1. Introduction
Animal-built structures vary widely in shape and size and have evolved through natural selection on their occupants [
1]. Burrows protect their occupants from predators and parasites [
2], provide shelter during periods of vulnerability, such as during molting or when rearing young [
3], and buffer their occupants from the vagaries of the environment, including from events like fire, flooding, and rain [
4,
5]. Also, these structures can serve as adaptive interfaces for managing fluxes of gases and energy between the inhabitant(s) and their physical environment [
1,
6]. Burrows are ubiquitous structures, and, on land, are dug by animals whose size ranges from small arthropods (e.g., ants and spiders) to large mammals (e.g., aardvarks and warthogs) [
7]. In arid environments such as deserts, they may protect their inhabitants from desiccation and thermal stress [
8].
The physical isolation of an animal in its burrow could potentially result in the depletion or accumulation of respiratory or other gases (O
2, CO
2, NH
3, etc.) due to limited ventilation and thus affect its normal physiological functions. This is, however, not necessarily the case, and several ventilation mechanisms have been reported in the literature for relatively large bi-entrance [
6,
9] and single entrance burrows [
10,
11,
12].
It has been proposed that forced ventilation of the lower parts of their burrows may be detrimental to ectothermic animals, as implied from the fact that some scorpions build burrows in a manner that reduces gas exchange [
12,
13], and many spider species directly occlude ventilation by capping the entrances of their burrows. An example of the latter is species of burrowing spiders that cover the entrances with trapdoors. The role of trapdoors in maintaining favorable thermal and hydric conditions within the burrow is indirectly supported by studies of spider behavior in arid environments. For example,
Nemesia caementaria (Latreille, 1799) seal their burrows during estivation [
14], presumably to maintain both cool and humid conditions. Also,
Aliatypus (Smith, 1908) trapdoor spiders that inhabit arid areas in western North America were found to seal their burrows more often than their conspecifics that live in more humid conditions [
15,
16,
17].
None of the above-mentioned studies, however, are supported by direct measurements of the relevant microclimatic variables in the field. Our aim was therefore to quantitatively assess the effects of the presence or absence of a trapdoor on the environmental conditions at the bottom of burrows of a common, but undescribed, lycosid spider species. To this end, we made detailed measurements in a set of artificial burrows, built in the field to resemble natural ones as closely as possible; we also made observations of spider behavior in their natural burrows after selectively removing trapdoors at different times of the day.
Our working hypotheses were that the presence of trapdoors would have measurable effects on air temperature (Ta) and relative humidity (RH) in the burrows and that their removal would affect environmental conditions at the bottom of the burrow and therefore induce spider responses depending on the prevailing environmental conditions at the surface. Specifically, we predicted that warmer and drier surface conditions would elicit faster responses to trapdoor removal.
2. Materials and Methods
Field site—Our research site was a 3-hectare plot on the Sede Zin plateau in the Negev Desert highlands, less than 50 m from the north-east corner of the Sede Boqer Campus of Ben-Gurion University of the Negev at Midreshet Ben-Gurion, Israel (30°51′38″ N, 34°46′40″ E), where the Jacob Blaustein Institutes for Desert Research (BIDR) meteorology station is located. The station provides hourly-averaged values of standard environmental variables. The loess soil in the study area is sparsely populated by shrubs, dominated by Hammada scoparia (Pomel) Iljin.
Study species—The many groups of spiders that build burrows include wolf spiders (reviewed in [
18]). We studied a trapdoor-building wolf spider (Lycosidae), that, although is locally abundant, is yet undescribed in the scientific literature. However, in March 2017, it was given the temporary working name, “
Lycosa hyraculus” (Igor Armiach Steinpress and colleagues, National Arachnid Collection, The Hebrew University of Jerusalem, personal communication) that we have used below. All the spiders studied were adults and we did not differentiate among sexes.
During the day, “L. hyraculus” generally remain in their burrows with the trapdoor closed. At night, they actively hunt on the soil surface within the vicinity of their burrows, typically less than 1 m away, and the trapdoors are left open (personal observations). Like other lycosids, “L. hyraculus” is a generalist predator and we observed them capturing or holding moths, beetles, termites, scorpions, smaller spiders, and other arthropods.
