R.; Destri, S.; Spencer, S.H.; Turillazzi, S. Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Anim. Behav. 2001, 62, 165–171. [Google Scholar] [CrossRef]
- Dani, F.R.; Jones, G.R.; Corsi, S.; Beard, R.; Pradella, D.; Turillazzi, S. Nestmate recognition cues in the honey bee: Differential importance of cuticular alkanes and alkenes. Chem. Senses 2005, 30, 477–489. [Google Scholar] [CrossRef]
- Van Wilgenburg, E.; Sulc, R.; Shea, K.J.; Tsutsui, N.D. Deciphering the chemical basis of nestmate recognition. J. Chem. Ecol. 2010, 36, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Dettner, K.; Liepert, C. Chemical mimicry and camouflage. Annu. Rev. Entomol. 1994, 39, 129–154. [Google Scholar] [CrossRef]
- Lorenzi, M.C. The result of an arms race: The chemical strategies of Polistes social parasites. Ann. Zool. Fenn. 2006, 43, 550–563. [Google Scholar]
- Bagnères, A.-G.; Lorenzi, M.C. Chemical deception/mimicry using cuticular hydrocarbons. In Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Blomquist, G., Bagnères, A.-G., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 283–324. [Google Scholar]
- Casacci, L.; Barbero, F.; Ślipiński, P.; Witek, M. The inquiline ant Myrmica karavajevi uses both chemical and vibroacoustic deception mechanisms to integrate into its host colonies. Biology 2021, 10, 654. [Google Scholar] [CrossRef]
- Von Beeren, C.; Brückner, A.; Maruyama, M.; Burke, G.; Wieschollek, J.; Kronauer, D.J.C. Chemical and behavioral integration of army ant-associated rove beetles—A comparison between specialists and generalists. Front. Zool. 2018, 15, 1–15. [Google Scholar] [CrossRef]
- Lorenzi, M.C.; d’Ettorre, P. Nestmate recognition in social insects: What does it mean to be chemically insignificant? Front. Ecol. Evol. 2020, 7, 488. [Google Scholar] [CrossRef]
- Neupert, S.; DeMilto, A.; Drijfhout, F.; Speller, S.; Adams, R.M. Host colony integration: Megalomyrmex guest ant parasites maintain peace with their host using weaponry. Anim. Behav. 2018, 139, 71–79. [Google Scholar] [CrossRef]
- d’Ettorre, P.; Errard, C. Chemical disguise during colony founding in the dulotic ant Polyergus rufescens Latr. (Hymenoptera, Formicidae). Insect Soc. Life 1998, 2, 71–77. [Google Scholar]
- Uboni, A.; Bagnères, A.-G.; Christidès, J.-P.; Lorenzi, M.C. Cleptoparasites, social parasites and a common host: Chemical insignificance for visiting host nests, chemical mimicry for living in. J. Insect Physiol. 2012, 58, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Lambardi, D.; Dani, F.R.; Turillazzi, S.; Boomsma, J.J. Chemical mimicry in an incipient leaf-cutting ant social parasite. Behav. Ecol. Sociobiol. 2006, 61, 843–851. [Google Scholar] [CrossRef]
- Nehring, V.; Dani, F.R.; Turillazzi, S.; Boomsma, J.J.; d’Ettorre, P. Integration strategies of a leaf-cutting ant social parasite. Anim. Behav. 2015, 108, 55–65. [Google Scholar] [CrossRef]
- Lorenzi, M.C.; Bagnères, A.G. Concealing identity and mimicking hosts: A dual chemical strategy for a single social parasite? (Polistes atrimandibularis, Hymenoptera: Vespidae). Parasitology 2002, 125, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, M.C.; Cervo, R.; Zacchi, F.; Turillazzi, S.; Bagnères, A.-G. Dynamics of chemical mimicry in the social parasite wasp Polistes semenowi (Hymenoptera: Vespidae). Parasitology 2004, 129, 643–651. [Google Scholar] [CrossRef]
- Gibbs, A.G. The role of lipid physical properties in lipid barriers. Am. Zool. 1998, 38, 268–279. [Google Scholar] [CrossRef]
- Gibbs, A.G. Lipid melting and cuticular permeability: New insights into an old problem. J. Insect Physiol. 2002, 48, 391–400. [Google Scholar] [CrossRef]
