Lupin Root Weevils (Charagmus spp., Curculionidae: Sitonini), a Lupin Pest: A Review of Their Distribution, Biology, and Challenges in Integrated Pest Management
Abstract
Simple Summary
Abstract
1. Introduction
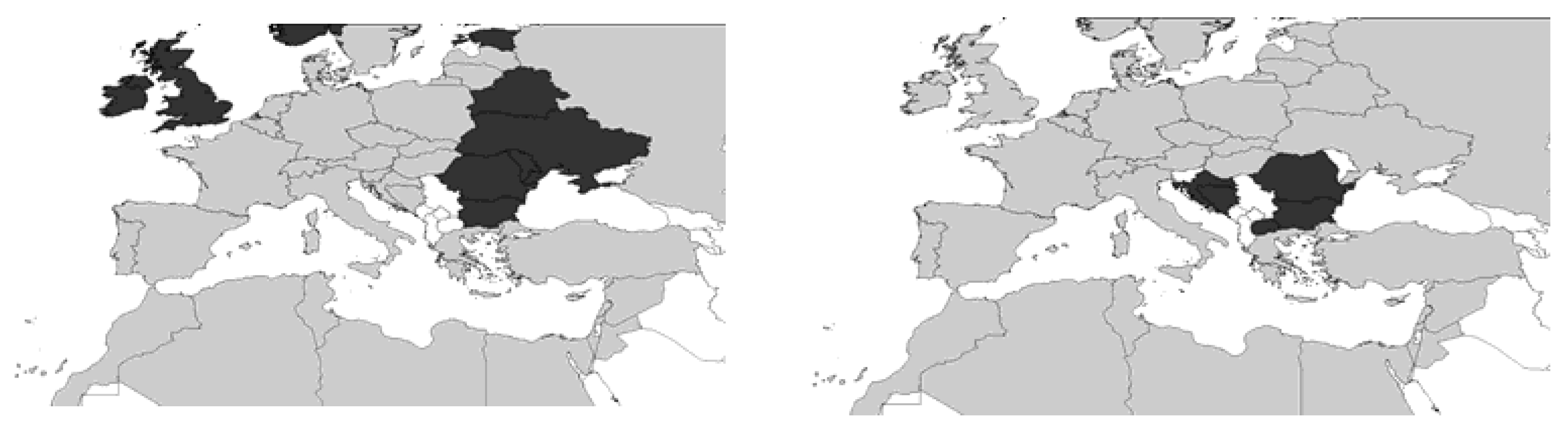
2. Systematics and Geographical Distribution
3. Biology and Ecology
3.1. Host Plants
3.2. Damage
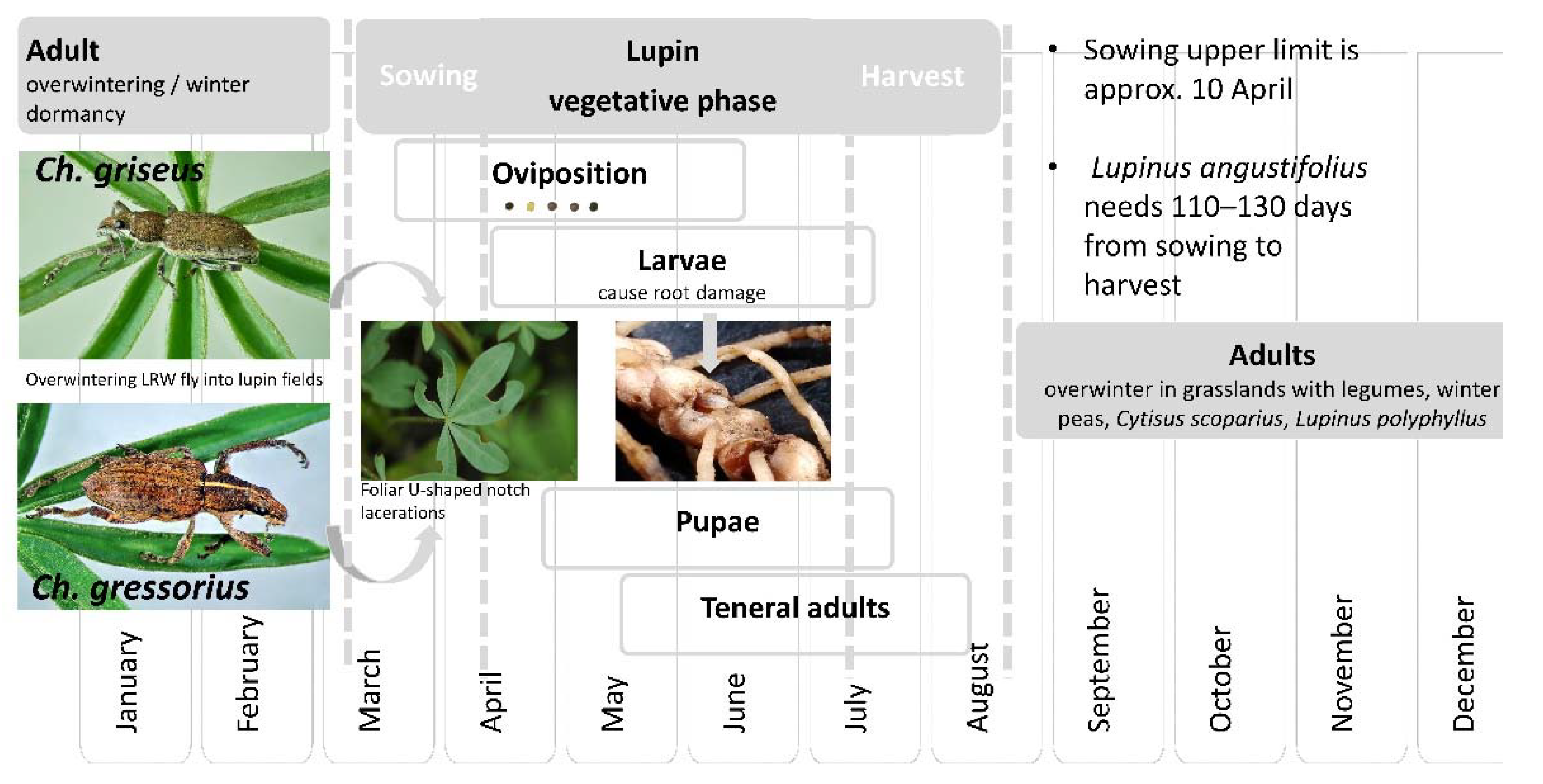
3.3. Life Stages
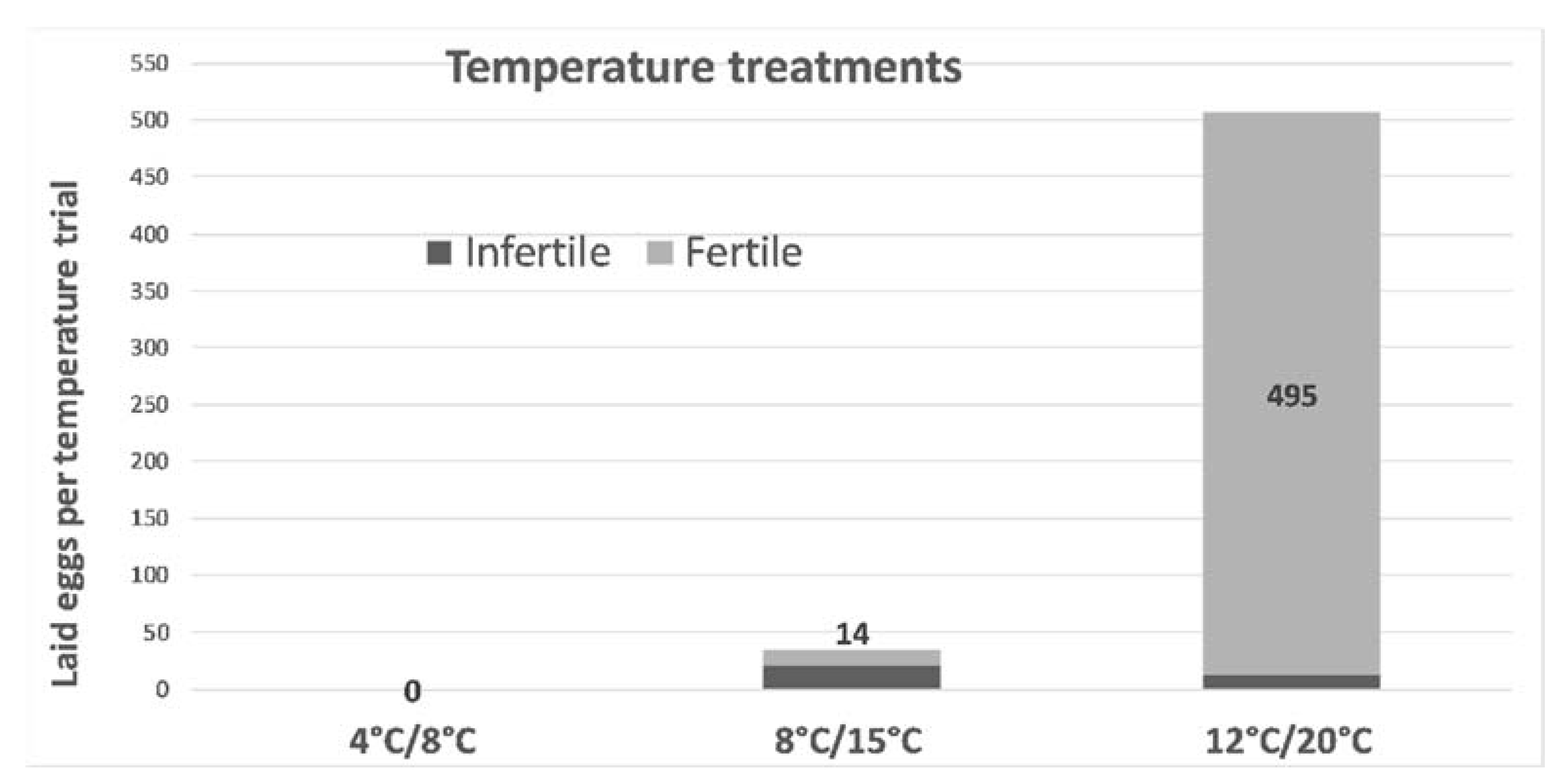
3.4. Phenology
4. Integrated Pest Management
4.1. Monitoring
4.2. Chemical Control
4.3. Crop Management Measures as Control Options
4.4. Host Plant Resistance
4.5. Natural Enemies and Potential Biological Control
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zander, P.; Amjath-Babu, T.S.; Preissel, S.; Reckling, M.; Bues, A.; Schläfke, N.; Kuhlman, T.; Bachinger, J.; Uthes, S.; Stoddard, F.; et al. Grain legume decline and potential recovery in European agriculture: A review. Agron. Sustain. Dev. 2016, 36, 26. [Google Scholar] [CrossRef]
- Lucas, M.M.; Stoddard, F.L.; Eannicchiarico, P.; Efrias, J.; Emartinez-Villaluenga, C.; Esussmann, D.; Eduranti, M.; Eseger, A.; Zander, P.M.; Pueyo, J.J. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015, 6, 705. [Google Scholar] [CrossRef] [PubMed]
- Gresta, F.; Wink, M.; Prins, U.; Abberton, M.; Capraro, J.; Scarafoni, A.; Hill, G. Lupins in European cropping systems. In Legumes in Cropping Systems; CABI: Wallingford, UK, 2017; pp. 88–108. [Google Scholar]
- Reckling, M.; Bergkvist, G.; Watson, C.; Stoddard, F.L.; Bachinger, J. Re-designing organic grain legume cropping systems using systems agronomy. Eur. J. Agron. 2020, 112, 125951. [Google Scholar] [CrossRef]
- Bebeli, P.J.; Lazaridi, E.; Chatzigeorgiou, T.; Suso, M.-J.; Hein, W.; Alexopoulos, A.A.; Canha, G.; Van Haren, R.J.; Jóhannsson, M.H.; Mateos, C.; et al. State and progress of andean lupin cultivation in Europe: A review. Agronomy 2020, 10, 1038. [Google Scholar] [CrossRef]
- Andersen, K.T. The weewils Sitona griseus F. and Sitona gressorius F. as lupin pests. Die Blattrandkäfer Sitona griseus F. und Sitona gressorius F. als Lupinenschädlinge. Anz. Schädlingskunde 1937, 13, 81–84. [Google Scholar] [CrossRef]
- Andersen, K.T. The lupin weevils Sitona griseus F. and Sitona gressorius F. Die Lupinenblattrandkäfer Sitona griseus F. und Sitona gressorius F. Z. Angew. Entomol. 2009, 24, 325–356. [Google Scholar] [CrossRef]
- Telnov, D.; Fägerström, C.; Gailis, J.; Kalnins, M.; Napolov, A.; Piterans, U.; Vilks, K. Contributions to the knowledge of Latvian Coleoptera. 5. Latv. Entomol. 2006, 43, 78–125. [Google Scholar]
- Tyler, G.; Tyler, T. Comparisons of the weevil fauna (Curculionidae, Apionidae, Bruchidae) of fifteen legume hosts (Fabaceae) in South Sweden. Balt. J. Coleopterol. 2016, 16, 157–165. [Google Scholar]
