Identification of Pheromone Components of Plagionotus detritus (Coleoptera: Cerambycidae), and Attraction of Conspecifics, Competitors, and Natural Enemies to the Pheromone Blend
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Insects
2.2. Field Observations of Behaviour
2.3. Microscopy of External Morphology
2.4. Collection and Analysis of Insect-Produced Volatiles
2.5. Electroantennography (EAG) with Clerid Beetle Antennae
2.6. Sources of Chemicals
2.7. Field Test 1
2.8. Field Test 2
2.9. Statistics
3. Results
3.1. Diurnal Activity of Beetles
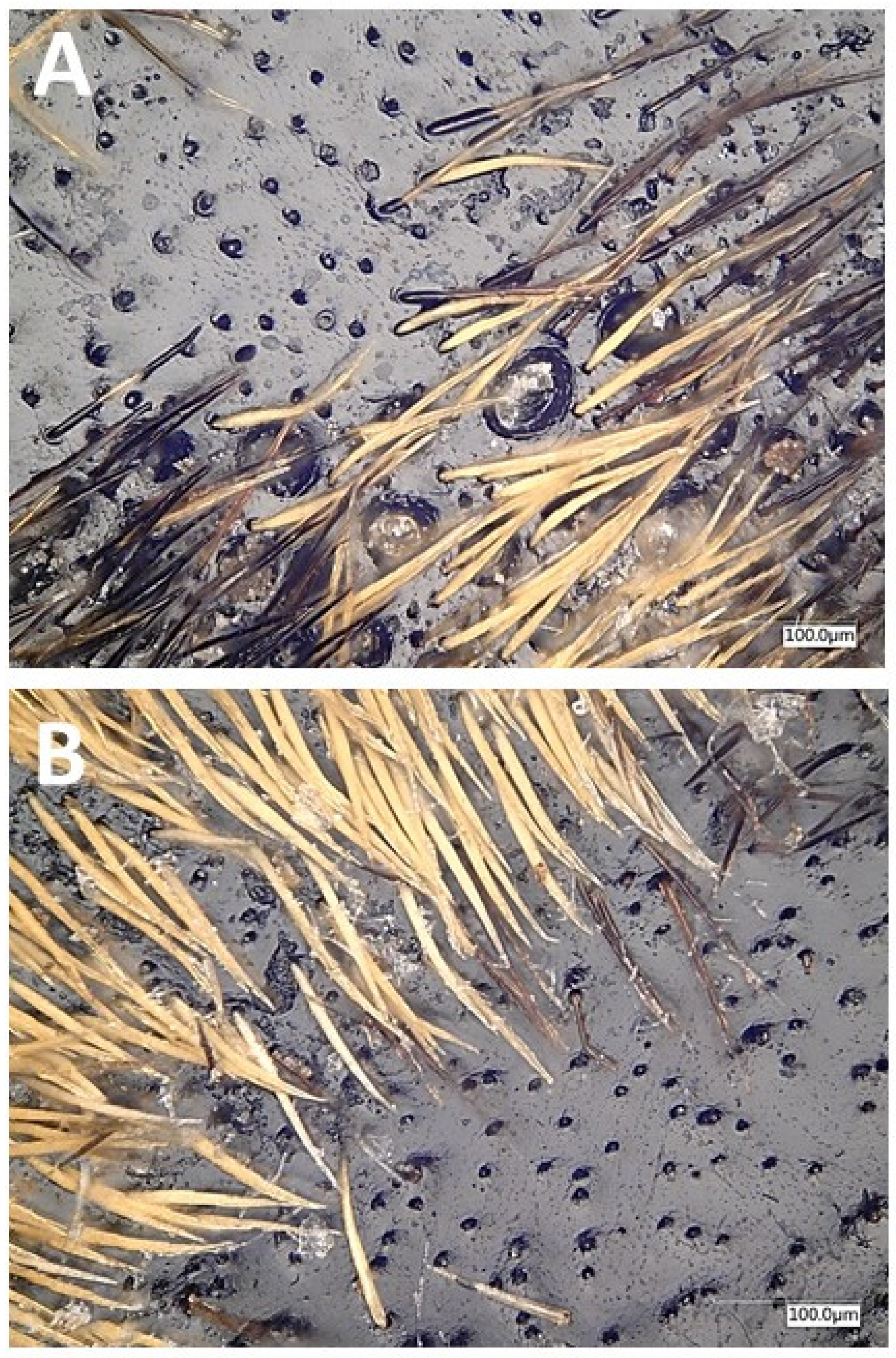
3.2. Sex-Specific Pheromone Gland Pores
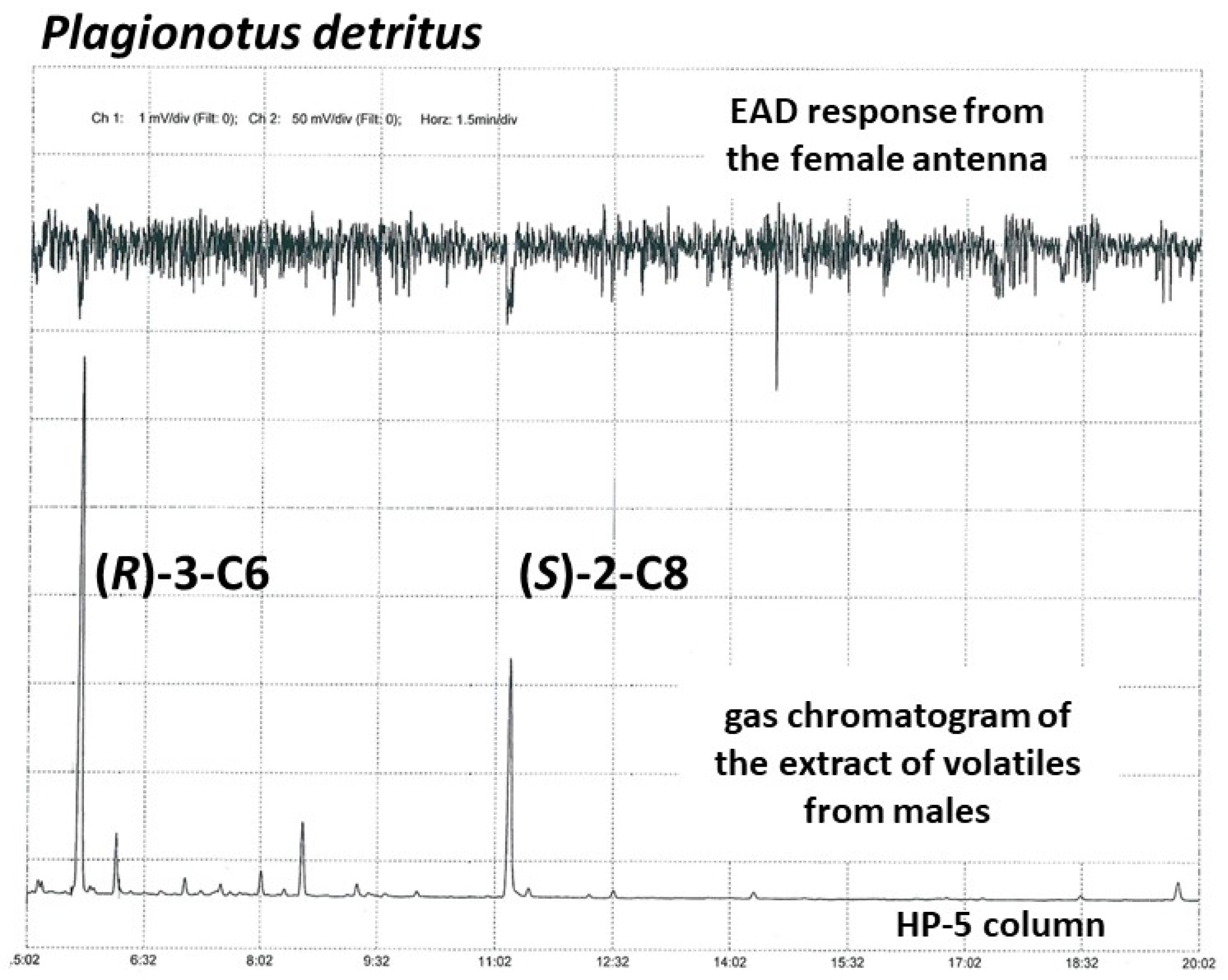
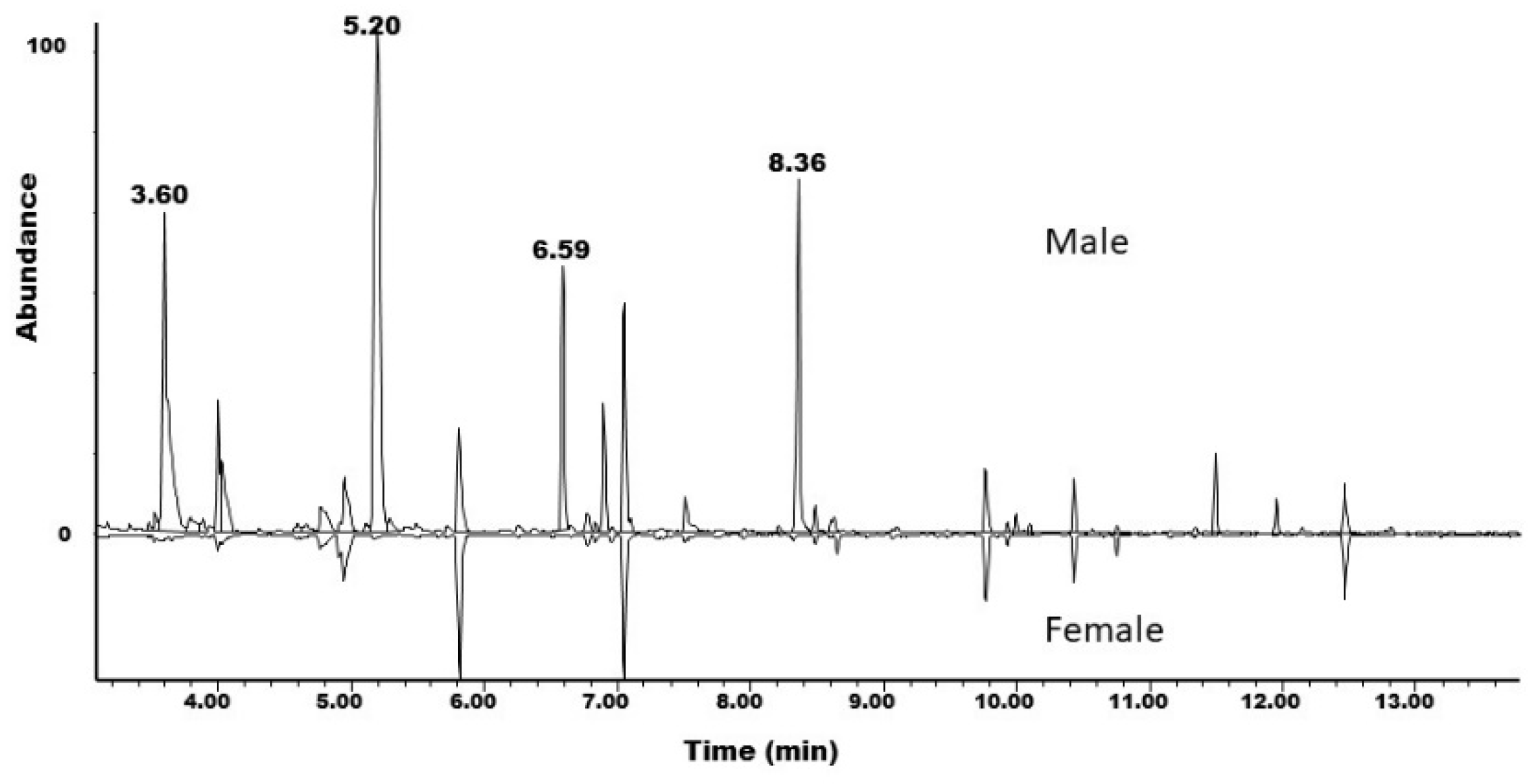
3.3. Collection and Analysis of Insect-Produced Volatiles
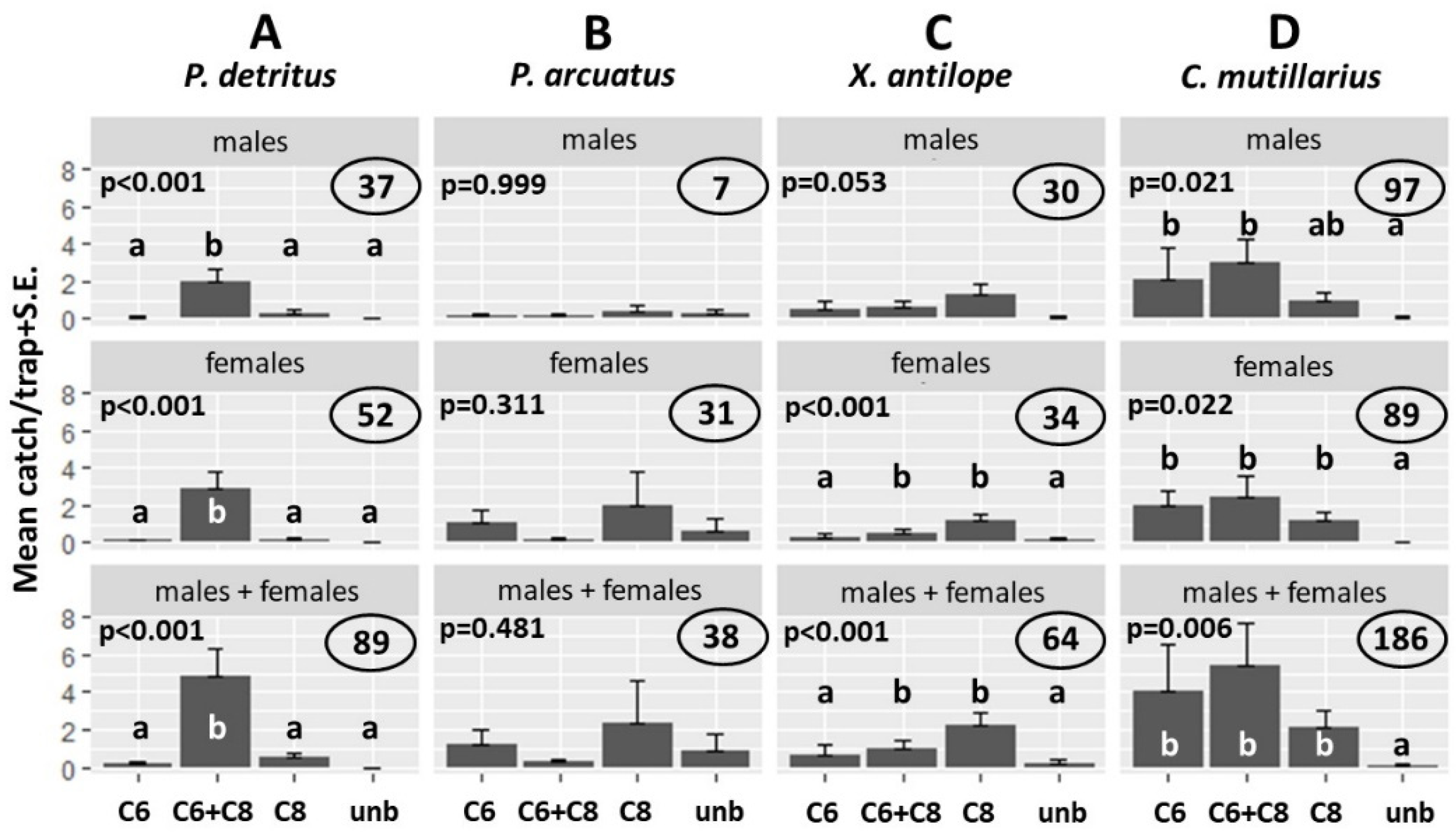
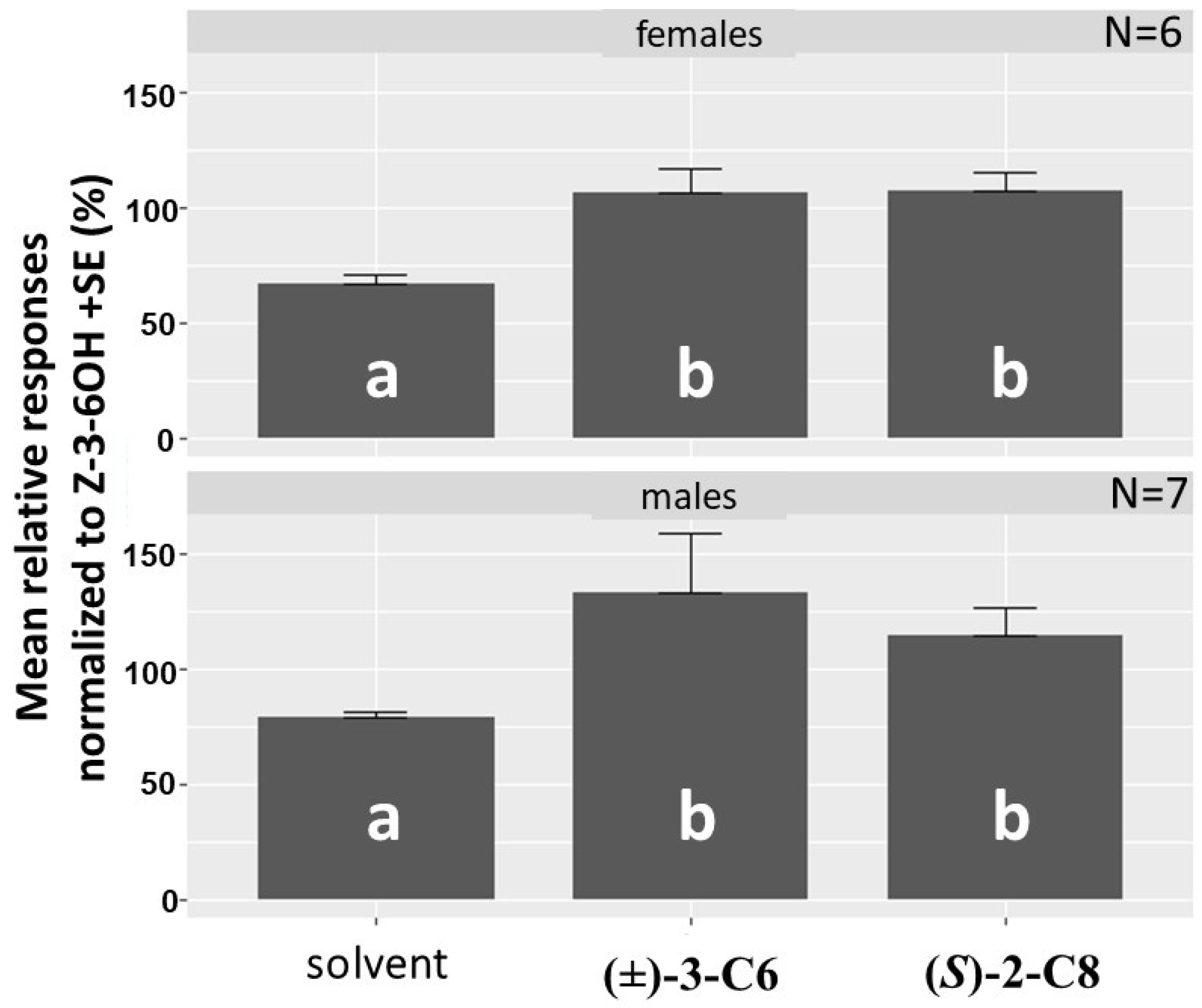
3.4. Field Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Seibold, S.; Brandl, R.; Buse, J.; Hothorn, T.; Schmidl, J.; Thorn, S.; Muller, J. Association of extinction risk of saproxylic beetles with ecological degradation of forests in Europe. Conserv. Biol. 2015, 29, 382–390. [Google Scholar] [CrossRef]
- Larsson, M.C. Pheromones and Other Semiochemicals for Monitoring Rare and Endangered Species. J. Chem. Ecol. 2016, 42, 853–868. [Google Scholar] [CrossRef] [Green Version]
- Kaszab, Z. Cincérek—Cerambycidae; Akadémiai Kiadó: Budapest, Hungary, 1971; Volume 106. [Google Scholar]