“
Lycosa hyraculus” co-occurs with
Lycosa olivieri Simon, 1876, also a widely distributed species, but the two differ in their coloration and the construction of their burrow entrances.
Lycosa hyraculus covers and camouflages its burrow entrance, usually with a trapdoor made from a piece of soil crust.
L. olivieri, in contrast, builds a grass or soil turret around its burrow entrance. The presence or absence of a turret was found to be a reliable species-specific indicator in other burrowing wolf spiders [
19]. Individuals of the two species can also be distinguished by the extent of the orange coloration on their abdomens, which is found only faintly around the spinnerets in “
L. hyraculus” but extends towards the cephalothorax in
L. olivieri (
Figure 1).
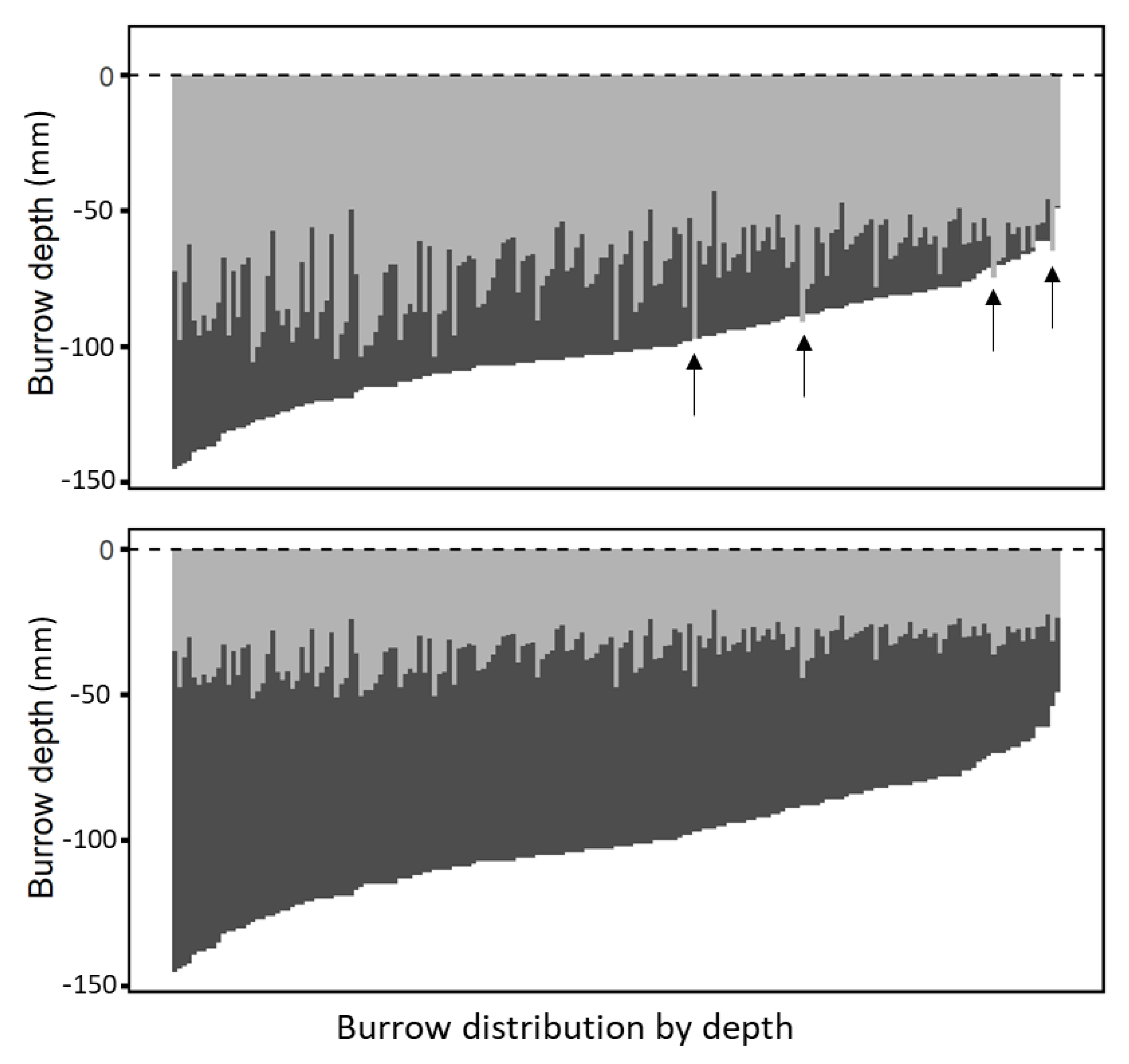
Natural burrow structure: We found that “
L. hyraculus" builds simple, vertical burrows that are between 49 and 145 mm deep (mean = 101.5 mm, SD = 20.0 mm,
n = 196) and between 7 and 21 mm in diameter (mean = 11.89 mm, SD = 2.56 mm,
n = 196). Ninety-eight percent of the burrow entrance diameters were less than 17 mm. Diameters and depths were linearly correlated (diameter (mm) = 4.51 + 0.073 × depth (mm); r
2 = 0.3228; F = 92.47;
p = 0.0001). Burrows are lined with a layer of silk, which may stabilize the soil [
16] and facilitate the movement of the spider inside [
20]. Like other trapdoor-building spiders, “
L. hyraculus" constructs the trapdoor from materials available in its environment, most often pieces of soil crust, but also small pebbles and snail shells, and attaches it to the burrow entrance with silk strands [
15,
21]. The undersides of “
L. hyraculus" trapdoors are usually lined with silk.
Natural burrow survey: At night, we found the spiders visually from reflections of their tapeta lucida in our headlamp beams. We located over 200 “L. hyraculus” and their burrows between May 2016 and July 2017, recorded the GPS location (MAP-330, Magellan, San Dimas, California) of each and marked it with a flag and identification number. We measured burrow entrance diameters and spider body lengths (from the anterior end of the cephalothorax to the posterior end of the abdomen) using digital calipers (Absolute Digimatic, Mituyo, Kawasaki, Japan). The spiders remained immobile on the soil surface near their burrow entrances in our headlamp beams and we could lay the caliper next to them to measure body length. Depth was manually measured with a thin rod against a steel ruler.