- Fabricius, J.C.; Metcalf Collection (North Carolina State University). Systema Entomologiae: Sistens Insectorvm Classes, Ordines, Genera, Species, Adiectis Synonymis, Locis, Descriptionibvs, Observationibvs/Io; Officina Libraria Kortii: Flensburg and Leipzig, Germany, 1775. [Google Scholar]
- Müller Frederik, O. Zoologiae Danicae Prodromus, seu Animalium Daniae et Norvegiae Indigenarum Characteres, Nomina, et Synonyma Imprimis Popularium; Havniae Typis Hallageriis: Copenhagen, Denmark, 1776. [Google Scholar]
- Velasquez De Castro, A.J.; Alonso-Zarazaga, M.Á.; Outerelo, R. Systematics of Sitonini (Coleoptera: Curculionidae: Entiminae), with a hypothesis on the evolution of feeding habits. Syst. Entomol. 2007, 32, 312–331. [Google Scholar] [CrossRef]
- Karsch, F.A.F. The weevil Sitones griseus (Fabr.) as a new enemy of agriculture. Der Rüssler Sitones griseus (Fabr.) als neuer feind der landwirthschaft. Entomologische Nachrichten 1884, 10, 157–159. [Google Scholar]
- Reitter, E. Fauna Germanica: Die Käfer des Deutschen Reiches; K.G. Lutz: Stuttgart, Germany, 1916. [Google Scholar]
- Dieckmann, L. Contributions to the insect fauna of the GDR: Curculionidae (Brachycerinae, Otiorhynchinae, Brachyderinae) Beiträge zur Insektenfauna der DDR: Coleoptera—Curculionidae (Brachycerinae, Otiorhynchinae, Brachyderinae). Beiträge Entomol. 1980, 30, 145–310. [Google Scholar]
- Balalaikins, M. Latvian Entiminae (Coleoptera: Curculionidae): 3. Tribe sitonini gistel, 1856. Acta Biol. Univ. Daugavp. 2012, 12, 6–23. [Google Scholar]
- Silfverberg, H. Renaming weevils (Coleoptera: Curculionoidea). Sahlbergia 2014, 20, 35–38. [Google Scholar]
- Velazquez de Castro, A.J.; Friedman, A.; Borovec, R. Sitonini (Curculionidae: Entiminae) of Israel. Israel J. Entomol. 2011, 40, 71–108. [Google Scholar]
- Vetter, V.M.S.; Walter, J.; Wilfahrt, P.A.; Buhk, C.; Braun, M.; Clemens, S.; Dinkel, E.; Dubbert, M.; Schramm, A.; Wegener, F.; et al. Invasion windows for a global legume invader are revealed after joint examination of abiotic and biotic filters. Plant Biol. 2019, 21, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Valtonen, A.; Jantunen, J.; Saarinen, K. Flora and lepidoptera fauna adversely affected by invasive Lupinus polyphyllus along road verges. Biol. Conserv. 2006, 133, 389–396. [Google Scholar] [CrossRef]
- Germann, C. Charagmus gressorius (Fabricius, 1792)—Neunachweis für die Schweiz (Curculionidae, Entiminae, Sitonini). Entomo Helv. 2009, 2, 7–10. [Google Scholar]
- Museum für Naturkunde Berlin. Fauna Europaea: All European Animal Species Online. Charagmus gressorius (Fabricius, 1792). Available online: https://fauna-eu.org/cdm_dataportal/taxon/efec4b58-cba5-480d-9086-9bf95ae55617 (accessed on 27 May 2021).
- Museum für Naturkunde Berlin. Fauna Europaea: All European Animal Species Online. Charagmus griseus (Fabricius, 1775). Available online: https://fauna-eu.org/cdm_dataportal/taxon/891a8784-ff7f-41c9-8c00-aee98f27353a (accessed on 27 May 2021).
