Effects of Agaricus bisporus Mushroom Extract on Honey Bees Infected with Nosema ceranae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Agaricus bisporus Extract Preparation
2.2. Bees and the Design of Cage Experiment
2.3. The Selection of an Appropriate Extract Concentration
2.4. Effects of A. bisporus Extract on Nosema Infection
2.5. Inoculum Preparation, Experimental Infection, and Bee Sampling
2.6. Nosema Spore Counting
2.7. RNA Extraction and cDNA Synthesis
2.8. Real-Time Quantitative PCR
3. Statistical Methods
4. Results
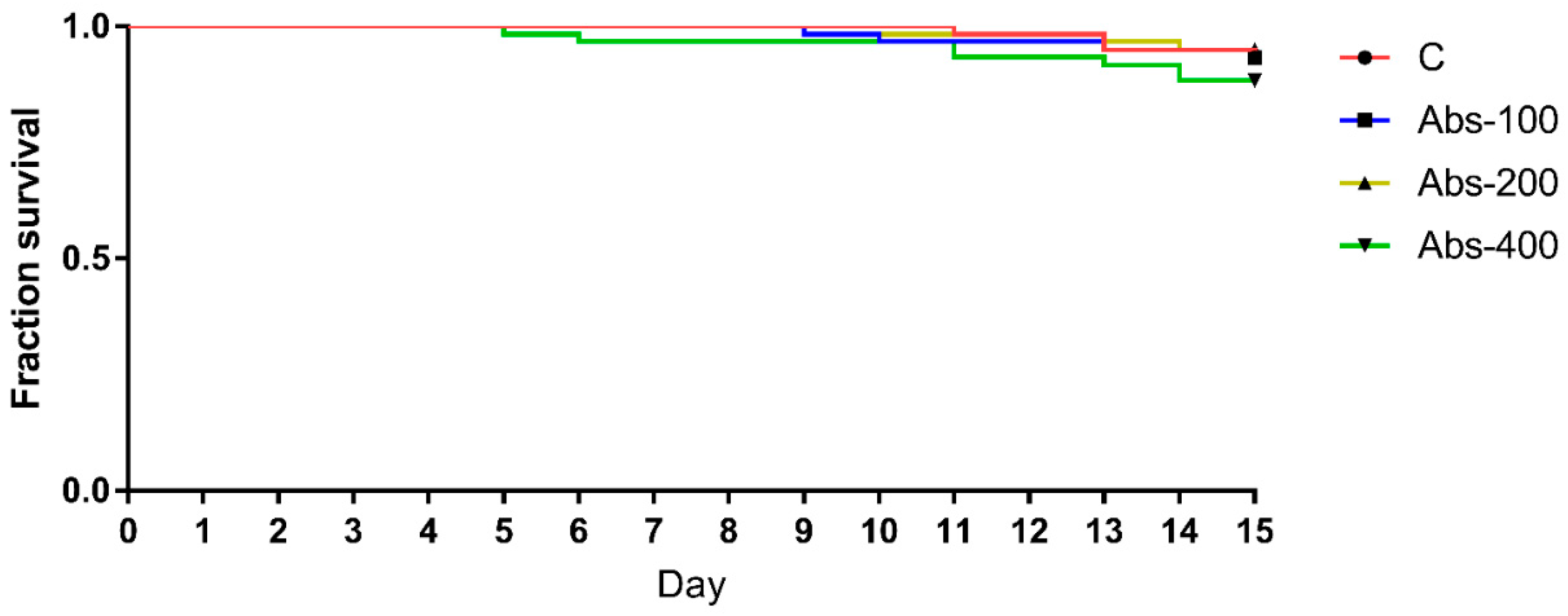
4.1. Bee Survival and Food Consumption
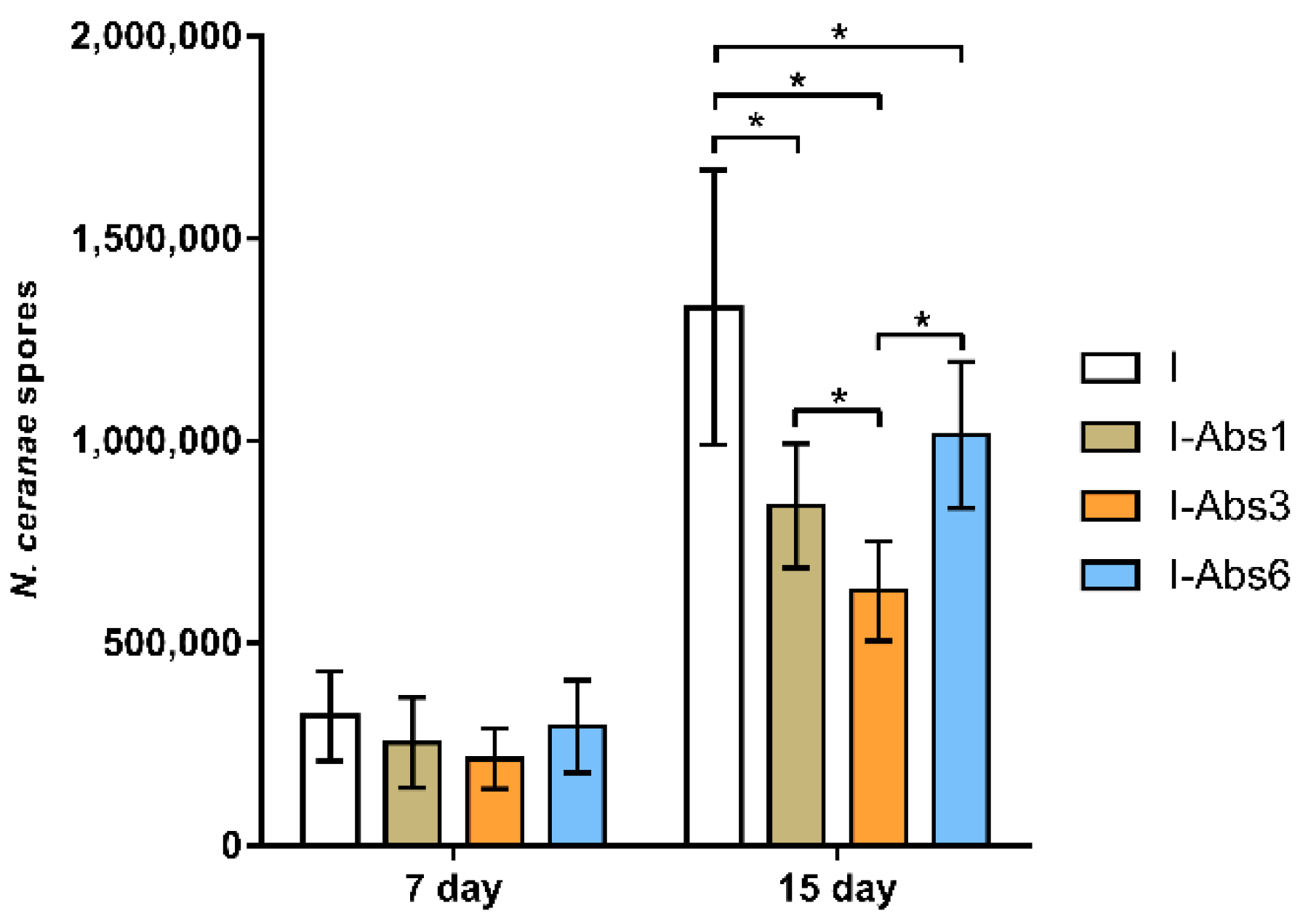
4.2. Quantification of N. ceranae Spores
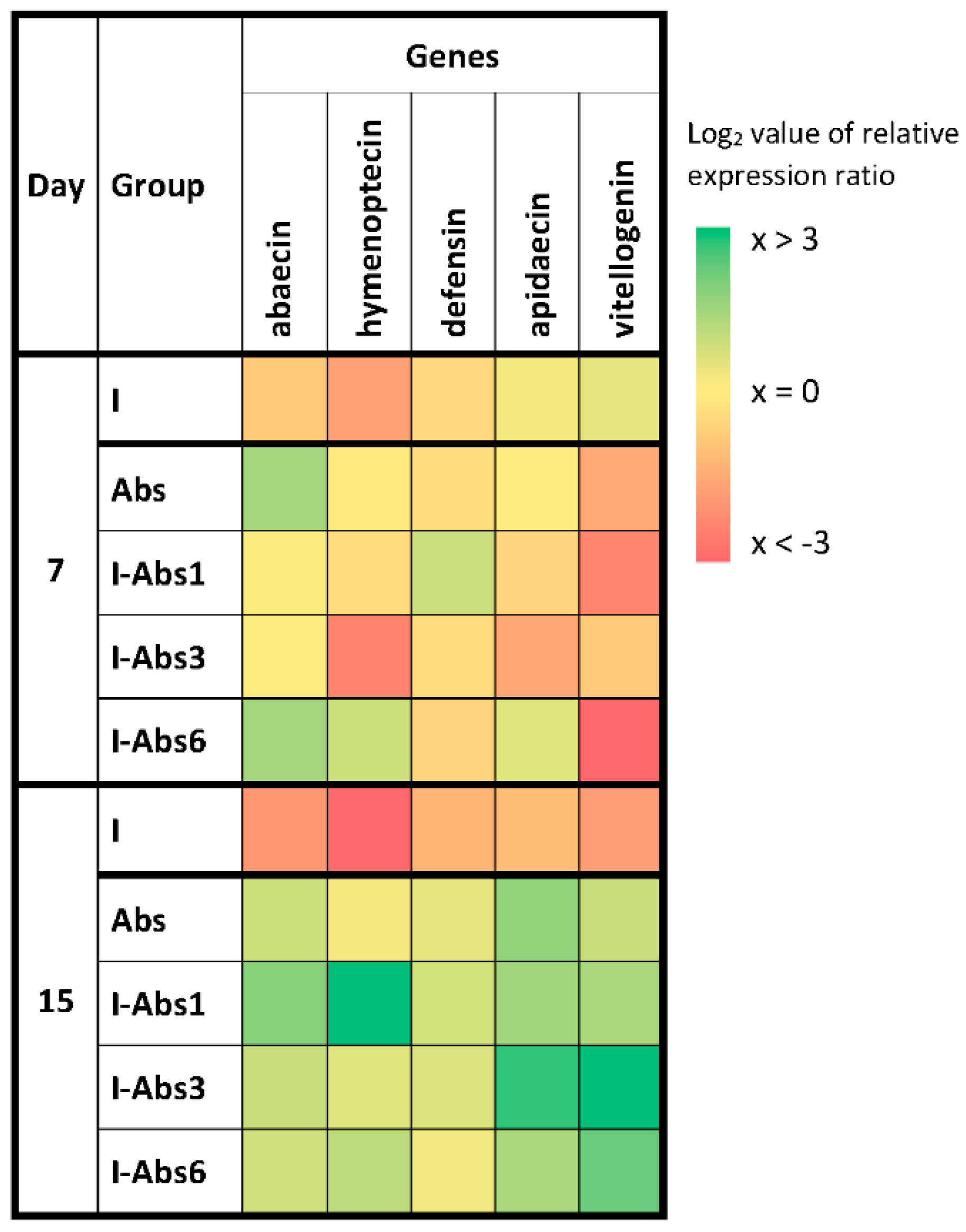
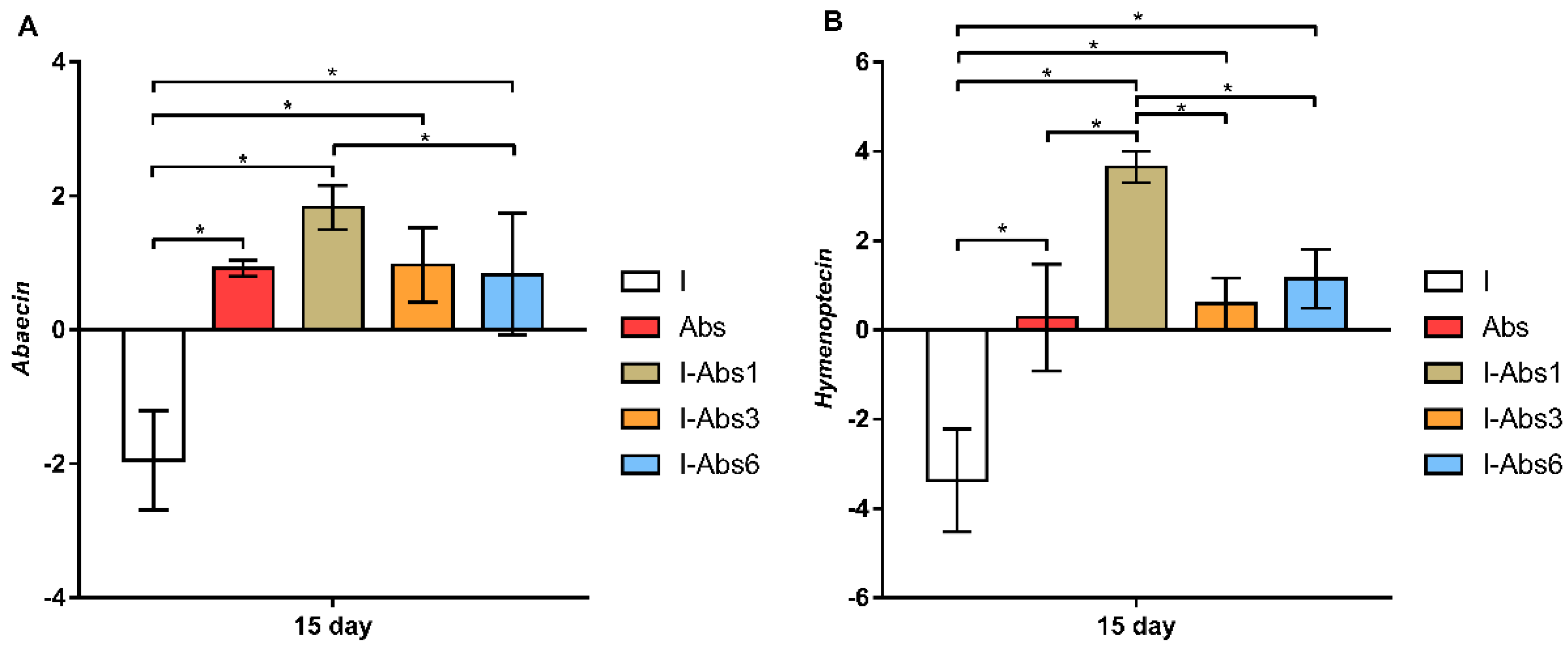
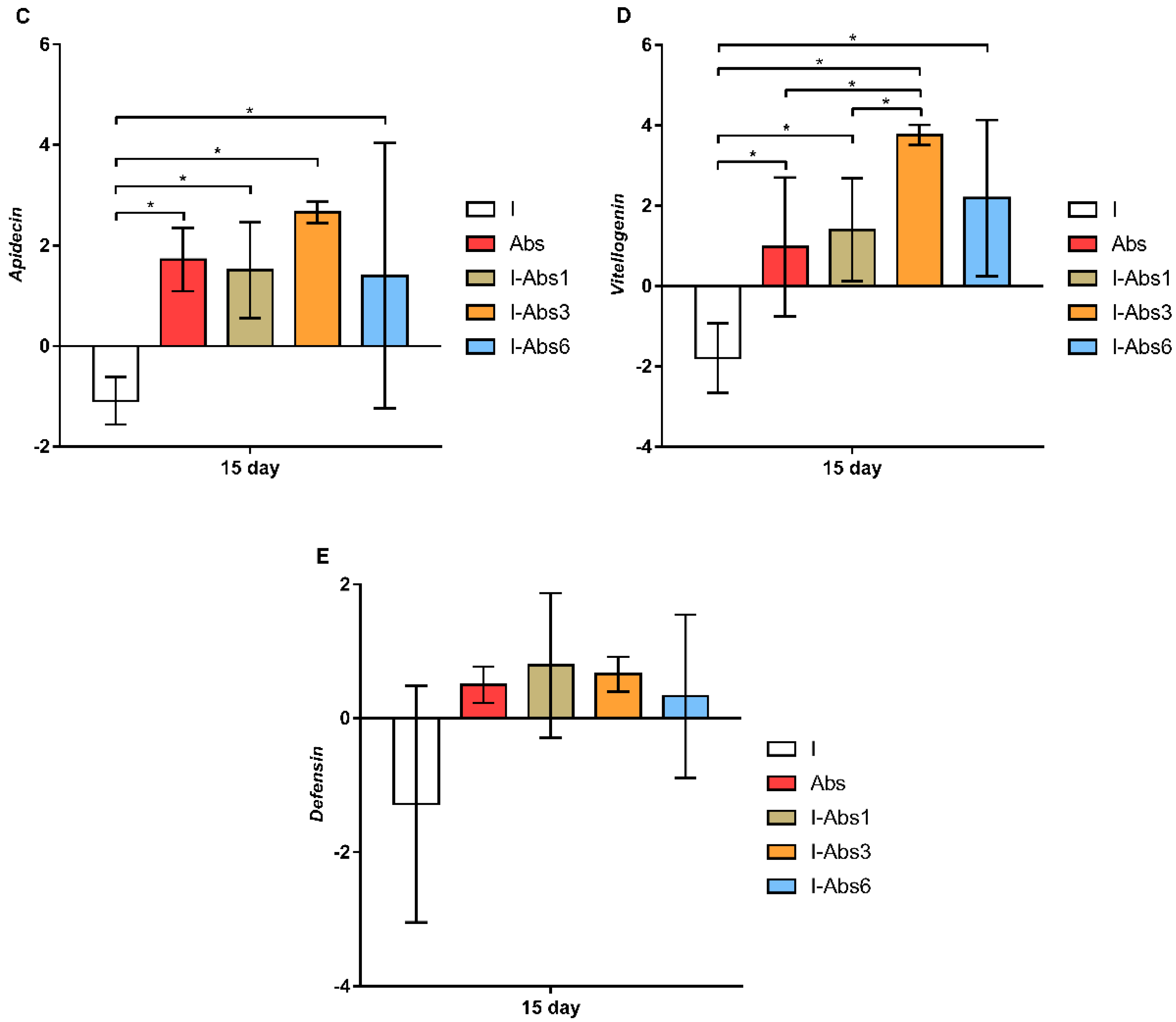
4.3. Gene Expression Analyses
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Burnham, A.J. Scientific Advances in Controlling Nosema ceranae (Microsporidia) Infections in Honey Bees (Apis mellifera). Front. Vet. Sci. 2019, 6, 79. [Google Scholar] [CrossRef]
- Reeves, A.M.; O’Neal, S.T.; Fell, R.D.; Brewster, C.C.; Anderson, T.D. In-Hive Acaricides Alter Biochemical and Morphological Indicators of Honey Bee Nutrition, Immunity, and Development. J. Insect Sci. 2018, 18. [Google Scholar] [CrossRef]
- Stanimirović, Z.; Glavinić, U.; Ristanić, M.; Aleksić, N.; Jovanović, N.M.; Vejnović, B.; Stevanović, J. Looking for the causes of and solutions to the issue of honey bee colony losses. Acta Vet. 2019, 69, 1–31. [Google Scholar] [CrossRef]
- Barroso-Arévalo, S.; Vicente-Rubiano, M.; Puerta, F.; Molero, F.; Sánchez-Vizcaíno, J.M. Immune related genes as markers for monitoring health status of honey bee colonies. BMC Vet. Res. 2019, 15, 72. [Google Scholar] [CrossRef]
- Glavinic, U.; Stevanovic, J.; Ristanic, M.; Rajkovic, M.; Davitkov, D.; Lakic, N.; Stanimirovic, Z. Potential of Fumagillin and Agaricus blazei Mushroom Extract to Reduce Nosema ceranae in Honey Bees. Insects 2021, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- Araneda, X.; Cumian, M.; Morales, D. Distribution, epidemiological characteristics and control methods of the pathogen Nosema ceranae Fries in honey bees Apis mellifera L. (Hymenoptera, Apidae). Arch. Med. Vet. 2015, 47, 129–138. [Google Scholar] [CrossRef][Green Version]
- Martín-Hernandez, R.; Bartolomé, C.; Chejanovsky, N.; Le Conte, Y.; Dalmon, A.; Dussaubat, C.; García-Palencia, P.; Meana, A.; Pinto, M.A.; Soroker, V.; et al. Nosema ceranae in Apis mellifera: A 12 years postdetection perspective. Environ. Microbiol. 2018, 20, 1302–1329. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Evans, J.; Murphy, C.; Gutell, R.; Zuker, M.; Gundensen-Rindal, D.; Pettis, J.S. Morphological, Molecular, and Phylogenetic Characterization ofNosema ceranae, a Microsporidian Parasite Isolated from the European Honey Bee, Apis mellifera. J. Eukaryot. Microbiol. 2009, 56, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Gisder, S.; Hedtke, K.; Möckel, N.; Frielitz, M.C.; Linde, A.; Genersch, E. Five-year cohort study of Nosema spp. in Germany: Does climate shape virulence and assertiveness of Nosema ceranae? Appl. Environ. Microb. 2010, 76, 3032–3038. [Google Scholar] [CrossRef]
