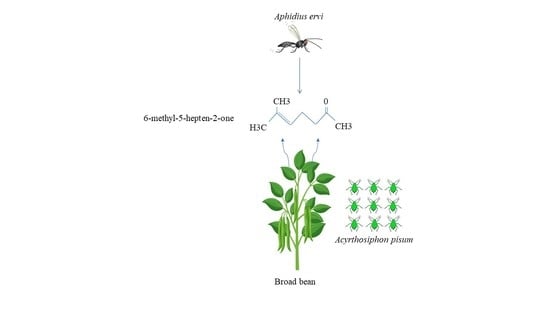
Aphids Facing Their Parasitoids: A First Look at How Chemical Signals May Make Higher Densities of the Pea Aphid Acyrthosiphon pisum Less Attractive to the Parasitoid Aphidius ervi
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant and Insect Rearing
2.2. Bio-Assay Behavioral Experiment
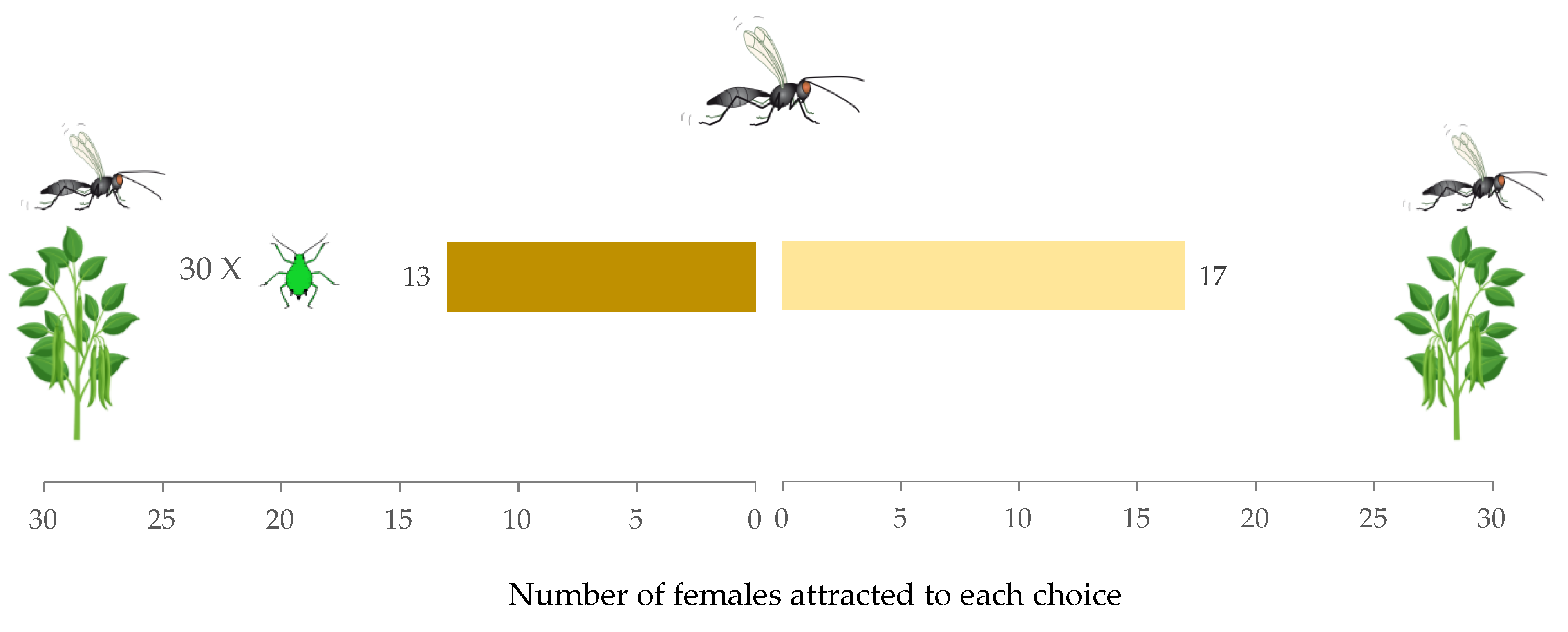
2.3. Experiment 1: Effect of Aphid Density on Parasitoid Behavioral Choice
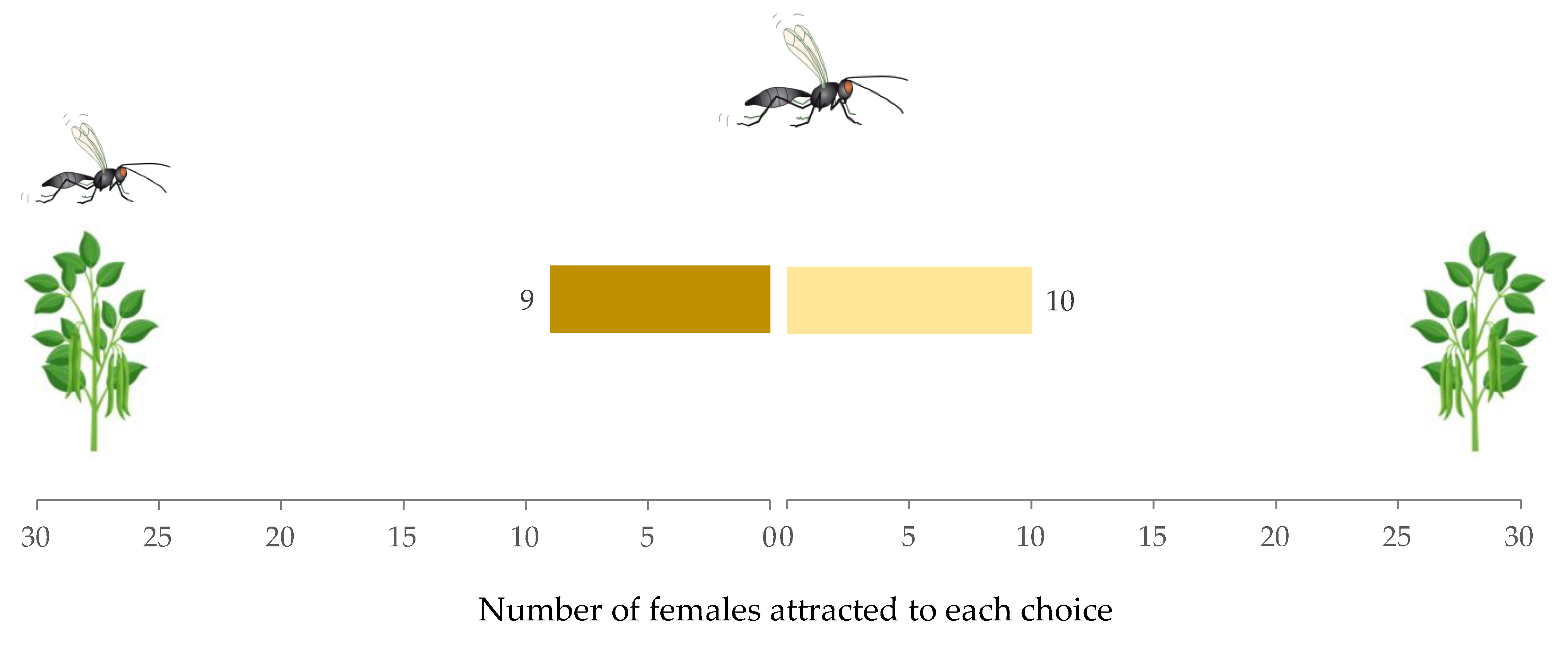
2.4. Experiment 2: Detectability of a Female Conspecific on a Host Colony
2.5. Chemical Extraction and Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
2.6. Statistical Analysis
3. Results
3.1. Experiment 1: Effect of Host Density on A. ervi Response
3.2. Experiment 2: Detectability of a Female Conspecific on a Host Colony
3.3. Volatile Blends
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, J.O. Chapter 68—Defensive Behavior. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 252–257. ISBN 978-0-12-374144-8. [Google Scholar]
- Pulliam, H. On the Advantages of Flocking. J. Theor. Biol. 1973, 38, 419–422. [Google Scholar] [CrossRef]
- Powell, G.V.N. Experimental Analysis of the Social Value of Flocking by Starlings (Sturnus vulgaris) in Relation to Predation and Foraging. Anim. Behav. 1974, 22, 501–505. [Google Scholar] [CrossRef]
- Beauchamp, G. A Comparative Analysis of Vigilance in Birds. Evol. Ecol. 2010, 24, 1267–1276. [Google Scholar] [CrossRef]
- Creel, S.; Schuette, P.; Christianson, D. Effects of Predation Risk on Group Size, Vigilance, and Foraging Behavior in an African Ungulate Community. Behav. Ecol. 2014, 25, 773–784. [Google Scholar] [CrossRef]
- Ward, A.J.W.; Herbert-Read, J.E.; Sumpter, D.J.T.; Krause, J. Fast and Accurate Decisions through Collective Vigilance in Fish Shoals. Proc. Acad. Natl. Sci. USA 2011, 108, 2312–2315. [Google Scholar] [CrossRef]
- Inman, A.; Krebs, J. Predation and Group Living. Trends Ecol. Evol. 1987, 2, 31–32. [Google Scholar] [CrossRef]
- Foster, W.A.; Treherne, J.E. Evidence for the Dilution Effect in the Selfish Herd from Fish Predation on a Marine Insect. Nature 1981, 293, 466–467. [Google Scholar] [CrossRef]