- Danilevsky, M.L.E. Catalogue of Palaearctic Coleoptera, Volume 6/1: Chrysomeloidea I (Vesperidae, Disteniidae, Cerambycidae). Updated and Revised Second Edition; Brill: Leiden, The Netherlands, 2020; Volume 6, p. 924. [Google Scholar]
- Molander, M.A.; Helgesson, J.; Winde, I.B.; Millar, J.G.; Larsson, M.C. The male-produced aggregation-sex pheromone of the cerambycid beetle Plagionotus detritus ssp. detritus. J. Chem. Ecol. 2019, 45, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Konwerski, S.; Gutowski, J.M.; Bloszyk, J. Patterns of distribution of phoretic deutonymphs of Uropodina on longhorn beetles in Bialowie(z) over dota primeval forest, Central Europe. Diversity 2020, 12, 239. [Google Scholar] [CrossRef]
- Flaherty, L.; Gutowski, J.M.G.; Hughes, C.; Mayo, P.; Mokrzycki, T.; Pohl, G.; Silk, P.; Van Rooyen, K.; Sweeney, J. Pheromone-enhanced lure blends and multiple trap heights improve detection of bark and wood-boring beetles potentially moved in solid wood packaging. J. Pest Sci. 2019, 92, 309–325. [Google Scholar] [CrossRef]
- Keszthelyi, S. Diversity and seasonal patterns of longhorn beetles (Coleoptera: Cerambycidae) in the Zselic region, Hungary. North-West. J. Zool. 2015, 11, 62–69. [Google Scholar]
- Rassati, D.; Marini, L.; Marchioro, M.; Rapuzzi, P.; Magnani, G.; Poloni, R.; Di Giovanni, F.; Mayo, P.; Sweeney, J. Developing trapping protocols for wood-boring beetles associated with broadleaf trees. J. Pest Sci. 2018, 92, 267–279. [Google Scholar] [CrossRef]