- Hefetz, A. The evolution of hydrocarbon pheromone parsimony in ants (Hymenoptera: Formicidae)—Interplay of colony odor uniformity and odor idiosyncrasy: A review. Myrmecol. News 2007, 10, 59–68. [Google Scholar]
- Quinlan, M.C.; Gibbs, A.G. Discontinuous gas exchange in insects. Respir. Physiol. Neurobiol. 2006, 154, 18–29. [Google Scholar] [CrossRef]
- Rajpurohit, S.; Vrkoslav, V.; Hanus, R.; Gibbs, A.G.; Cvačka, J.; Schmidt, P.S. Post-eclosion temperature effects on insect cu-ticular hydrocarbon profiles. Ecol. Evol. 2021, 11, 352–364. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Ginzel, M.D. Chemical ecology, biochemistry, and molecular biology of insect hydrocarbons. Annu. Rev. Entomol. 2021, 66, 45–60. [Google Scholar] [CrossRef]
- Gibbs, A.G.; Rajpurohit, S. Cuticular lipids and water balance. In Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology; Blomquist, G., Bagnères, A.-G., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 100–119. [Google Scholar]
- Chung, H.; Carroll, S.B. Wax, sex and the origin of species: Dual roles of insect cuticular hydrocarbons in adaptation and mating. BioEssays 2015, 37, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Reeve, H.K. Polistes. In The Social Biology of Wasps; Ross, K.G., Matthews, R.W., Eds.; Comstock Publishing Associates: Ithaca, NY, USA, 1991; pp. 99–148. [Google Scholar]
- West-Eberhard, M.J. Wasp societies as microcosms for the study of development and evolution. In Natural History and Evolution of Paper Wasps; Turillazzi, S., West-Eberhard, M.J., Eds.; Oxford University Press: Oxford, UK, 1996; pp. 290–317. [Google Scholar]
- Hozumi, S.; Kudô, K.; Zucchi, R. Promotion of thermoregulatory insulation in nests of neotropical wasps by building extra-combs with empty cells. Neotropical Entomol. 2008, 37, 159–166. [Google Scholar] [CrossRef][Green Version]
- Hozumi, S.; Mateus, S.; Kudô, K.; Kuwahara, T.; Yamane, S.; Zucchi, R. Nest thermoregulation in Polybia scutellaris (White) (Hymenoptera: Vespidae). Neotropical Entomol. 2010, 39, 826–828. [Google Scholar] [CrossRef]
- Höcherl, N.; Kennedy, S.; Tautz, J. Nest thermoregulation of the paper wasp Polistes dominula. J. Therm. Biol. 2016, 60, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.; Aron, S. Adaptations to thermal stress in social insects: Recent advances and future directions. Biol. Rev. 2020, 95, 1535–1553. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.; Strassmann, J.E.; Queller, D.C.; Turillazzi, S.; Cervo, R. Social parasites in polistine wasps are monophyletic: Implications for sympatric speciation. Proc. R. Soc. B Biol. Sci. 1994, 257, 31–35. [Google Scholar] [CrossRef]
- Cervo, R. Polistes wasps and their social parasites: An overview. Ann. Zool. Fenn. 2006, 43, 531–549. [Google Scholar]
- Lorenzi, M.C.; Turillazzi, S. Behavioural and ecological adaptations to the high mountain environment of Polistes biglumis bimaculatus. Ecol. Entomol. 1986, 11, 199–204. [Google Scholar] [CrossRef]
- Lorenzi, M.C.; Thompson, J.N. The geographic structure of selection on a coevolving interaction between social parasitic wasps and their hosts hampers social evolution. Evolution 2011, 65, 3527–3542. [Google Scholar] [CrossRef]
- Turillazzi, S.; Sledge, M.F.; Dani, F.R.; Cervo, R.; Massolo, A.; Fondelli, L. Social hackers: Integration in the host chemical recognition system by a paper wasp social parasite. Naturwissenschaften 2000, 87, 172–176. [Google Scholar] [CrossRef]
- Cervo, R.; Lorenzi, M.C.; Turillazzi, S. Nonaggressive usurpation of the nest of Polistes biglumis bimaculatus by the social parasite Sulcopolistes atrimandibularis (Hymenoptera Vespidae). Insectes Sociaux 1990, 37, 333–347. [Google Scholar] [CrossRef]
