Tackling the Taxonomic Challenges in the Family Scoliidae (Insecta, Hymenoptera) Using an Integrative Approach: A Case Study from Southern China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Collection and Identification
2.2. DNA Extraction, Amplification, and Sequencing
2.3. Sequence Analysis and Molecular Species Delimitation
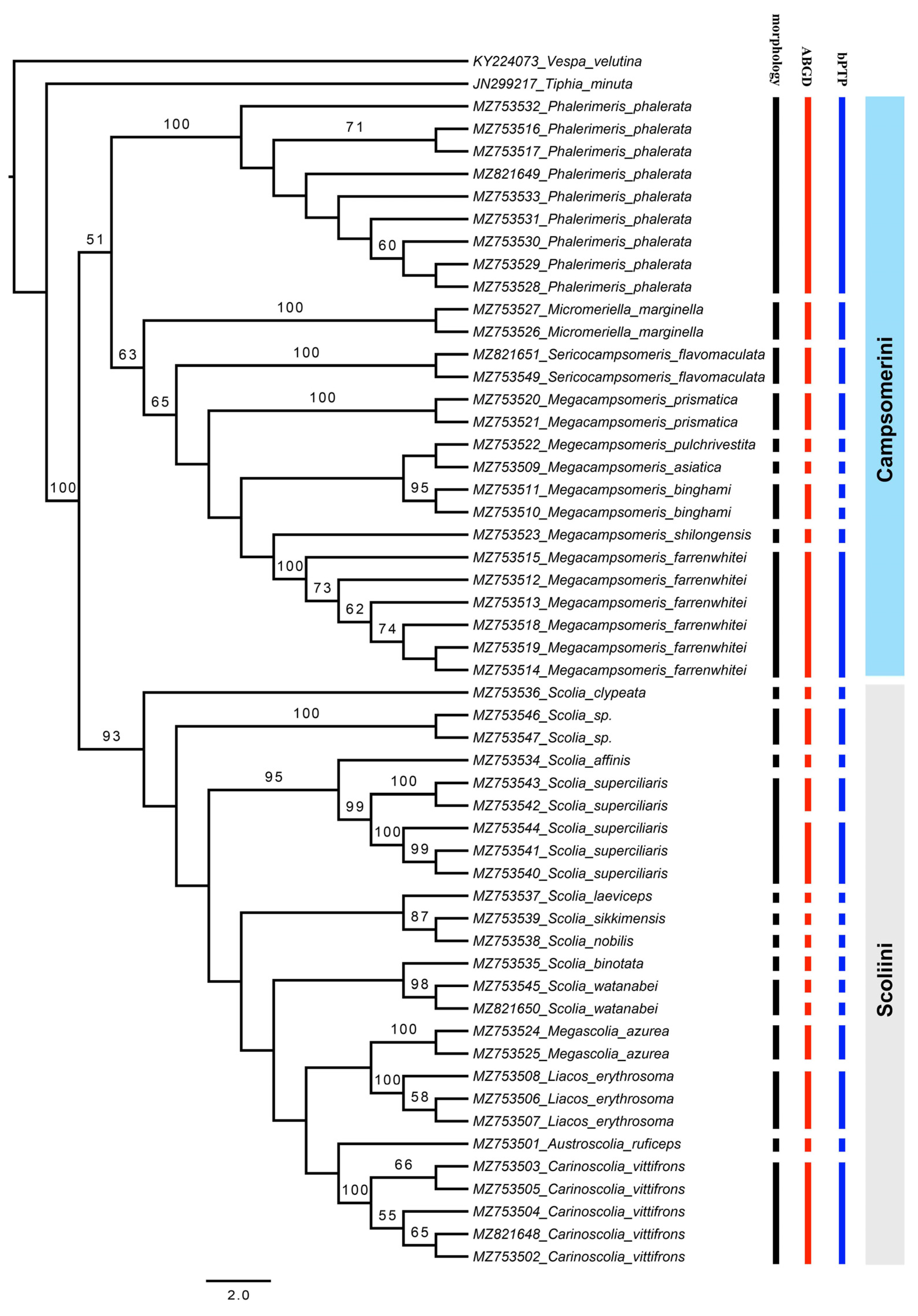
3. Results and Discussion
3.1. Tribe Scoliini
3.2. Tribe Campsomerini
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Illingworth, J.F. Monthly notes on grubs and other cane pests. Qld. Bur. BSES Div. Entomol. Bull. 1919, 7, 1–29. [Google Scholar]
- Illingworth, J.F. Natural enemies of sugar-cane beetles in Queensland. Bur. BSES Div. Entomol. Bull. 1921, 13, 1–47. [Google Scholar]
- Askew, R.R. Parasitic Insects; Elsevier Publishing: New York, NY, USA, 1971; pp. 1–316. [Google Scholar]
- Naumann, I.D. Hymenoptera (Wasps, bees, ants, sawflies). In The Insects of Australia: A Textbook for Students and Research Workers, 2nd ed.; Naumann, I.D., Carne, P.B., Lawrence, J.F., Nielsen, E.S., Spradberry, J.P., Taylor, R.W., Whitten, M.J., Littlejohn, M.J., Eds.; Melbourne University Press: Carlton, VIC, Australia, 1991; pp. 916–1000. [Google Scholar]
- Osten, T. Checkliste der Dolchwespen der Welt (Insecta: Hymenoptera, Scoliidae). Naturforsch. Ges. 2005, 220, 1–62. [Google Scholar]
- Elliott, M.G. Annotated catalogue of the Australian scoliidae (Hymenoptera). Tech. Rep. Aust. Mus. Online 2011, 22, 1–17. [Google Scholar] [CrossRef]
- Abbate, A.; Campbell, J.; Bremer, J.; Kern, W.H. The introduction and establishment of Campsomeris dorsata (Hymenoptera: Scoliidae) in Florida. Fla. Entomol. 2018, 101, 543–545. [Google Scholar] [CrossRef]
- Clausen, C.P. Entomophagous Insects; McGraw-Hill: New York, NY, USA, 1940; pp. 1–688. [Google Scholar]
- O’Neill, K. Solitary Wasps: Behavior and Natural History; Cornell University Press: Ithaca, NY, USA; New York, NY, USA, 2001; p. xiv + 406. [Google Scholar]
- Elliott, N.B. Flower-feeding activities of Campsomeris trifasciata nassauensis Bradley (Hymenoptera: Scoliidae). In Proceedings of the 3rd Symposium on the Botany of the Bahamas, San Salvador, El Salvador, 6–9 June 1989; Volume 3, pp. 1–6. [Google Scholar]
- Minagi, K.; Maeta, Y.; Kitamura, K. Studies on the conservation of pollination ecosystem in sand dune 1. Pollinators and their seasonal fluctuation at the Taisya sand dune in San-in district. Bull. Hoshizaki Green Found. 2000, 4, 139–160, (In Japanese with English summary). [Google Scholar]
- Inoue, M.; Endo, T. Species composition of flowervisiting insect community in the coastal sand dune of Hakoishi, Kyoto Prefecture. Hum. Sci. 2006, 9, 39–46, (In Japanese with English summary). [Google Scholar]
- Inoue, M.; Endo, T. Spatiotemporal distribution and resource use of scoliid wasps (Hymenoptera) in coastal sand dunes. Entomol. Sci. 2006, 9, 359–371. [Google Scholar] [CrossRef]