- Copley, T.; Jabaji, S. Honeybee glands as possible infection reservoirs of Nosema ceranae and Nosema apis in naturally infected forager bees. J. Appl. Microbiol. 2011, 112, 15–24. [Google Scholar] [CrossRef]
- Glavinic, U.; Stevanovic, J.; Gajic, B.; Simeunovic, P.; Đuric, S.; Vejnovic, B.; Stanimirovic, Z. Nosema ceranae DNA in honey bee haemolymph and honey bee mite Varroa destructor. Acta Vet.-Beograd 2014, 64, 349–357. [Google Scholar]
- Gajger, I.; Kozaric, Z.; Berta, D.; Nejedli, S.; Petrinec, Z. Effect of the Herbal preparation Nozevict on the mid-gut structure of honeybees (Apis melífera) infected with Nosema sp. Spores. Vet. Med. 2011, 56, 344–351. [Google Scholar] [CrossRef]
- Traver, B.E.; Fell, R.D. Nosema ceranae in drone honey bees (Apis mellifera). J. Invertebr. Pathol. 2011, 107, 234–236. [Google Scholar] [CrossRef]
- Dussaubat, C.; Brunet, J.-L.; Higes, M.; Colbourne, J.; Lopez, J.; Choi, J.H.; Hernández, R.M.; Botías, C.; Cousin, M.; McDonnell, C.; et al. Gut Pathology and Responses to the Microsporidium Nosema ceranae in the Honey Bee Apis mellifera. PLoS ONE 2012, 7, e37017. [Google Scholar] [CrossRef] [PubMed]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef]
- Aliferis, K.A.; Copley, T.; Jabaji, S. Gas chromatography–mass spectrometry metabolite profiling of worker honey bee (Apis mellifera L.) hemolymph for the study of Nosema ceranae infection. J. Insect Physiol. 2012, 58, 1349–1359. [Google Scholar] [CrossRef]
- Vidau, C.; Panek, J.; Texier, C.; Biron, D.G.; Belzunces, L.P.; Le Gall, M.; Broussard, C.; Delbac, F.; El Alaoui, H. Differential proteomic analysis of midguts from Nosema ceranae-infected honeybees reveals manipulation of key host functions. J. Invertebr. Pathol. 2014, 121, 89–96. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Parasitic infection leads to decline in hemolymph sugar levels in honeybee foragers. J. Insect Physiol. 2010, 56, 1572–1575. [Google Scholar] [CrossRef]
- Hernández, R.M.; Botías, C.; Barrios, L.; Martínez-Salvador, A.; Meana, A.; Mayack, C.; Higes, M. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol. Res. 2011, 109, 605–612. [Google Scholar] [CrossRef]
- Aufauvre, J.; Misme-Aucouturier, B.; Viguès, B.; Texier, C.; Delbac, F.; Blot, N. Transcriptome Analyses of the Honeybee Response to Nosema ceranae and Insecticides. PLoS ONE 2014, 9, e91686. [Google Scholar] [CrossRef]