- Brandt, M.; Foitzik, S.; Fischer-Blass, B.; Heinze, J. The coevolutionary dynamics of obligate ant social parasite systems—between prudence and antagonism. Biol. Rev. 1999, 80, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Bagnères, A.-G.; Lorenzi, M.C.; Dusticier, G.; Turillazzi, S.; Clément, J.-L. Chemical usurpation of a nest by paper wasp parasites. Science 1996, 272, 889–892. [Google Scholar] [CrossRef]
- Wilson, E.O. Social modifications related to rareness in ant species. Evolution 1963, 17, 249–253. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Belknap (Harvard University Press): Cambridge, MA, USA, 1990; p. 732. [Google Scholar]
- Thomas, J.A.; Schönrogge, K.; Elmes, G.W. Specializations and host associations of social parasites of snts. In Insect Evolutionary Ecology; Fellowes, M., Holloway, G., Rolff, J., Eds.; CABI Publishing: Wallingford Oxon, UK; Cambridge, MA, USA, 2005; pp. 475–514. [Google Scholar]
- Nash, D.R.; Boomsma, J.J. Communication between hosts and social parasites. In Sociobiology of Communication: An Interdisci-plinary Perspective; d’Ettorre, P., Hughes, D.P., Eds.; Oxford Scholarship Online: Oxford, UK, 2008; pp. 55–79. [Google Scholar]
- Trontti, K.; Aron, S.; Sundström, L. The genetic population structure of the ant Plagiolepis xene—implications for genetic vulnerability of obligate social parasites. Conserv. Genet. 2006, 7, 241–250. [Google Scholar] [CrossRef]
- Thomas, J.A.; Simcox, D.J.; Clarke, R.T. Successful conservation of a threatened Maculinea butterfly. Science 2009, 325, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Settele, J.; Barbero, F.; Musche, M.; Thomas, J.A.; Schönrogge, K. Singing the blues: From experimental biology to conservation application. J. Exp. Biol. 2011, 214, 1407–1410. [Google Scholar] [CrossRef][Green Version]
- Pekkarinen, A.; Gustafsson, B. The Polistes species in northern Europe (Hymenoptera: Vespidae). Entomol. Fenn. 1999, 10, 191–197. [Google Scholar] [CrossRef]
- Seppä, P.; Bonelli, M.; Dupont, S.; Hakala, S.M.; Bagnères, A.-G.; Lorenzi, M.C. Strong gene flow undermines local adaptations in a host parasite system. Insects 2020, 11, 585. [Google Scholar] [CrossRef]
- Kovac, H.; Käfer, H.; Stabentheiner, A. The respiratory metabolism of Polistes biglumis, a paper wasp from mountainous regions. Insects 2020, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Guiglia, D. Les guêpes sociales (Hymenoptera Vespidae) d’Europe Occidentale et Septentrionale. In Faune de l’Europe et du Bassin Mediterraneen; Masson: Paris, France, 1972; Volume 6, pp. 1–181. [Google Scholar]
- Fanelli, D.; Cervo, R.; Turillazzi, S. Three new host species of the social wasp parasite, Polistes atrimandibularis (Hymenoptera, Vespidae). Insectes Sociaux 2001, 48, 352–354. [Google Scholar] [CrossRef]
- Thompson, J.N. The Coevolutionary Process; University of Chicago Press: Chicago, IL, USA, 1994; p. 383. [Google Scholar]
- Thompson, J.N. The Geographic Mosaic of Coevolution; University of Chicago Press: Chicago, IL, USA, 2005; 400p. [Google Scholar]
- Michalakis, Y. Parasitism and the evolution of life-history traits. In Ecology and Evolution of Parasitism; Thomas, F., Guégan, J.-F., Renaud, F., Eds.; Oxford University Press: Oxford, UK, 2009; pp. 19–30. [Google Scholar]
- Ortolani, I.; Cervo, R. Intra-specific body size variation in Polistes paper wasps as a response to social parasite pressure. Ecol. Entomol. 2010, 35, 352–359. [Google Scholar] [CrossRef]
- Fucini, S.; Di Bona, V.; Mola, F.; Piccaluga, C.; Lorenzi, M.C. Social wasps without workers: Geographic variation of caste expression in the paper wasp Polistes biglumis. Insectes Sociaux 2009, 56, 347–358. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B. lme4: Linear Mixed-Effects Models using Eigen and S4. R Package Version 1.1–7. 2014. Available online: http://CRAN.R-project.org/package=lme4 (accessed on 20 July 2021).