- Alekseev, V.I.; Bukejs, A.; Drotikova, A.M.; Rozhina, V.I. Contributions to the knowledge of beetles (Insecta: Coleoptera) in the Kaliningrad region. 5. Zoöl. Ecol. 2015, 25, 247–256. [Google Scholar] [CrossRef]
- Gosik, R.; Sprick, P. Description of the larvae and pupae of eight species of the tribe Sitonini gistel, 1848 (Coleoptera, Curculionidae) from Central Europe. Zootaxa 2017, 4324, 401. [Google Scholar] [CrossRef]
- Hondelmann, W. The lupin—Ancient and modern crop plant. Theor. Appl. Genet. 1984, 68, 1–9. [Google Scholar] [CrossRef]
- Wink, M.; Meißner, C.; Witte, L. Patterns of quinolizidine alkaloids in 56 species of the genus Lupinus. Phytochemistry 1995, 38, 139–153. [Google Scholar] [CrossRef]
- Philippi, J.; Schliephake, E.; Jürgens, H.-U.; Jansen, G.; Ordon, F. Feeding behavior of aphids on narrow-leafed lupin (Lupinus angustifolius) genotypes varying in the content of quinolizidine alkaloids. Entomol. Exp. Appl. 2015, 156, 37–51. [Google Scholar] [CrossRef]
- Ströcker, K.; Wendt, S.; Kirchner, W.H.; Struck, C. Feeding preferences of the weevils Sitona gressorius and Sitona griseus on different lupin genotypes and the role of alkaloids. Arthropod-Plant Interact. 2013, 7, 579–589. [Google Scholar] [CrossRef]
- Stępkowski, T.; Moulin, L.; Krzyżańska, A.; McInnes, A.; Law, I.J.; Howieson, J. European origin of Bradyrhizobium populations infecting lupins and serradella in soils of Western Australia and South Africa. Appl. Environ. Microbiol. 2005, 71, 7041–7052. [Google Scholar] [CrossRef]
- Stępkowski, T.; Banasiewicz, J.; Granada, C.E.; Andrews, M.; Passaglia, L.M.P. Phylogeny and phylogeography of rhizobial symbionts nodulating legumes of the tribe Genisteae. Genes 2018, 9, 163. [Google Scholar] [CrossRef]
- Parker, M.A. The spread of Bradyrhizobium lineages across host legume clades: From abarema to zygia. Microb. Ecol. 2014, 69, 630–640. [Google Scholar] [CrossRef]
- Johnson, S.; Rasmann, S. Root-feeding insects and their interactions with organisms in the rhizosphere. Annu. Rev. Èntomol. 2015, 60, 517–535. [Google Scholar] [CrossRef]
- Johnson, S.; Gregory, P.J.; Greenham, J.R.; Zhang, X.; Murray, P.J. Attractive properties of an isoflavonoid found in white clover root nodules on the clover root weevil. J. Chem. Ecol. 2005, 31, 2223–2229. [Google Scholar] [CrossRef]
- Johnson, S.N.; Birch, A.N.E.; Gregory, P.J.; Murray, P.J. The ‘mother knows best’ principle: Should soil insects be included in the preference-performance debate? Ecol. Entomol. 2006, 31, 395–401. [Google Scholar] [CrossRef]
- Ströcker, K.; Kaufmann, K.; Schachler, B.; Struck, C. Influence of insect herbivory on yield and infestation by lupin weevils (Sitona spp.) on different lupin genotypes. In Proceedings of the 13th International Lupin Conference, Poznan, Poland, 6–10 June 2011; pp. 262–268. [Google Scholar]