- Campbell, J.W.; Irvin, A.; Stanley-Stahr, C.; Ellis, J.D. Insect visitors to flowering buckwheat, Fagopyrum esculentum (Polygonales: Polygonaceae), in north–central Florida. Fla. Entomol. 2016, 99, 264–268. [Google Scholar] [CrossRef] [Green Version]
- Ciotek, L.; Giorgis, P.; Benitez-Vieyra, S.; Cocucci, A.A. First confirmed case of pseudocopulation in terrestrial orchids of South America: Pollination of Geoblasta pennicillata (Orchidaceae) by Campsomeris bistrimacula (Hymenoptera, Scoliidae). Flora 2006, 201, 365–369. [Google Scholar] [CrossRef]
- Betrem, J.G. Monographie der Indo-Australischen Scoliiden mit zoogeographischen Betrachtungen. Treubia 1928, 9, 1–388. [Google Scholar]
- Betrem, J.G. Étude systematique des Scoliidae de Chine et leurs relations avec les autres groups de Scoliidae. In Notes d’Entomologie Chinoise; Chen, T.T.H., Ed.; Université l’Aurore: Shanghai, China, 1941; pp. 47–188. [Google Scholar]
- Liu, Z.; van Achterberg, C.; He, J.H.; Chen, X.X. A checklist of Scoliidae (Insecta: Hymenoptera) from China. Zootaxa 2021, 4966, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; Dewaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebert, P.D.N.; Ratnasingham, S.; Dewaard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. B Biol. Sci. 2003, 270, S96–S99. [Google Scholar] [CrossRef] [Green Version]
- Branstetter, M.G.; Danforth, B.N.; Pitts, J.P.; Faircloth, B.C.; Ward, P.S.; Buffington, M.L.; Gates, M.W.; Kula, R.R.; Brady, S.G. Phylogenomic insights into the evolution of stinging wasps and the origins of ants and bees. Curr. Biol. 2017, 27, 1019–1025. [Google Scholar] [CrossRef] [Green Version]
- Dowton, M.; Austin, A.D. Molecular phylogeny of the insect order Hymenoptera: Apocritan relationships. Proc. Natl. Acad. Sci. USA 1994, 91, 9911–9915. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; van Achterberg, C.; He, J.H.; Chen, X.X.; Chen, H.Y. Illustrated keys to Scoliidae (Insecta, Hymenoptera, Scolioidea) from China. ZooKeys 2021, 1025, 139–175. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol. Marine Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, automatic barcode gap discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [Green Version]
- Peters, R.S.; Krogmann, L.; Mayer, C.; Donath, A.; Gunkel, S.; Meusemann, K.; Kozlov, A.; Podsiadlowski, L.; Petersen, M.; Lanfear, R.; et al. Evolutionary history of the Hymenoptera. Curr. Biol. 2017, 27, 1013–1018. [Google Scholar] [CrossRef] [Green Version]
- Day, M.C.; George, R.E.; David, M. The most primitive Scoliidae (Hymenoptera). J. Nat. Hist. 1981, 15, 671–684. [Google Scholar] [CrossRef]
- Wilson, J.J. Assessing the Value of DNA Barcodes and Other Priority Gene Regions for Molecular Phylogenetics of Lepidoptera. PLoS ONE 2010, 5, e10525. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.K.; Jonathan, J.K. Fauna of India and the adjacent countries, Hymenoptera: Scoliidae; Zoological Survey of India: Kolkata, India, 2003; pp. 1–277. [Google Scholar]