- R Core Team. R: A language and Environment for Statistical Computing. Vienna (Austria): R Foundation for Statisti-cal Computing. 2014. Available online: www.R-project.org (accessed on 20 July 2021).
- Fucini, S.; Uboni, A.; Lorenzi, M.C. Cuckoo wasps manipulate foraging and resting activities in their hosts. Behav. Ecol. Sociobiol. 2014, 68, 1753–1759. [Google Scholar] [CrossRef]
- Faraway, J.J. Extending the Linear Model with R. Generalized Linear, Mixed Effects and Nonparametric Regression Models; Chapman & Hall: Boca Raton, FL, USA, 2006; p. 345. [Google Scholar]
- Ichinose, K.; Lenoir, A. Ontogeny of hydrocarbon profiles in the ant Aphaenogaster senilis and effects of social isolation. Comptes Rendus Biol. 2009, 332, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Monnin, T. Chemical recognition of reproductive status in social insects. Ann. Zool. Fenn. 2006, 43, 515–530. [Google Scholar]
- Martin, S.J.; Drijfhout, F.P. Nestmate and task cues are influenced and encoded differently within ant cuticular hydrocarbon profiles. J. Chem. Ecol. 2009, 35, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Liebig, J. Hydrocarbon profiles indicate fertility and dominance status in ant, bee, and wasp colonies. In Insect Hydro-Carbons: Biology, Biochemistry, and Chemical Ecology; Blomquist, G., Bagnères, A.-G., Eds.; Cambridge University Press: New York, NY, USA, 2010; pp. 282–324. [Google Scholar]
- Holman, L.; Lanfear, R.; d’Ettorre, P. The evolution of queen pheromones in the ant genus Lasius. J. Evol. Biol. 2013, 26, 1549–1558. [Google Scholar] [CrossRef]
- van Oystaeyen, A.; Oliveira, R.C.; Holman, L.; van Zweden, J.S.; Romero, C.; Oi, C.A.; d’Ettorre, P.; Khalesi, M.; Billen, J.; Wäckers, F.; et al. Conserved class of queen pheromones stops social insect workers from reproducing. Science 2014, 287, 287–290. [Google Scholar] [CrossRef]
- Oi, C.A.; Van Zweden, J.S.; Oliveira, R.C.; Van Oystaeyen, A.; Nascimento, F.; Wenseleers, T. The origin and evolution of social insect queen pheromones: Novel hypotheses and outstanding problems. BioEssays 2015, 37, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Brown, M.J.F.; Broun, P.; Cuevas, W.; Moses, L.E.; Chao, D.; Gordon, D.M. Task-related differences in the cuticular hydrocarbon composition of harvester ants, Pogonomyrmex barbatus. J. Chem. Ecol. 1998, 24, 2021–2037. [Google Scholar] [CrossRef]
- Kleeberg, I.; Menzel, F.; Foitzik, S. The influence of slavemaking lifestyle, caste and sex on chemical profiles in Temnothorax ants: Insights into the evolution of cuticular hydrocarbons. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162249. [Google Scholar] [CrossRef] [PubMed]
- Beros, S.; Foitzik, S.; Menzel, F. What are the mechanisms behind a parasite-induced decline in nestmate recognition in ants? J. Chem. Ecol. 2017, 43, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Dani, F.R.; Turillazzi, S. Chemical communication and reproduction partitioning in social wasps. J. Chem. Ecol. 2018, 44, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Cervo, R.; Lorenzi, M.C.; Turillazzi, S. Sulcopolistes atrimandibularis, social parasite and predator of an alpine Polistes (Hyme-noptera, Vespidae). Ethology 1990, 86, 71–78. [Google Scholar] [CrossRef]
- Cervo, R.; Macinai, V.; Dechigi, F.; Turillazzi, S. Fast growth of immature brood in a social parasite wasp: A convergent evo-lution between avian and insect cuckoos. Am. Nat. 2004, 164, 814–820. [Google Scholar] [CrossRef]
- Casacci, L.P.; Thomas, J.A.; Sala, M.; Treanor, D.; Bonelli, S.; Balletto, E.; Schönrogge, K. Ant pupae employ acoustics to communicate social status in their colony’s hierarchy. Curr. Biol. 2013, 23, 323–327. [Google Scholar] [CrossRef]