- Lohaus, K.; Vidal, S. Abundance of Sitona lineatus L. (Col., Curculionidae) in peas (Pisum sativum L.): Effects on yield parameters and nitrogen balance. Crop. Prot. 2010, 29, 283–289. [Google Scholar] [CrossRef]
- Quinn, M.A.; Hall, M.H. Compensatory response of a legume root-nodule system to nodule herbivory by Sitona hispidulus. Entomol. Exp. Appl. 1992, 64, 167–176. [Google Scholar] [CrossRef]
- Vankosky, M.A.; Cárcamo, H.A.; Dosdall, L.M. Response of Pisum sativum (Fabales: Fabaceae) to Sitona lineatus (Coleoptera: Curculionidae) infestation: Effect of adult weevil density on damage, larval population, and yield loss. J. Econ. Entomol. 2011, 104, 1550–1560. [Google Scholar] [CrossRef]
- Rim, K.; Price, S.J.; Wenninger, E.J.; Long, R.; Ramirez, R.A. Biology and management of clover root curculio (Coleoptera: Curculionidae). J. Integr. Pest Manag. 2019, 10, 403. [Google Scholar] [CrossRef]
- Kaufmann, K.; Thalmann, R.; Schachler, B.; Saal, B.; Struck, C. Characterization of soil-borne root and stem rot diseases in narrow-leafed lupin cultivation in North-East Germany and development of screening methods for resistance breeding. In Lupin crops—An opportunity for today, a promise for the future. In Proceedings of the 13th International Lupin Conference, Poznań, Poland, 6–10 June 2011; pp. 257–261. [Google Scholar]
- Thalmann, R.; Kaufmann, K.; Struck, C. Black root rot on lupins – An early species-specific detection of the pathogen Thielaviopsis basicola Schwarze Wurzelfäule bei Blauen Lupinen—frühzeitige und spezifische Detektion des Erregers Thielaviopsis basicola. Gesunde Pflanz. 2008, 60, 67–75. [Google Scholar] [CrossRef]
- Hatcher, P.E. Three-way interactions between plant pathogenic fungi, herbivorous insects and their host plants. Biol. Rev. 1995, 70, 639–694. [Google Scholar] [CrossRef]
- Willsey, T.; Chatterton, S.; Cárcamo, H. Interactions between the root rot pathogen Fusarium avenaceum and the pea leaf weevil (Sitona lineatus) in field pea. Crop. Prot. 2018, 116, 108–114. [Google Scholar] [CrossRef]
- Unkovich, M.; Pate, J.S. An appraisal of recent field measurements of symbiotic N2 fixation by annual legumes. Field Crop. Res. 2000, 65, 211–228. [Google Scholar] [CrossRef]
- Peoples, M.B.; Brockwell, J.; Herridge, D.F.; Rochester, I.J.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; Maskey, S.L.; et al. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Kalembasa, S.; Szukała, J.; Faligowska, A.; Kalembasa, D.; Symanowicz, B.; Becher, M.; Gebus-Czupyt, B. Quantification of biologically fixed nitrogen by white lupin (Lupins albus L.) and its subsequent uptake by winter wheat using the 15N isotope dilution method. Agronomy 2020, 10, 1392. [Google Scholar] [CrossRef]
- Corre-Hellou, G.; Crozat, Y. N2 fixation and N supply in organic pea (Pisum sativum L.) cropping systems as affected by weeds and peaweevil (Sitona lineatus L.). Eur. J. Agron. 2005, 22, 449–458. [Google Scholar] [CrossRef]