- Glavinic, U.; Stankovic, B.; Draskovic, V.; Stevanovic, J.; Petrovic, T.; Lakic, N.; Stanimirovic, Z. Dietary amino acid and vitamin complex protects honey bee from immunosuppression caused by Nosema ceranae. PLoS ONE 2017, 12, e0187726. [Google Scholar] [CrossRef]
- Glavinic, U.; Tesovnik, T.; Stevanovic, J.; Zorc, M.; Cizelj, I.; Stanimirovic, Z.; Narat, M. Response of adult honey bees treated in larval stage with prochloraz to infection with Nosema ceranae. PeerJ 2019, 7, e6325. [Google Scholar] [CrossRef]
- Tesovnik, T.; Zorc, M.; Ristanić, M.; Glavinić, U.; Stevanović, J.; Narat, M.; Stanimirović, Z. Exposure of honey bee larvae to thiamethoxam and its interaction with Nosema ceranae infection in adult honey bees. Environ. Pollut. 2020, 256, 113443. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; Barrios, L.; Nanetti, A.; Meana, A.; Martín-Hernández, R.; Garrido-Bailón, E.; Higes, M. Nosema spp. parasitization decreases the effectiveness of acaricide strips (Apivar®) in treating varroosis of honey bee (Apis mellifera iberiensis) colonies. Environ. Microbiol. Rep. 2011, 4, 57–65. [Google Scholar] [CrossRef]
- Tihelka, E. Effects of synthetic and organic acaricides on honey bee health: A review. Slov. Vet.-Res. 2018, 55. [Google Scholar] [CrossRef]
- Katznelson, H.; Jamieson, C.A. Control of Nosema Disease of Honeybees with Fumagillin. Science 1952, 115, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L. Effect of Fumagillin upon Nosema apis (Zander). Nature 1953, 171, 212–213. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-F.; Solter, L.F.; Yau, P.; Imai, B.S. Nosema ceranae Escapes Fumagillin Control in Honey Bees. PLOS Pathog. 2013, 9, e1003185. [Google Scholar] [CrossRef] [PubMed]
- Heever, J.P.V.D.; Thompson, T.; Otto, S.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. Evaluation of Fumagilin-B® and other potential alternative chemotherapies against Nosema ceranae-infected honeybees (Apis mellifera) in cage trial assays. Apidologie 2015, 47, 617–630. [Google Scholar] [CrossRef]
- Heever, J.P.V.D.; Thompson, T.; Otto, S.; Curtis, J.M.; Ibrahim, A.; Pernal, S.F. The effect of dicyclohexylamine and fumagillin on Nosema ceranae-infected honey bee (Apis mellifera) mortality in cage trial assays. Apidologie 2015, 47, 663–670. [Google Scholar] [CrossRef]
- Stanimirovic, Z.; Stevanovic, J.; Bajic, V.; Radovic, I. Evaluation of genotoxic effects of fumagillin (dicyclohexylamine) by citogenetic tests in vivo. Mut. Res.-Genet. Toxicol. Environ. Mutagenes. 2007, 628, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, J.; Stanimirović, Z.; Radakovic, M.; Stojić, V. In vitro evaluation of the clastogenicity of fumagillin. Environ. Mol. Mutagenes. 2008, 49, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Nozal, M.J.; Alvaro, A.; Barrios, L.; Meana, A.; Hernández, R.M.; Bernal, J.L. The stability and effectiveness of fumagillin in controlling Nosema ceranae (Microsporidia) infection in honey bees (Apis mellifera) under laboratory and field conditions. Apidologie 2011, 42, 364–377. [Google Scholar] [CrossRef]