- Matsumoto, R.; Hasegawa, M.; Ichikawa, A. Scolia watanabei, an adventive wasp newly discovered in Japan (Hymenoptera, Scoliidae, Scoliinae). Bull. Osaka Mus. Nat. Hist. 2019, 73, 1–5. [Google Scholar]
- Ilyasov, R.A.; Park, J.; Takahashi, J.; Kwon, H.W. Phylogenetic uniqueness of honeybee Apis cerana from the Korean peninsula inferred from the mitochondrial, nuclear, and morphological data. J. Apic. Sci. 2018, 62, 189–214. [Google Scholar] [CrossRef] [Green Version]
- Tsuneki, K. Studies on the scoliid wasps of eastern Asia (Hymenoptera). Etizenia 1972, 62, 1–41. [Google Scholar]
- Luo, A.R.; Chen, L.; Simon, Y.W.H.; Zhu, C.D. Comparison of Methods for Molecular Species Delimitation across a Range of Speciation Scenarios. Syst. Biol. 2018, 67, 830–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Code | Species | Sex | GenBank Accession No. |
|---|---|---|---|
| SCAU3043675 | Austroscolia ruficeps (Smith) | male | MZ753501 |
| SCAU3043670 | Carinoscolia vittifrons (Sichel) | male | MZ753502 |
| SCAU3043671 | Carinoscolia vittifrons (Sichel) | female | MZ753503 |
| SCAU3043672 | Carinoscolia vittifrons (Sichel) | female | MZ753504 |
| SCAU3043673 | Carinoscolia vittifrons (Sichel) | male | MZ753505 |
| En-418584 | Carinoscolia vittifrons (Sichel) | female | MZ821648 |
| SCAU3043661 | Liacos erythrosoma (Burmeister) | female | MZ753506 |
| SCAU3043662 | Liacos erythrosoma (Burmeister) | male | MZ753507 |
| SCAU3048016 | Liacos erythrosoma (Burmeister) | male | MZ753508 |
| SCAU3043656 | Megacampsomeris asiatica (Saussure) | male | MZ753509 |
| SCAU3043660 | Megacampsomeris binghami (Betrem) | male | MZ753510 |
| SCAU3048011 | Megacampsomeris binghami (Betrem) | male | MZ753511 |
| SCAU3043653 | Megacampsomeris farrenwhitei (Betrem) | female | MZ753512 |
| SCAU3043654 | Megacampsomeris farrenwhitei (Betrem) | male | MZ753513 |
| SCAU3043658 | Megacampsomeris farrenwhitei (Betrem) | male | MZ753514 |
| SCAU3043659 | Megacampsomeris farrenwhitei (Betrem) | male | MZ753515 |
| SCAU3043686 | Megacampsomeris farrenwhitei (Betrem) | female | MZ753517 |
| SCAU3048009 | Megacampsomeris farrenwhitei (Betrem) | male | MZ753518 |
| SCAU3048012 | Megacampsomeris farrenwhitei (Betrem) | male | MZ753519 |
| SCAU3043657 | Megacampsomeris prismatica (Smith) | female | MZ753520 |
| SCAU3048015 | Megacampsomeris prismatica (Smith) | male | MZ753521 |
| NZ4760 | Megacampsomeris pulchrivestita (Cameron) | male | MZ753522 |
| SCAU3043655 | Megacampsomeris shillongensis (Betrem) | male | MZ753523 |
| SCAU3043665 | Megascolia (Regiscolia) azurea (Christ) | female | MZ753524 |
| SCAU3043666 | Megascolia (Regiscolia) azurea (Christ) | male | MZ753525 |
| SCAU3043667 | Micromeriella marginella (Klug) | female | MZ753526 |
| SCAU3043668 | Micromeriella marginella (Klug) | male | MZ753527 |
| SCAU3043685 | Phalerimeris phalerata (Saussure) | male | MZ753516 |