- Brodie, B.S.; Popescu, V.D.; Iosif, R.; Ciocanea, C.; Manolache, S.; Vanau, G.; Gavrilidis, A.A.; Serafim, R.; Rozylowicz, L. Non-lethal monitoring of longicorn beetle communities using generic pheromone lures and occupancy models. Ecol. Indic. 2019, 101, 330–340. [Google Scholar] [CrossRef]
- Dovhaniuk, I.Y.; Zamoroka, A.M. The longhorn beetles (Coleoptera: Cerambycidae) of National Park «Kremenetski Hory». Proc. State Nat. Hist. Mus. 2020, 36, 129–140. [Google Scholar] [CrossRef]
- Zamoroka, A.M. The longhorn beetles (Coleoptera: Cerambycidae) of the Eastern Carpathian Mountains in Ukraine. Munis Entomol. Zool. 2018, 13, 655–691. [Google Scholar]
- Ruchin, A.B.; Egorov, L.V. Fauna of longicorn beetles (Coleoptera: Cerambycidae) of Mordovia. Russ. Entomol. J. 2018, 27, 161–177. [Google Scholar] [CrossRef] [Green Version]
- Keszthelyi, S.; Pónya, Z.; Pál-Fám, F. Climate-induced seasonal activity and flight period of cerambycid beetles in the Zselic forests, Hungary. J. For. Sci. 2017, 63, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Ray, A.M.; Lacey, E.S.; Hanks, L.M. Predicted taxonomic patterns in pheromone production by longhorned beetles. Naturwissenschaften 2006, 93, 543–550. [Google Scholar] [CrossRef]
- Corey, E.J.; Schmidt, G. Useful procedures for the oxidation of alcohols involving pyridinium dichromate in aprotic media. Tetrahedron Lett. 1979, 20, 399–402. [Google Scholar] [CrossRef]