Artificial burrows: We made artificial burrows of two diameters that we chose to represent the range of natural burrow dimensions (cylinders with a diameter of 11 or 17 mm, which bracket the range of natural burrow diameters and 100 mm deep, the mean depth of natural burrows) to standardize burrow shape and size. To make a burrow, we first slightly wetted the soil surface to stabilize the soil crust. We then drove a hollow pipe (1 mm thick, 10 mm, or 16 mm external diameter) 100 mm into the ground, and slowly twisted it up to extract soil. We fitted the burrows with sensors and immediately covered them with opaque, soil-colored plaster discs (Glastone Dental Stone, DENTSPLY International, York, PA, USA), 10–20 mm in diameter and 2–3 mm thick, to mimic natural cover and to prevent the soil from drying out at the bottom of the burrow. The covered burrows were allowed to equilibrate with the surrounding soil at least 24 h before measurements were made.
Measurements: (1) Temperature: We measured
Ta in ten artificial burrows of each size (11 mm or 17 mm diameter,) twice a day (early morning and midday) on six days in June 2017. Each burrow was fitted with a type-T thermocouple (24 SWG, 0.511 mm diameter) at the bottom of the burrow with the junctions bent upwards to ensure that they did not touch the bottom or sides. The thus measured air temperatures are indicative of the environment in which spiders likely reside on hot days [
22]. We concurrently measured
Ta 10 mm above the soil surface with a thermocouple shaded by a white polyurethane board. All thermocouples were connected to dataloggers (CR23X Micrologger, Campbell Scientific, Inc., Logan, UT, USA), which recorded
Ta at 10 s intervals and averaged once per minute.
(2) Relative humidity: We measured relative humidity (RH) and temperature simultaneously using compact probes (5.6 mm diameter, USB-TRH300, Dracal Technologies, Brossard, Canada), whose overall accuracy was at 25 °C is ±2% RH. We calibrated the probes against a humidity sensor conforming to the Israel Meteorological Service standard (HC2-S3 humidity sensor, Rotronic AG, Bassersdorf, Switzerland) at the BIDR meteorology station for 24 h on 30 May and 8 August 2017.