- Barbero, F.; A Thomas, J.; Bonelli, S.; Balletto, E.; Schönrogge, K. Queen ants make distinctive sounds that are mimicked by a butterfly social parasite. Science 2009, 323, 782–785. [Google Scholar] [CrossRef]
- Morrell, L.J.; Ruxton, G.D.; James, R. Spatial positioning in the selfish herd. Behav. Ecol. 2010, 22, 16–22. [Google Scholar] [CrossRef]
- Jandt, J.M.; Dornhaus, A. Spatial organization and division of labour in the bumblebee Bombus impatiens. Anim. Behav. 2009, 77, 641–651. [Google Scholar] [CrossRef]
- Jandt, J.M.; Dornhaus, A. Competition and cooperation: Bumblebee spatial organization and division of labor may affect worker reproduction late in life. Behav. Ecol. Sociobiol. 2011, 65, 2341–2349. [Google Scholar] [CrossRef]
- Powell, S.; Tschinkel, W.R. Ritualized conflict in Odontomachus brunneus and the generation of interaction-based task allocation: A new organizational mechanism in ants. Anim. Behav. 1999, 58, 965–972. [Google Scholar] [CrossRef]
- Robson, S.; Bean, K.; Hansen, J.; Norling, K.; Rowe, R.; White, D. Social and spatial organisation in colonies of a primitively eusocial wasp, Ropalidia revolutionalis (de Saussure) (Hymenoptera: Vespidae). Aust. J. Entomol. 2000, 39, 20–24. [Google Scholar] [CrossRef]
- Pardi, L. Dominance order in Polistes wasps. Physiol. Zool. 1948, 21, 1–13. [Google Scholar] [CrossRef]
- Gamboa, G.J.; Dropkin, J.A. Comparisons of behaviors in early vs. late foundress associations of the paper wasp, Polistes metricus (Hymenoptera: Vespidae). Can. Entomol. 1979, 111, 919–926. [Google Scholar] [CrossRef]
- Baracchi, D.; Zaccaroni, M.; Cervo, R.; Turillazzi, S. Home range analysis in the study of spatial organization on the comb in the paper wasp Polistes dominulus. Ethology 2010, 116, 579–587. [Google Scholar] [CrossRef]
- Dapporto, L.; Theodora, P.; Spacchini, C.; Pieraccini, G.; Turillazzi, S. Rank and epicuticular hydrocarbons in different pop-ulations of the paper wasp Polistes dominulus (Christ) (Hymenoptera, Vespidae). Insectes Soc. 2004, 51, 279–286. [Google Scholar] [CrossRef]
- Cervo, R.; Lorenzi, M.C. Inhibition of host queen reproductive capacity by the obligate social parasite Polistes atrimandibularis (Hymenoptera, Vespidae). Ethology 2010, 102, 1042–1047. [Google Scholar] [CrossRef]
- Gibbs, A.G.; Chippindale, A.K.; Rose, M.R. Physiological mechanisms of evolved desiccation resistance in Drosophila melanogaster. J. Exp. Biol. 1997, 200, 1821–1832. [Google Scholar] [CrossRef]
- Menzel, F.; Zumbusch, M.; Feldmeyer, B. How ants acclimate: Impact of climatic conditions on the cuticular hydrocarbon profile. Funct. Ecol. 2017, 32, 657–666. [Google Scholar] [CrossRef]
- Lenoir, A.; Aron, S.; Cerdá, X.; Hefetz, A. Cataglyphis desert ants: A good model for evolutionary biology in Darwin’s anni-versary year-A review. Israel J. Entomol. 2009, 39, 1–32. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzi, M.C. Chemically Insignificant Social Parasites Exhibit More Anti-Dehydration Behaviors than Their Hosts. Insects 2021, 12, 1006. https://doi.org/10.3390/insects12111006
Lorenzi MC. Chemically Insignificant Social Parasites Exhibit More Anti-Dehydration Behaviors than Their Hosts. Insects. 2021; 12(11):1006. https://doi.org/10.3390/insects12111006
Chicago/Turabian StyleLorenzi, Maria Cristina. 2021. "Chemically Insignificant Social Parasites Exhibit More Anti-Dehydration Behaviors than Their Hosts" Insects 12, no. 11: 1006. https://doi.org/10.3390/insects12111006
APA StyleLorenzi, M. C. (2021). Chemically Insignificant Social Parasites Exhibit More Anti-Dehydration Behaviors than Their Hosts. Insects, 12(11), 1006. https://doi.org/10.3390/insects12111006