- Murray, P.J.; Hatch, D.J.; Cliquet, J.B. Impact of insect root herbivory on the growth and nitrogen and carbon contents of white clover (Trifolium repens) seedlings. Can. J. Bot. 1996, 74, 1591–1595. [Google Scholar] [CrossRef]
- Paak, M.-L.; Struck, C. Two small beetles–one big damage: Weevils in the lupin field. Zwei kleine Käfer—Ein großer Schaden: Blattrandkäfer im Lupinenfeld. In Legumes-Contributions to Sustainable Agriculture/Mecklenburg-Western Pomerania, State Research Center for Agriculture and Fisheries; State Research Center for Agriculture and Fisheries Mecklenburg-Western Pomerania, Ed.; State Research Center for Agriculture and Fisheries Mecklenburg-Western Pomerania (LFA): Gulzow-Pluzen, Germany, 2020; Volume 62, pp. 61–64. [Google Scholar]
- Tan, Y.; Hower, A.A. Development and feeding behavior of clover root Curculio (Coleoptera: Curculionidae) Larvae on alfalfa. Environ. Entomol. 1991, 20, 1013–1018. [Google Scholar] [CrossRef]
- Vankosky, M. Distribution, biology and integrated management of the pea leaf weevil, Sitona lineatus L. (Coleoptera: Curculionidae), with an analysis of research needs. CAB Rev. Perspect. Agric. Veter. Sci. Nutr. Nat. Resour. 2009, 4, 1–18. [Google Scholar] [CrossRef]
- Humphreys, R.K.; Ruxton, G.D. A review of thanatosis (death feigning) as an anti-predator behaviour. Behav. Ecol. Sociobiol. 2018, 72, 1–16. [Google Scholar] [CrossRef]
- Hamon, N.; Bardner, R.; Lee, J.B.; Allen-Williams, L. Flight periodicity and infestation size of Sitona lineatus. Ann. Appl. Biol. 1987, 111, 271–284. [Google Scholar] [CrossRef]
- Cantot, P.; Rolet, F. Quantification des populations de Sitona lineatus L. et de leurs attaques sur pois protéagineux (Pisum sativum L.). Agronomie 1986, 6, 481–486. [Google Scholar] [CrossRef]
- Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL). Verzeichnis Zugelassener Pflanzenschutzmittel. Available online: https://apps2.bvl.bund.de/psm/jsp/index.jsp (accessed on 19 March 2021).
- Matyjaszczyk, E. Protection possibilities of agricultural minor crops in the European Union: A case study of soybean, lupin and camelina. J. Plant Dis. Prot. 2019, 127, 55–61. [Google Scholar] [CrossRef]
- Cárcamo, H.A.; Vankosky, M.A.; Wijerathna, A.; Olfert, O.O.; Meers, S.B.; Evenden, M.L. Progress toward integrated pest management of pea leaf weevil: A review. Ann. Entomol. Soc. Am. 2018, 111, 144–153. [Google Scholar] [CrossRef]
- Struck, C. Fungal diseases and pests and their control. In Lupins Cultivation and Uses; Gesellschaft zur Förderung der Lupine, Ed.; Altstadt-Druck GmbH: Rostock, Germany, 2020; pp. 33–37. [Google Scholar]
- Niu, Y.; Bainard, L.D.; May, W.E.; Hossain, Z.; Hamel, C.; Gan, Y. Intensified pulse rotations buildup pea rhizosphere pathogens in cereal and pulse based cropping systems. Front. Microbiol. 2018, 9, 1909. [Google Scholar] [CrossRef]