Since RH depends on temperature, we examined changes in the water content of the air at the bottom of the burrow by calculating the water vapor pressure (
e). We first solved for the saturation water vapor pressure (
es, hPa) at the given temperature (using Equations (2.5) and (2.6) of [
23] and calculated actual vapor pressure
e, as
e = RH ×
es.
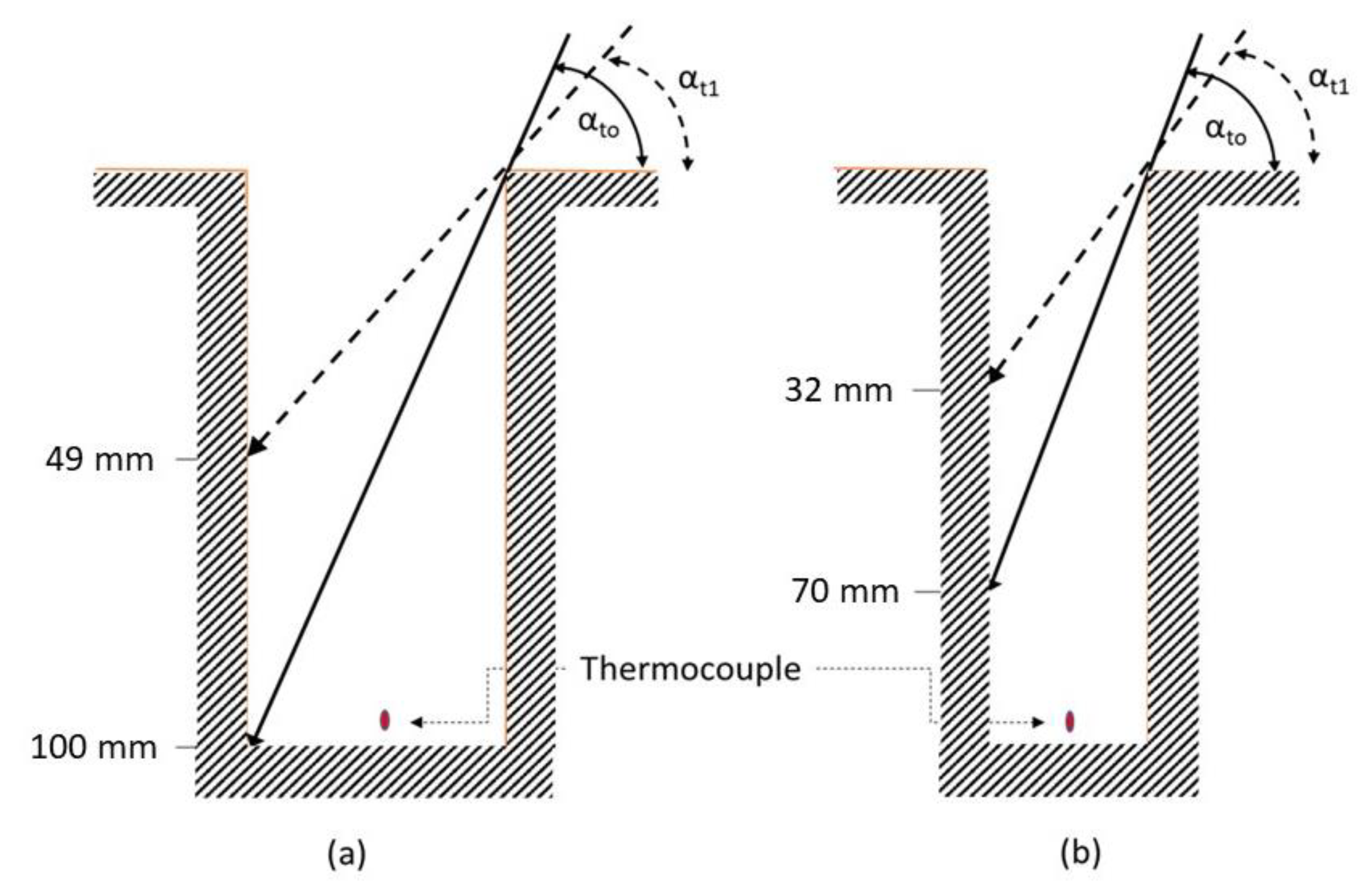
(3) Estimation of solar radiation penetration: The thermal and hydric conditions in the burrow may be affected by diffuse and direct radiation entering open burrows. Diffuse radiation from the celestial hemisphere that enters the burrow can be neglected because the solid angle subtended by the burrow’s entrance is extremely small. Direct radiation penetration into the burrow depends, however, on the elevation of the sun and the size of the burrow opening. Assuming a straight, vertical burrow, the maximum depth that direct radiation could penetrate the burrow (
drad) is:
where
D is the burrow diameter and
α is the solar elevation angle. We calculated the elevation angles for the time of opening and closing of the burrows at midday using the NOAA Solar Position Calculator (