- McCallum, R.; Olmstead, S.; Shaw, J.; Glasgow, K. Evaluating efficacy of Fumagilin-B® against Nosemosis and tracking seasonal srends of Nosema spp. in Nova Scotia Honey bee colonies. J. Apic. Sci. 2020, 64, 277–286. [Google Scholar]
- Sarlo, E.G.; Medici, S.K.; Porrini, M.P.; Garrido, P.M.; Floris, I.; Eguaras, M.J. Comparison between different fumagillin dosage and evaluation method in the apiary control of Nosemosis type C. Redia 2011, 94, 39–44. [Google Scholar]
- Charistos, L.; Parashos, N.; Hatjina, F. Long term effects of a food supplement HiveAlive™ on honey bee colony strength and Nosema ceranae spore counts. J. Apic. Res. 2015, 54, 420–426. [Google Scholar] [CrossRef]
- Roussel, M.; Villay, A.; Delbac, F.; Michaud, P.; Laroche, C.; Roriz, D.; El Alaoui, H.; Diogon, M. Antimicrosporidian activity of sulphated polysaccharides from algae and their potential to control honeybee nosemosis. Carbohydr. Polym. 2015, 133, 213–220. [Google Scholar] [CrossRef]
- Stevanovic, J.; Stanimirovic, Z.; Simeunovic, P.; Lakic, N.; Radovic, I.; Sokovic, M.; Van Griensven, L.J. The effect of Agaricus brasiliensis extract supplementation on honey bee colonies. Acad. Bras. Ciências 2018, 90, 219–229. [Google Scholar] [CrossRef]
- Glavinic, U. The effects of various antimicrobials and supplements on the expression of immune-related genes, oxidative stress and survival of honey bee Apis mellifera infected with microsporidium Nosema ceranae. Ph.D. Thesis, Faculty of Veterinary Medicine, University of Belgrade, Belgrade, Serbia, 2019. [Google Scholar]
- Valizadeh., P.; Guzman-Novoa, E.; Goodwin, P.H. Effect of immune inducers on Nosema ceranae multiplication and their impact on Honey Bee (Apis mellifera L.) survivorship and behaviors. Insects 2020, 11, 572. [Google Scholar] [CrossRef]
- Borges, D.; Guzman-Novoa, E.; Goodwin, P.H. Control of the microsporidian parasite Nosema ceranae in honey bees (Apis mellifera) using nutraceutical and immuno-stimulatory compounds. PLoS ONE 2020, 15, e0227484. [Google Scholar] [CrossRef]
- Borges., D.; Guzman-Novoa., E.; Goodwin, P.H. Effects of prebiotics and probiotics on honey bees (Apis mellifera) infected with the microsporidian parasite Nosema ceranae. Microorganisms 2021, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Nanetti, A.; Ugolini, L.; Cilia, G.; Pagnotta, E.; Malaguti, L.; Cardaio, I.; Matteo, R.; Lazzeri, L. Seed meals from Brassica nigra and Eruca sativa control artificial Nosema ceranae infections in Apis mellifera. Microorganisms 2021, 9, 949. [Google Scholar] [CrossRef]