- Lacey, E.S.; Moreira, J.A.; Millar, J.G.; Ray, A.M.; Hanks, L.M. Male-produced aggregation pheromone of the cerambycid beetle Neoclytus mucronatus mucronatus. Entomol. Exp. Et Appl. 2007, 122, 171–179. [Google Scholar] [CrossRef]
- Toshova, T.B.; Atanasova, D.I.; Tóth, M.; Subchev, M.A. Seasonal activity of Plagionotus (Echinocerus) floralis (Pallas) (Coleoptera: Cerambycidae, Cerambycinae) adults in Bulgaria established by attractant baited fluorescent yellow funnel traps. Acta Phytopath. Entomol. Hung. 2010, 45, 391–399. [Google Scholar] [CrossRef]
- Imrei, Z.; Kováts, Z.; Toshova, T.B.; Subchev, M.; Harmincz, K.; Szarukán, I.; Domingue, M.J.; Tóth, M. Development of a trap combining visual and chemical cues for the alfalfa longhorn beetle, Plagionotus floralis. Bull Insectol 2014, 67, 161–166. [Google Scholar]
- Imrei, Z.; Millar, J.G.; Janik, G.; Tóth, M. Field screening of known pheromone components of longhorned beetles in the subfamily Cerambycinae (Coleoptera: Cerambycidae) in Hungary. Z. Für Nat. C 2012, 68, 236–242. [Google Scholar] [CrossRef]
- Imrei, Z.; Molander, M.A.; Winde, I.B.; Lohonyai, Z.; Bálintné Csonka, É.; Fail, J.; Hanks, L.M.; Zou, Y.F.; Millar, J.G.; Tóth, M.; et al. Identification of the aggregation-sex pheromone of Plagionotus arcuatus ssp. arcuatus (Coleoptera: Cerambycidae) from two geographically separated European populations. Sci. Nat. 2019, 106, 9. [Google Scholar] [CrossRef] [Green Version]
- Graham, E.E.; Poland, T.M. Efficacy of fluon conditioning for capturing cerambycid beetles in different trap designs and persistence on panel traps over time. J. Econ. Entomol. 2012, 105, 395–401. [Google Scholar] [CrossRef]