https://www.esrl.noaa.gov/gmd/grad/solcalc/) (accessed on 1 September 2021). Typical values were 81° and 71° at the beginning and end of the measurement trial (13:00–14:00) during the period of 14–18 June 2017 (
Figure 2). We also calculated
drad for the diameters of the natural burrow and compared them to their corresponding depths (
n = 181). Thus, we could determine if and for how long direct radiation reached the bottom of the natural “
L. hyraculus" burrows.
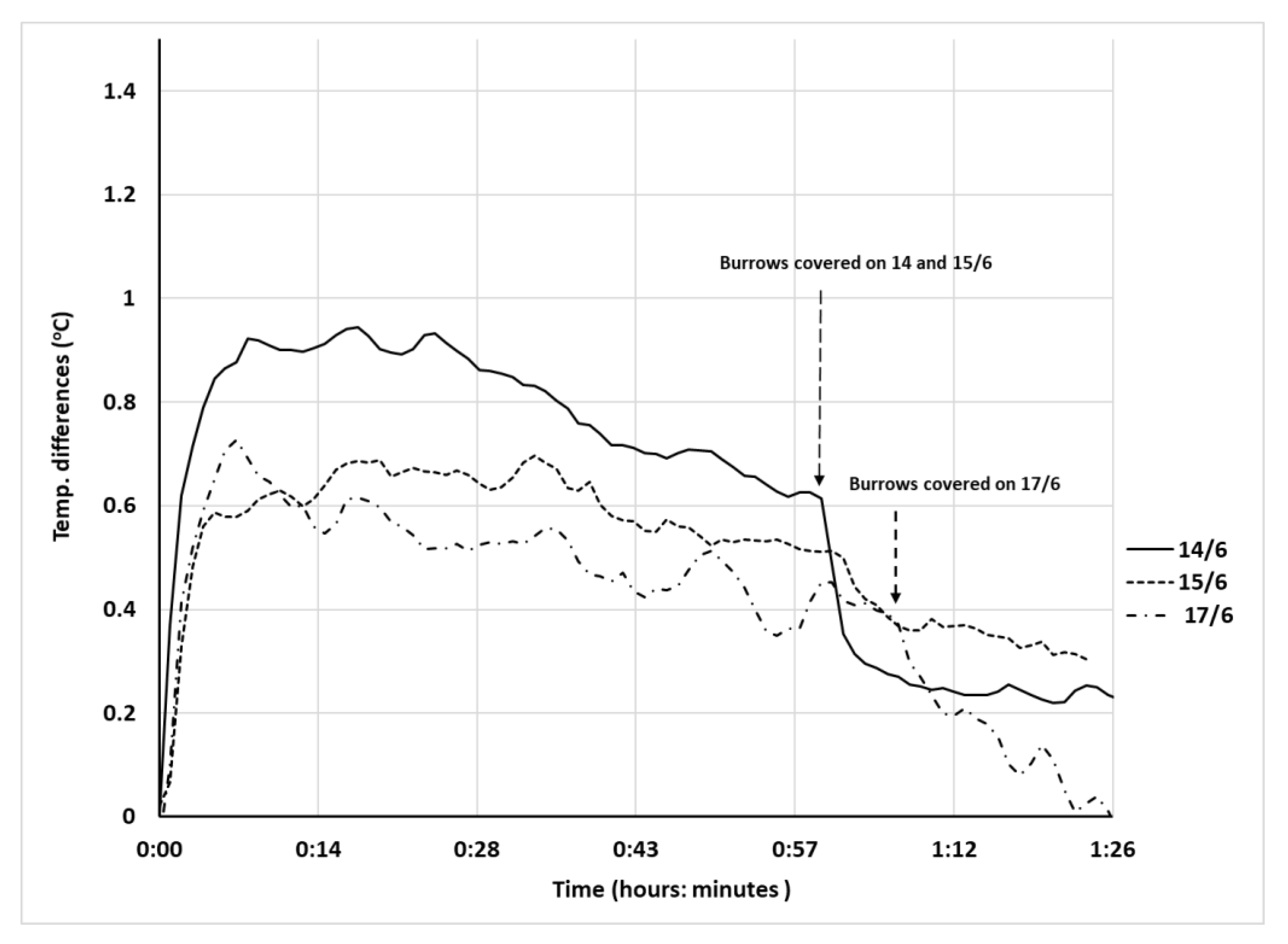
Field trials in artificial burrows: Trial (1) On each trial day, we randomly chose three burrows and opened them for one hour at 07:00, and then three different burrows were opened for one hour at 13:00, leaving four burrows with trapdoors untouched as controls. Opened burrows were closed after each trial. Trials were repeated on three different days for both large (17 mm diameter) and medium-sized (11 mm) burrows. Trial (2) We monitored RH on the 30 July 2017, only at the bottom of the large (17 mm diameter) burrows due to the size of the sensors. We measured RH and