- Ioannou, C.; Tosh, C.; Neville, L.; Krause, J. The Confusion Effect—From Neural Networks to Reduced Predation Risk. Behav. Ecol. 2008, 19, 126–130. [Google Scholar] [CrossRef]
- Wiesel, I. Killing of Cape Fur Seal (Arctocephalus pusillus Pusillus) Pups by Brown Hyenas (Parahyaena brunnea) at Mainland Breeding Colonies along the Coastal Namib Desert. Acta Ethol. 2010, 13, 93–100. [Google Scholar] [CrossRef]
- Sugiura, S. Predators as Drivers of Insect Defenses. Entomol. Sci. 2020, 23, 316–337. [Google Scholar] [CrossRef]
- Théry, M.; Gomez, D. Insect Colours and Visual Appearance in the Eyes of Their Predators. Adv. Insect Physiol. 2010, 38, 267–363. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification and Information Guide, 2nd ed.; John Wiley and Sons Ltd.: Chichester, UK, 2000. [Google Scholar]
- Boivin, G.; Hance, T.; Brodeur, J. Aphid Parasitoids in Biological Control. Can. J. Plant Sci. 2012, 92, 1–12. [Google Scholar] [CrossRef]
- Hance, T.; Kohandani-Trafresh, F.; Munaut, F. Biological control. In Aphids as Crop Pests; van Emden, H.F., Harrington, R., Eds.; CABI: Wallingford, UK, 2017; pp. 448–493. [Google Scholar]
- Wang, Z.; Liu, Y.; Shi, M.; Huang, J.; Chen, X. Parasitoid Wasps as Effective Biological Control Agents. J. Integr. Agric. 2019, 18, 705–715. [Google Scholar] [CrossRef]
- Dixon, A.F.G. The Escape Responses Shown by Certain Aphids to the Presence of the Coccinellid Adalia decempunctata (L.). Trans. R. Entomol. Soc. Lond. 1958, 110, 319–334. [Google Scholar] [CrossRef]
- Siddiqui, J.; Xuting, Z.; Qian, L.; Zhang, H.; Xiaolan, L.; Huang, X. Functional Morphology and Defensive Behavior in a Social Aphid. Insects 2019, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, R.; Ruxton, G.; Karley, A. Drop When the Stakes Are High: Adaptive, Flexible Use of Dropping Behaviour by Aphids. Behaviour 2021, 1, 1–21. [Google Scholar] [CrossRef]
- Fan, L.-P.; Ouyang, F.; Su, J.-W.; Ge, F. Adaptation of Defensive Strategies by the Pea Aphid Mediates Predation Risk from the Predatory Lady Beetle. J. Chem. Ecol. 2018, 44, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Vandermoten, S.; Mescher, M.; Francis, F.; Haubruge, E.; Verheggen, F. Aphid Alarm Pheromone: An Overview of Current Knowledge on Biosynthesis and Functions. Insect Biochem. Mol. Biol. 2011, 42, 155–163. [Google Scholar] [CrossRef]