- Hanavan, R.P.; Bosque-Pérez, N.A.; Schotzko, D.J.; Guy, S.O.; Eigenbrode, S.D. Early-season aerial adult colonization and ground activity of pea leaf weevil (Coleoptera: Curculionidae) in pea as influenced by tillage system. J. Econ. Entomol. 2008, 101, 1606–1613. [Google Scholar] [CrossRef]
- Hanavan, R.P.; Bosque-Pérez, N.A.; Schotzko, D.J.; Eigenbrode, S.D. Influence of tillage on adult and immature pea leaf weevil (Coleoptera: Curculionidae) densities in pea. J. Econ. Entomol. 2010, 103, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Hanavan, R.P.; Bosque-Pérez, N.A. Influence of no-tillage practices and later planting date on the pea leaf weevil, Sitona lineatus, in pea, Pisum sativum. Entomol. Exp. Appl. 2017, 162, 77–85. [Google Scholar] [CrossRef]
- González-Chang, M.; Tiwari, S.; Sharma, S.; Wratten, S.D. Habitat management for pest management: Limitations and prospects. Ann. Entomol. Soc. Am. 2019, 112, 302–317. [Google Scholar] [CrossRef]
- He, H.-M.; Liu, L.-N.; Munir, S.; Bashir, N.H.; Wang, Y.; Yang, J.; Li, C.-Y. Crop diversity and pest management in sustainable agriculture. J. Integr. Agric. 2019, 18, 1945–1952. [Google Scholar] [CrossRef]
- Hurej, M.; Twardowski, J.P.; Kozak, M. Weevil (Coleoptera: Curculionidae) assemblages in the fields of narrow-leafed lupin sown as pure stand and intercropped with spring triticale. Zemdirb. Agric. 2013, 100, 393–400. [Google Scholar] [CrossRef]
- Stout, M.J. Host-plant resistance in pest management. In Integrated Pest Management; Abrol, D.P., Ed.; Elsevier: Oxford, UK, 2014; pp. 1–21. ISBN 9780123985293. [Google Scholar]
- Chang, G.C.; Rutledge, C.E.; Biggam, R.C.; Eigenbrode, S.D. Arthropod diversity in peas with normal or reduced waxy bloom. J. Insect Sci. 2004, 4, 1–11. [Google Scholar] [CrossRef]
- Murray, P.J.; Cheung, A.K.M.; Abberton, M.T. Intraspecific variation in Trifolium pratense: Impact on feeding and host location by Sitona lepidus (Coleoptera, Curculionidae). J. Pest Sci. 2007, 80, 51–57. [Google Scholar] [CrossRef]
- El-Bouhssini, M.; Sarker, A.; Erskine, W.; Joubi, A. First sources of resistance to Sitona weevil (Sitona crinitus Herbst) in wild Lens species. Genet. Resour. Crop. Evol. 2007, 55, 1–4. [Google Scholar] [CrossRef]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Quinolizidine and pyrrolizidine alkaloid chemical ecology—a mini-review on their similarities and differences. J. Chem. Ecol. 2018, 45, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, G.; Gurr, G.; Kühne, S.; Wade, M.R.; Wratten, S.; Wyss, E. Arthropod pest management in organic crops. Annu. Rev. Entomol. 2007, 52, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Barratt, B.I.P.; Oberprieler, R.G.; Barton, D.M.; Mouna, M.; Stevens, M.; Alonso-Zarazaga, M.A.; Vink, C.J.; Ferguson, C.M. Could research in the native range, and non-target host range in Australia, have helped predict host range of the parasitoid Microctonus aethiopoides Loan (Hymenoptera: Braconidae), a biological control agent introduced for Sitona discoideus Gyllenhal (Coleoptera: Curculionidae) in New Zealand? BioControl 2012, 57, 735–750. [Google Scholar] [CrossRef]