- Graham, E.; Mitchell, R.; Reagel, P.; Barbour, J.; Millar, J.; Hanks, L. Treating panel traps with a fluoropolymer enhances their efficiency in capturing cerambycid beetles. J. Econ. Entomol. 2010, 103, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Francois, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation. R Package Version 0.7.4. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 20 June 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Englewood Cliffs, NJ, USA, 1999. [Google Scholar]
- Hoshino, K.; Nakaba, S.; Inoue, H.; Iwabuchi, K. Structure and development of male pheromone gland of longicorn beetles and its phylogenetic relationships within the tribe Clytini. J. Exp. Zool. (Mol. Dev. Evol. ) 2015, 324B, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kaszab, Z. Különböző Csápú Bogarak Diversicornia I. Lágytestű bogarak Malacodermata; Akadémiai Kiadó: Budapest, Hungary, 1955; Volume 8. [Google Scholar]
- Millar, J.G.; Hanks, L.M. Chemical ecology of cerambycid beetles. In Cerambycidae of the World: Biology and Management; Wang, Q., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2016. [Google Scholar]
- Rassati, D.; Marchioro, M.; Flaherty, L.; Poloni, R.; Edwards, S.; Faccoli, M.; Sweeney, J. Response of native and exotic longhorn beetles to common pheromone components provides partial support for the pheromone-free space