| NZ4762 | Phalerimeris phalerata (Saussure) | female | MZ753528 |
| NZ4792 | Phalerimeris phalerata (Saussure) | male | MZ753529 |
| NZ4832 | Phalerimeris phalerata (Saussure) | male | MZ753530 |
| NZ4835 | Phalerimeris phalerata (Saussure) | female | MZ753531 |
| SCAU3043669 | Phalerimeris phalerata (Saussure) | male | MZ753532 |
| SCAU3048017 | Phalerimeris phalerata (Saussure) | male | MZ753533 |
| En-418587 | Phalerimeris phalerata (Saussure) | female | MZ821649 |
| SCAU3043683 | Scolia (Discolia) affinis Guérin-Méneville | male | MZ753534 |
| SCAU3043684 | Scolia (Discolia) binotata Fabricius | female | MZ753535 |
| SCAU3043682 | Scolia (Discolia) clypeata Sickman | female | MZ753536 |
| SCAU3043680 | Scolia (Discolia) laeviceps Smith | male | MZ753537 |
| SCAU3043681 | Scolia (Discolia) nobilis Saussure | male | MZ753538 |
| SCAU3048008 | Scolia (Discolia) sikkimensis Bingham | male | MZ753539 |
| SCAU3043676 | Scolia (Discolia) superciliaris Saussure | female | MZ753540 |
| SCAU3043677 | Scolia (Discolia) superciliaris Saussure | male | MZ753541 |
| SCAU3043678 | Scolia (Discolia) superciliaris Saussure | male | MZ753542 |
| SCAU3048010 | Scolia (Discolia) superciliaris Saussure | male | MZ753543 |
| SCAU3048013 | Scolia (Discolia) superciliaris Saussure | male | MZ753544 |
| SCAU3043679 | Scolia (Discolia) watanabei (Matsumura) | male | MZ753545 |
| En-418585 | Scolia (Discolia) watanabei (Matsumura) | male | MZ821650 |
| SCAU3043674 | Scolia sp. | male | MZ753546 |
| SCAU3048014 | Scolia sp. | male | MZ753547 |
| SCAU3043664 | Sericocampsomeris flavomacula Gupta and Jonathan | male | MZ753549 |
| En-418591 | Sericocampsomeris flavomacula Gupta and Jonathan | male | MZ821651 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yang, S.-J.; Wang, Y.-Y.; Peng, Y.-Q.; Chen, H.-Y.; Luo, S.-X. Tackling the Taxonomic Challenges in the Family Scoliidae (Insecta, Hymenoptera) Using an Integrative Approach: A Case Study from Southern China. Insects 2021, 12, 892. https://doi.org/10.3390/insects12100892
Liu Z, Yang S-J, Wang Y-Y, Peng Y-Q, Chen H-Y, Luo S-X. Tackling the Taxonomic Challenges in the Family Scoliidae (Insecta, Hymenoptera) Using an Integrative Approach: A Case Study from Southern China. Insects. 2021; 12(10):892. https://doi.org/10.3390/insects12100892
Chicago/Turabian StyleLiu, Zhen, Sheng-Jie Yang, Yu-Yuan Wang, Yan-Qiong Peng, Hua-Yan Chen, and Shi-Xiao Luo. 2021. "Tackling the Taxonomic Challenges in the Family Scoliidae (Insecta, Hymenoptera) Using an Integrative Approach: A Case Study from Southern China" Insects 12, no. 10: 892. https://doi.org/10.3390/insects12100892
APA StyleLiu, Z., Yang, S.-J., Wang, Y.-Y., Peng, Y.-Q., Chen, H.-Y., & Luo, S.-X. (2021). Tackling the Taxonomic Challenges in the Family Scoliidae (Insecta, Hymenoptera) Using an Integrative Approach: A Case Study from Southern China. Insects, 12(10), 892. https://doi.org/10.3390/insects12100892