- Cilia, G.; Garrido, C.; Bonetto, M.; Tesoriero, D.; Nanetti, A. Effect of Api-Bioxal® and ApiHerb® treatments against Nosema ceranae infection in Apis mellifera investigated by two qPCR methods. Vet. Sci. 2020, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Cilia, G.; Fratini, F.; Tafi, E.; Turchi, B.; Mancini, S.; Sagona, S.; Nanetti, A.; Cerri, D.; Felicioli, A. Microbial profile of the ven-triculum of honey bee (Apis mellifera ligustica Spinola, 1806) fed with veterinary drugs, dietary supplements and non-protein amino acids. Vet. Sci. 2020, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, S.; Rousseau, A.; Lecoeur, A.; Cheaib, B.; Bouslama, S.; Mercier, P.-L.; Demey, V.; Castex, M.; Giovenazzo, P.; Derome, N. Deleterious Interaction Between Honeybees (Apis mellifera) and its Microsporidian Intracellular Parasite Nosema ceranae Was Mitigated by Administrating Either Endogenous or Allochthonous Gut Microbiota Strains. Front. Ecol. Evol. 2018, 6. [Google Scholar] [CrossRef]
- Michalczyk, M.; Sokół, R. Estimation of the influence of selected products on co-infection with N. apis/N. ceranae in Apis mellifera using real-time PCR. Invertebr. Reprod. Dev. 2018, 62, 92–97. [Google Scholar] [CrossRef]
- Jovanovic, N.M.; Glavinic, U.; Delic, B.; Vejnovic, B.; Aleksic, N.; Mladjan, V.; Stanimirovic, Z. Plant-based supplement containing B-complex vitamins can improve bee health and increase colony performance. Prev. Vet.-Med. 2021, 190, 105322. [Google Scholar] [CrossRef]
- Ishii, P.L.; Prado, C.K.; de Oliveira Mauro, M.; Carreira, C.M.; Mantovani, M.S.; Ribeiro, L.R.; Dichi, J.B.; Oliveira, R.J. Eval-uation of Agaricus blazei in vivo for antigenotoxic, anticarcinogenic, phagocytic and immunomodulatory activities. Regul. Toxicol. Pharm. 2011, 59, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Soković, M.; Ćirić, A.; Glamočlija, J.; Nikolić, M.; Van Griensven, L.J.L.D. Agaricus Blazei Hot Water Extract Shows Anti Quorum Sensing Activity in the Nosocomial Human Pathogen Pseudomonas Aeruginosa. Molecules 2014, 19, 4189–4199. [Google Scholar] [CrossRef]
- Stojković, D.; Reis, F.S.; Glamočlija, J.; Ćirić, A.; Barros, L.; Van Griensven, L.J.; Ferreira, C.F.R.I.; Soković, M. Cultivated strains of Agaricus bisporus and A. brasiliensis: Chemical characterization and evaluation of antioxidant and antimicrobial properties for the final healthy product–natural preservatives in yoghurt. Food Funct. 2014, 5, 1602–1612. [Google Scholar] [CrossRef]
- Stojkovic, D.; Smiljkovic, M.; Ciric, A.; Glamočlija, J.; Van Griensven, L.; Ferreira, I.; Sokovic, M. An insight into antidiabetic properties of six medicinal and edible mushrooms: Inhibition of α-amylase and α-glucosidase linked to type-2 diabetes. S. Afr. J. Bot. 2019, 120, 100–103. [Google Scholar] [CrossRef]