hypothesis. Insect Sci. 2021, 28, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Nakagawa, Y.; Takahashi, J.; Iwabuchi, K.; Lshii, A. Isolation and identification of the male sex pheromone of the grape borer Xylotrechus pyrrhoderus Bates (Coleoptera: Cerambycidae). Chem. Lett. 1984, 13, 263–264. [Google Scholar] [CrossRef] [Green Version]
- Ehnström, B.; Holmer, M. Skalbaggar: Långhorningar. Coleoptera: Cerambycidae; Swedish Species Information Centre: Uppsala, Sweden, 2007. [Google Scholar]
- Jeniš, I. Long-Horned Beetles Vesperidae & Cerambycidae of Europe 1; Ateliér Regulus: Prague, Czech Republic, 2001. [Google Scholar]
- Klausnitzer, B.; Klausnitzer, U.; Wachmann, E.; Hromádko, Z. Die Bockkäfer Mitteleuropas; Die Neue Brehm-Bücherei: Magdeburg, Germany, 2016. [Google Scholar]
- Csóka, G.; Kovács, T. Xilofág Rovarok—Xylophagous Insects; Agroinform: Budapest, Hungary, 1999. [Google Scholar]
- Bílý, S.; Mehl, O. Longhorn Beetles (Coleoptera, Cerambycidae) of Fennoscandia and Denmark; EJ Brill/Scandinavian Science Press Ltd.: Leiden, The Netherlands, 1989; Volume 22. [Google Scholar]
- Ehnström, B.; Axelsson, R. Insektsgnag i Bark Och Ved; Swedish Species Information Centre: Uppsala, Sweden, 2002. [Google Scholar]
- Nieto, A.; Dodelin, B.; Campanaro, A.; Mannerkoski, I.; Tezcan, S.; Horák, J.; Istrate, P.; Alexander, K.; Méndez, M. Xylotrechus antilope. The IUCN Red List of Threatened Species 2010. Available online: https://www.iucnredlist.org/species/157809/5150727 (accessed on 23 April 2021).