- Goldson, S.L.; McNeill, M.; Gerard, P.J.; Proffitt, J.R.; Phillips, C.B.; Cane, R.P.; Murray, P.J. British-based search for natural enemies of the clover root weevil, Sitona lepidus in Europe. N. Z. J. Zool. 2004, 31, 233–240. [Google Scholar] [CrossRef][Green Version]
- Aeschlimann, J.-P. The Sitona [Col.: Curculionidae] species occurring on Medicago and their natural enemies in the Mediterranean region. BioControl 1980, 25, 139–153. [Google Scholar] [CrossRef]
- Vankosky, M.A.; Cárcamo, H.A.; Dosdall, L.M. Identification of potential natural enemies of the pea leaf weevil, Sitona lineatus L. in western Canada. J. Appl. Entomol. 2011, 135, 293–301. [Google Scholar] [CrossRef]
- Cullen, J.M.; Hopkins, D.C. Rearing, release and recovery of Microtonus aethiopoides Loan (Hymenoptera: Braconidae) imported for the control of Sitona discoideus Gyllenhal (Coleoptera: Curculionidae) in South Eastern Australia. J. Aust. Ent. Soc. 1992, 21, 279–284. [Google Scholar] [CrossRef]
- Stufkens, M.; Farrell, J.; Goldson, S. Establishment of Microctonus aethiopoides, a parasitoid of the Sitona weevil, in New Zealand. In Proceedings of the New Zealand Weed and Pest Control Conference, Nelson, BC, Canada, 11 August 1987; New Zealand Plant Protection Society: Auckland, New Zealand, 1987; Volume 40, pp. 31–32. [Google Scholar]
- Gerard, P.J.; Wilson, D.J.; Eden, T.M. Field release, establishment and initial dispersal of Irish Microctonus aethiopoides in Sitona lepidus populations in northern New Zealand pastures. BioControl 2011, 56, 861–870. [Google Scholar] [CrossRef]
- Vink, C.J.; Barratt, B.I.; Phillips, C.; Barton, D.M. Moroccan specimens of Microctonus aethiopoides spice our understanding of genetic variation in this internationally important braconid parasitoid of adult weevils. BioControl 2012, 57, 751–758. [Google Scholar] [CrossRef]
- Willoughby, B.; Glare, T.; Kettlewell, F.; Nelson, T. Beauveria bassiana as a potential biocontrol agent against the clover root weevil, Sitona lepidus. In Proceedings of the New Zealand Plant Protection Conference, Hamilton, ON, USA, 11–13 August 1998; New Zealand Plant Protection Society: Auckland, New Zealand, 1998; Volume 51, pp. 9–15. [Google Scholar]
- Verkleij, F.N.; Van Amelsvoort, P.A.M.; Smits, P.H. Control of the pea weevil (Sitona lineatus L.) (Col., Curculionidae) by the entomopathogenic fungus Metarhizium anisopliaein field beans. J. Appl. Entomol. 1992, 113, 183–193. [Google Scholar] [CrossRef]
- Klingen, I.; Westrum, K.; Meyling, N.V. Effect of Norwegian entomopathogenic fungal isolates against Otiorhynchus sulcatus larvae at low temperatures and persistence in strawberry rhizospheres. Biol. Control. 2015, 81, 1–7. [Google Scholar] [CrossRef]