- da Silva de Souza, A.C.; Correa, V.G.; Goncalves, G.A.; Soares, A.A.; Bracht, A.; Peralta, R.M. Agaricus blazei bioactive com-pounds and their effects on human health: Benefits and controversies. Curr. Pharm. Des. 2017, 23, 2807–2834. [Google Scholar] [CrossRef] [PubMed]
- Gargano, M.L.; van Griensven, L.J.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Medicinal mushrooms: Valuable biological resources of high exploitation potential. Plant Biosyst. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Gallego, P.; Rojas, Á.; Falcón, G.; Carbonero, P.; García-Lozano, M.R.; Gil, A.; Grande, L.; Cremades, O.; Romero-Gómez, M.; Bautista, D.J.; et al. Water-soluble extracts from edible mushrooms (Agaricus bisporus) as inhibitors of hepatitis C viral replication. Food Funct. 2019, 10, 3758–3767. [Google Scholar] [CrossRef]
- Vunduk, J.; Kozarski, M.; Djekic, I.; Tomašević, I.; Klaus, A. Effect of modified atmosphere packaging on selected functional characteristics of Agaricus bisporus. Eur. Food Res. Technol. 2021, 247, 829–838. [Google Scholar] [CrossRef]
- Stamets, P.E.; Naeger, N.L.; Evans, J.D.; Han, J.O.; Hopkins, B.K.; Lopez, D.; Moershel, H.M.; Nally, R.; Sumerlin, D.; Taylor, A.W.; et al. Extracts of polypore mushroom mycelia reduce viruses in honey bees. Sci. Rep. 2018, 8, 13936. [Google Scholar] [CrossRef] [PubMed]
- Klaus, A.; Kozarski, M.; Niksic, M.; Jakovljevic, D.; Todorovic, N.; Van Griensven, L.J.L.D. Antioxidative activities and chemical char-acterization of polysaccharides extracted from the basidiomycete Schizophyllum commune. LWT-Food Sci. Technol. 2011, 44, 1–7. [Google Scholar] [CrossRef]
- Cantwell, G.E. Standard methods for counting Nosema spores. Am. Bee J. 1970, 110, 222–223. [Google Scholar]
- Williamson, S.M.; Wright, G.A. Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honey-bees. J. Exp. Biol. 2013, 216, 1799–1807. [Google Scholar]
- OIE–Office International Des Epizooties. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Chapter 2.2.4. Nosemosis of Honey Bees. 2018. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.02.04_NOSEMOSIS_FINAL.pdf (accessed on 30 August 2021).
- Simone, M.; Evans, J.D.; Spivak, M. Resin collection and social immunity in honey bees. Evolution 2009, 63, 3016–3022. [Google Scholar] [CrossRef]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef]
- Jehlík, T.; Kodrík, D.; Krištůfek, V.; Koubová, J.; Sábová, M.; Danihlík, J.; Tomčala, A.; Frydrychová, R.Č. Effects of Chlorella sp. on biological characteristics of the honey bee Apis mellifera. Apidologie 2019, 50, 564–577. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Simone-Finstrom, M. Nutritional and prebiotic efficacy of the microalga Arthrospira platensis (spirulina) in honey bees. Apidologie 2020, 51, 898–910. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Ihle, K.E.; Williams, S.T. Nutrigenetic comparison of two Varroa-resistant honey bee stocks fed pollen and spirulina microalgae. Apidologie 2021, 52, 873–886. [Google Scholar] [CrossRef]