temperature simultaneously in six burrows (three of them randomly uncovered) at 1-minute intervals, for 1 h (13:00–14:00), for at least 15 min before and after uncovering. Each set of trials (early morning or midday, mid-sized or large burrows) was analyzed separately. Opened burrows were closed after each trial.
Spider responses to trapdoor removal: We examined changes in “L. hyraculus" responses to trapdoor removal at different times of the day and in different seasons. For each trial, we removed the trapdoor and monitored the response of the spider for up to 24 h.
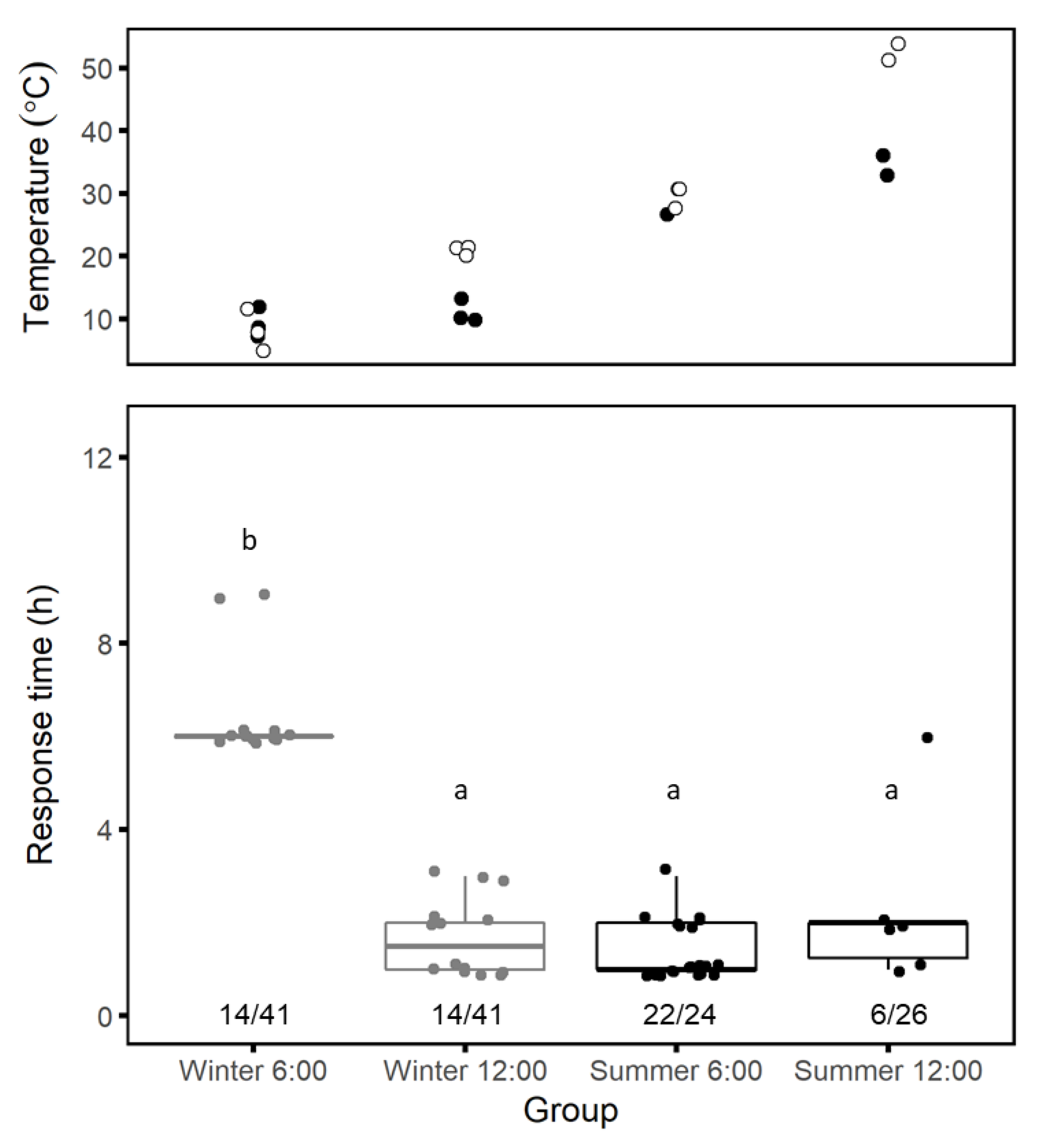
Seasonal patterns: We compared spider responses to trapdoor removal during the summer (June 2016, n = 16; July 2017, n = 33) and the winter (January 2017, n = 18, 30, or 34, depending on the day). For each trial day, we removed half of the trapdoors in the early morning (06:00) and half at midday (12:00). We monitored burrows at 1-hour intervals for 3 h following trapdoor removal and at 3-hour intervals thereafter until sundown. We defined tweb,h as the time in hours from trapdoor removal until when we first