- Ansari, M.A.; Butt, T. Influence of the application methods and doses on the susceptibility of black vine weevil larvae Otiorhynchus sulcatus to Metarhizium anisopliae in field-grown strawberries. BioControl 2013, 58, 257–267. [Google Scholar] [CrossRef]
- Hirsch, J.; Reineke, A. Efficiency of commercial entomopathogenic fungal species against different members of the genus Otiorhynchus (Coleoptera: Curculionidae) under laboratory and semi-field conditions. J. Plant Dis. Prot. 2014, 121, 211–218. [Google Scholar] [CrossRef]
- Jaworska, M.; Ropek, D. Influence of host-plant on the susceptibility of Sitona lineatus L. (Col., Curculionidae) to Steinernema carpocapsae Weiser. J. Invertebr. Pathol. 1994, 64, 96–99. [Google Scholar] [CrossRef]
- Loya, L.J.; Hower, A.A. Infectivity and reproductive potential of the Oswego strain of Heterorhabditis bacteriophora associated with life stages of the clover root curculio, Sitona hispidulus. J. Invertebr. Pathol. 2003, 83, 63–72. [Google Scholar] [CrossRef]
- Guy, A.; Gaffney, M.; Kapranas, A.; Griffin, C.T. Conditioning the entomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis megidis by pre-application storage improves efficacy against black vine weevil, Otiorhynchus sulcatus (Coleoptera: Curculionidae) at low and moderate temperatures. Biol. Control. 2017, 108, 40–46. [Google Scholar] [CrossRef]
- Haukeland, S.; Lola-Luz, T. Efficacy of the entomopathogenic nematodes Steinernema kraussei and Heterorhabditis megidis against the black vine weevil Otiorhynchus sulcatus in open field-grown strawberry plants. Agric. For. Entomol. 2010, 12, 363–369. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piedra-García, D.; Struck, C. Lupin Root Weevils (Charagmus spp., Curculionidae: Sitonini), a Lupin Pest: A Review of Their Distribution, Biology, and Challenges in Integrated Pest Management. Insects 2021, 12, 950. https://doi.org/10.3390/insects12100950
Piedra-García D, Struck C. Lupin Root Weevils (Charagmus spp., Curculionidae: Sitonini), a Lupin Pest: A Review of Their Distribution, Biology, and Challenges in Integrated Pest Management. Insects. 2021; 12(10):950. https://doi.org/10.3390/insects12100950
Chicago/Turabian StylePiedra-García, Diego, and Christine Struck. 2021. "Lupin Root Weevils (Charagmus spp., Curculionidae: Sitonini), a Lupin Pest: A Review of Their Distribution, Biology, and Challenges in Integrated Pest Management" Insects 12, no. 10: 950. https://doi.org/10.3390/insects12100950
APA StylePiedra-García, D., & Struck, C. (2021). Lupin Root Weevils (Charagmus spp., Curculionidae: Sitonini), a Lupin Pest: A Review of Their Distribution, Biology, and Challenges in Integrated Pest Management. Insects, 12(10), 950. https://doi.org/10.3390/insects12100950