- Shumkova, R.; Balkanska, R.; Hristov, P. The Herbal Supplements NOZEMAT HERB® and NOZEMAT HERB PLUS®: An Alternative Therapy for N. ceranae Infection and Its Effects on Honey Bee Strength and Production Traits. Pathogens 2021, 10, 234. [Google Scholar] [CrossRef]
- Williams, G.R.; Shutler, D.; Burgher-MacLellan, K.L.; Rogers, R.E. Infra-population and-community dynamics of the parasites Nosema apis and Nosema ceranae, and consequences for honey bee (Apis mellifera) hosts. PLoS ONE 2014, 9, e99465. [Google Scholar] [CrossRef]
- Huang, W.F.; Solter, L.; Aronstein, K.; Huang, Z. Infectivity and virulence of Nosema ceranae and Nosema apis in commercially available North American honey bees. J. Invertebr. Pathol. 2015, 124, 107–113. [Google Scholar] [CrossRef]
- Lull, C.; Wichers, H.J.; Savelkoul, H.F. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediat. Inflamm. 2005, 2, 63–80. [Google Scholar] [CrossRef]
- Vunduk, J.; Djekic, I.; Petrović, P.; Tomašević, I.; Kozarski, M.; Despotović, S.; Nikšić, M.; Klaus, A. Challenging the difference between white and brown Agaricus bisporus mushrooms: Science behind consumers choice. Br. Food J. 2018, 120, 1381–1394. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory effects of edible and medicinal mushrooms and their bioactive immunoregulatory products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef]
- Arismendi, N.; Vargas, M.; López, M.D.; Barría, Y.; Zapata, N. Promising antimicrobial activity against the honey bee parasite Nosema ceranae by methanolic extracts from Chilean native plants and propolis. J. Apic. Res. 2018, 57, 522–535. [Google Scholar] [CrossRef]
- Fries, I.; Chauzat, M.P.; Chen, Y.P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, P.D.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard methods for Nosema research. J. Apicult. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Higes, M.; Garcia-Palencia, P.; Martín-Hernández, R.; Meana, A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 2007, 94, 211–217. [Google Scholar] [CrossRef]
- Hayman, J.R.; Southern, T.R.; Nash, T.E. Role of sulfated glycans in adherence of the microsporidian Encephalitozoon intestinalis to host cells in vitro. Infect. Immun. 2005, 73, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Vunduk, J.; Wan-Mohtar, W.A.A.Q.I.; Mohamad, S.A.; Halim, N.H.A.; Dzomir, A.Z.M.; Žižak, Ž.; Klaus, A. Polysaccharides of Pleurotus flabellatus strain Mynuk produced by submerged fermentation as a promising novel tool against adhesion and biofilm formation of foodborne pathogens. LWT-Food Sci. Technol. 2019, 112, 108221. [Google Scholar] [CrossRef]
- Antunez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune-suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef]