saw a complete mesh in the burrow entrance. If a spider partially covered its burrow entrance but never completed it, we assumed that we had disturbed the process during our examination and noted the time of completion rounded up to the next full hour. We also used data collected at the BIDR meteorology station to relate changes in spider responses to soil and air temperatures.
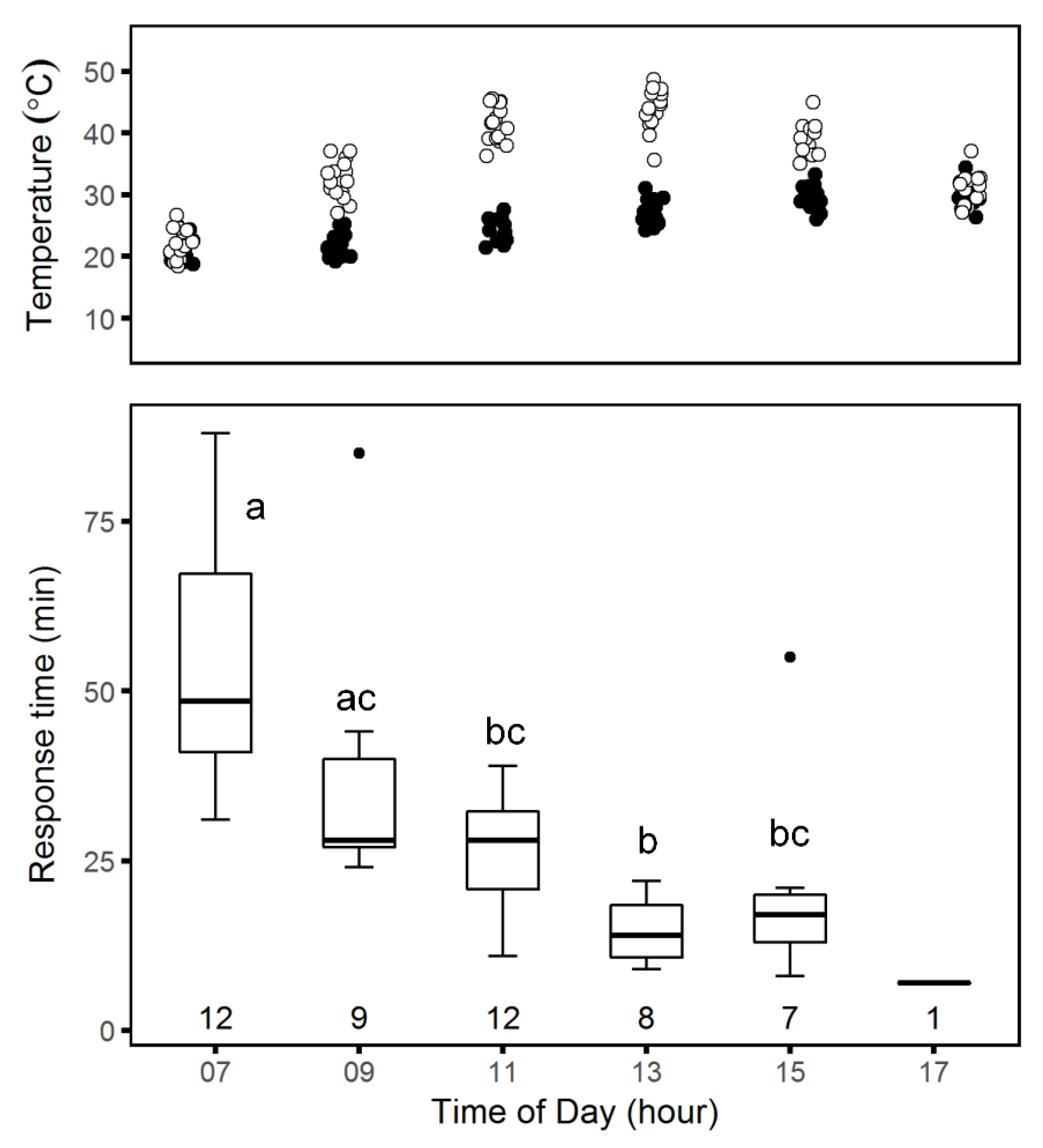
Daily patterns: We examined changes in response time by taking series of time-lapse photographs of spiders at their burrow entrances with a camera (SP-570UZ, Olympus, Tokyo) positioned on a short tripod above and to the north of a burrow entrance to avoid shadows and set the camera to take 100 photographs at 1-minute intervals. The first picture was taken with the burrow covered and a ruler next to the trapdoor. Thereafter, we carefully removed the burrow trapdoor and left the area. We moved the camera to a different burrow every 2 h, starting at 07:00 and ending at 19:00 over 15 days between 6 April and 5 May 2017. Since creating the mesh often involved several bouts of activity, it was difficult to define exactly when the repair behavior ended. Thus, we defined tweb,m as the time in minutes from trapdoor removal to the beginning of mesh-building, when either silk strands or a spider’s spinnerets first became visible at the burrow entrance.
Data analysis of daily spider activity patterns: For each experiment, we compared mean
tweb among groups using one-way ANOVA [
24]. We tested the assumptions of normality of distribution and homoscedasticity using the Shapiro–Wilk test and F test, respectively. For group means that were significantly different, we followed the ANOVA with a Tukey post-hoc test.
We examined differences in the fraction of spiders that spun meshes among groups using chi-square analyses. To determine whether and how this fraction changes over the course of a day, we ran a test of equal proportions followed by a chi-squared test for trends in proportions [
24]. We also used the Welch–Satterthwaite
t-test for unequal variances, where necessary. In our comparison between seasons, we ran a post-hoc test with a Bonferroni correction for multiple comparisons after chi-squared analysis [
25].