- Molander, M.A.; Eriksson, B.; Winde, I.B.; Zou, Y.F.; Millar, J.G.; Larsson, M.C. The aggregation-sex pheromones of the cerambycid beetles Anaglyptus mysticus and Xylotrechus antilope ssp. antilope: New model species for insect conservation through pheromone-based monitoring. Chemoecology 2019, 29, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Schröder, F.C. Identifizierung und Synthese Neuer Alkaloide, Hydroxyketone und Bicyclischer Acetale aus Insekten. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 1996. [Google Scholar]
- Iwabuchi, K. Sex pheromones in Cerambycidae. In Environmental Entomology–Behavior, Physiology and Chemical Ecology; Hidaka, T., Matsumoto, Y., Honda, K., Eds.; University of Tokyo Press: Tokyo, Japan, 1999; pp. 436–451. [Google Scholar]
- Varli, S.V.; Surgut, H.; Tuven, A.; Jansson, N. Faunistic studies on Carabidae, Staphylinidae, Elateridae, Cleridae, Cerambycidae and Chrysomelidae (Coleoptera) families of Cataldag (Balikesir-Susurluk) Province, Western Turkey. KSU Tarim Doga Derg. 2020, 23, 740–747. [Google Scholar] [CrossRef]
- Kovács, T.; Bátori, G.; Huber, A.; Urbán, L. Ritka és természetvédelmi szempontból jelentős bogarak (Coleoptera) a Bükk, az Aggteleki-karszt és a Putnoki-dombság környékéről. Folia Hist. -Nat. Musei Matra. 2017, 41, 167–180. [Google Scholar]
- Schmidt, U. Beetles of the World. Available online: www.kaefer-der-welt.de/clerus_mutillarius.htm (accessed on 17 October 2020).