- Chaimanee, V.; Chantawannakul, P.; Chen, Y.; Evans, J.D.; Pettis, J.S. Differential expression of immune genes of adult honey bee (Apis mellifera) after inoculated by Nosema ceranae. J. Insect. Physiol. 2012, 58, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Badaoui, B.; Fougeroux, A.; Petit, F.; Anselmo, A.; Gorni, C.; Cucurachi, M.; Cersini, A.; Granato, A.; Cardeti, G.; Formato, G.; et al. RNA-sequence analysis of gene expression from honeybees (Apis mellifera) infected with Nosema ceranae. PLoS ONE 2017, 12, e0173438. [Google Scholar] [CrossRef] [PubMed]
- Djekic, I.; Vunduk, J.; Tomašević, I.; Kozarski, M.; Petrovic, P.; Niksic, M.; Pudja, P.; Klaus, A. Total quality index of Agaricus bisporus mushrooms packed in modified atmosphere. J. Sci. Food Agric. 2017, 97, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; Van Griensven, L. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef]

| GROUP 1 | Day of Starting the Treatment 2 | N. ceranae Infection Day 2 | Sampling Day 2 | |
|---|---|---|---|---|
| NI | – | – | 7 | 15 |
| I | – | 3 | 7 | 15 |
| Abs | 1 | – | 7 | 15 |
| I-Abs1 | 1 | 3 | 7 | 15 |
| I-Abs3 | 3 | 3 | 7 | 15 |
| I-Abs6 | 6 | 3 | 7 | 15 |
| Primer | Sequence 5′–3′ | Reference |
|---|---|---|
| Beta actin-F | TTGTATGCCAACACTGTCCTTT | [62] |
| Beta actin-R | TGGCGCGATGATCTTAATTT | |
| Abaecin-F | CAGCATTCGCATACGTACCA | [63] |
| Abaecin-R | GACCAGGAAACGTTGGAAAC | |
| Hymenopt-F | CTCTTCTGTGCCGTTGCATA | [63] |
| Hymenopt-R | GCGTCTCCTGTCATTCCATT | |
| Defensin-F | TGCGCTGCTAACTGTCTCAG | [63] |
| Defensin-R | AATGGCACTTAACCGAAACG | |
| ApidNT-F | TTTTGCCTTAGCAATTCTTGTTG | [62] |
| ApidNT-R | GTAGGTCGAGTAGGCGGATCT | |
| VgMC-F | AGTTCCGACCGACGACGA | [62] |
| VgMC-R | TTCCCTCCCACGGAGTCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glavinic, U.; Rajkovic, M.; Vunduk, J.; Vejnovic, B.; Stevanovic, J.; Milenkovic, I.; Stanimirovic, Z. Effects of Agaricus bisporus Mushroom Extract on Honey Bees Infected with Nosema ceranae. Insects 2021, 12, 915. https://doi.org/10.3390/insects12100915
Glavinic U, Rajkovic M, Vunduk J, Vejnovic B, Stevanovic J, Milenkovic I, Stanimirovic Z. Effects of Agaricus bisporus Mushroom Extract on Honey Bees Infected with Nosema ceranae. Insects. 2021; 12(10):915. https://doi.org/10.3390/insects12100915
Chicago/Turabian StyleGlavinic, Uros, Milan Rajkovic, Jovana Vunduk, Branislav Vejnovic, Jevrosima Stevanovic, Ivanka Milenkovic, and Zoran Stanimirovic. 2021. "Effects of Agaricus bisporus Mushroom Extract on Honey Bees Infected with Nosema ceranae" Insects 12, no. 10: 915. https://doi.org/10.3390/insects12100915
APA StyleGlavinic, U., Rajkovic, M., Vunduk, J., Vejnovic, B., Stevanovic, J., Milenkovic, I., & Stanimirovic, Z. (2021). Effects of Agaricus bisporus Mushroom Extract on Honey Bees Infected with Nosema ceranae. Insects, 12(10), 915. https://doi.org/10.3390/insects12100915