- Cavaletto, G.; Faccoli, M.; Marini, L.; Spaethe, J.; Magnani, G.; Rassati, D. Effect of trap color on captures of bark-and wood-boring beetles (Coleoptera; Buprestidae and Scolytinae) and associated predators. Insects 2020, 11, 749. [Google Scholar] [CrossRef]
- Cebeci, H.H.; Baydemir, M. Predators of bark beetles (Coleoptera) in the Balikesir region of Turkey. Rev. Colomb. Entomol. 2018, 44, 283–287. [Google Scholar] [CrossRef]
- Merkl, O.; Vig, K. Bogarak a Pannon Régióban; B.K.L. Kiadó; Magyar Természettudományi Múzeum: Szombathely, Hungary, 2009. [Google Scholar]
- Miller, D.R.; Crowe, C.M.; Mayo, P.D.; Silk, P.J.; Sweeney, J.D. Responses of Cerambycidae and other insects to traps baited with ethanol, 2,3-hexanediol, and 3,2-hydroxyketone lures in North-Central Georgia. J. Econ. Entomol. 2015, 108, 2354–2365. [Google Scholar] [CrossRef] [PubMed]
- Bakke, A.; Kvamme, T. Kairomone response in Thanasimus predators to pheromone components of Ips typographus. J. Chem. Ecol. 1981, 7, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Cavaletto, G.; Faccoli, M.; Marini, L.; Spaethe, J.; Giannone, F.; Moino, S.; Rassati, D. Exploiting trap color to improve surveys of longhorn beetles. J. Pest Sci. 2021, 94, 871–883. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imrei, Z.; Domingue, M.J.; Lohonyai, Z.; Moreira, J.A.; Bálintné Csonka, É.; Fail, J.; Csóka, G.; Hanks, L.M.; Tóth, M.; Millar, J.G. Identification of Pheromone Components of Plagionotus detritus (Coleoptera: Cerambycidae), and Attraction of Conspecifics, Competitors, and Natural Enemies to the Pheromone Blend. Insects 2021, 12, 899. https://doi.org/10.3390/insects12100899
Imrei Z, Domingue MJ, Lohonyai Z, Moreira JA, Bálintné Csonka É, Fail J, Csóka G, Hanks LM, Tóth M, Millar JG. Identification of Pheromone Components of Plagionotus detritus (Coleoptera: Cerambycidae), and Attraction of Conspecifics, Competitors, and Natural Enemies to the Pheromone Blend. Insects. 2021; 12(10):899. https://doi.org/10.3390/insects12100899
Chicago/Turabian StyleImrei, Zoltán, Michael J. Domingue, Zsófia Lohonyai, Jardel A. Moreira, Éva Bálintné Csonka, József Fail, György Csóka, Lawrence M. Hanks, Miklós Tóth, and Jocelyn G. Millar. 2021. "Identification of Pheromone Components of Plagionotus detritus (Coleoptera: Cerambycidae), and Attraction of Conspecifics, Competitors, and Natural Enemies to the Pheromone Blend" Insects 12, no. 10: 899. https://doi.org/10.3390/insects12100899
APA StyleImrei, Z., Domingue, M. J., Lohonyai, Z., Moreira, J. A., Bálintné Csonka, É., Fail, J., Csóka, G., Hanks, L. M., Tóth, M., & Millar, J. G. (2021). Identification of Pheromone Components of Plagionotus detritus (Coleoptera: Cerambycidae), and Attraction of Conspecifics, Competitors, and Natural Enemies to the Pheromone Blend. Insects, 12(10), 899. https://doi.org/10.3390/insects12100899