- Treherne, J.E.; Foster, W.A. Group Size and Anti-Predator Strategies in a Marine Insect. Anim. Behav. 1982, 30, 536–542. [Google Scholar] [CrossRef]
- Wellings, P.W. Foraging Behaviour in Aphid Parasitoids: Spatial Scale and Resource Assessment. Eur. J. Entomol. 1993, 90, 377–382. [Google Scholar]
- Quilici, S.; Rousse, P. Location of Host and Host Habitat by Fruit Fly Parasitoids. Insects 2012, 3, 1220–1235. [Google Scholar] [CrossRef]
- De Moraes, C.; Lewis, W.J.; Pare, P.; Alborn, H.; Tumlinson, J. Herbivore-Infested Plants Selectively Attract Parasitoids. Nature 1998, 393, 570–573. [Google Scholar] [CrossRef]
- Guo, H.; Wang, C.-Z. The Ethological Significance and Olfactory Detection of Herbivore-Induced Plant Volatiles in Interactions of Plants, Herbivorous Insects, and Parasitoids. Arthropod-Plant Interact. 2019, 13, 161–179. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive Function of Herbivore-Induced Plant Volatile Emissions in Nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Schuman, M.C.; Baldwin, I.T. Field Studies Reveal Functions of Chemical Mediators in Plant Interactions. Chem. Soc. Rev. 2018, 47, 5338–5353. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Tumlinson, J.H.; Lewis, W.J. Exploitation of Herbivore-Induced Plant Odors by Host-Seeking Parasitic Wasps. Science 1990, 250, 1251–1253. [Google Scholar] [CrossRef]
- Bezemer, T.M.; Harvey, J.A.; Kamp, A.F.D.; Wagenaar, R.; Gols, R.; Kostenko, O.; Fortuna, T.; Engelkes, T.; Vet, L.E.M.; van Der Putten, E.W.; et al. Behaviour of Male and Female Parasitoids in the Field: Influence of Patch Size, Host Density, and Habitat Complexity. Ecol. Entomol. 2010, 35, 341–351. [Google Scholar] [CrossRef]
- Powell, W.; Pennacchio, F.; Poppy, G.M.; Tremblay, E. Strategies Involved in the Location of Hosts by the Parasitoid Aphidius Ervi Haliday (Hymenoptera: Braconidae: Aphidiinae). Biol. Control. 1998, 11, 104–112. [Google Scholar] [CrossRef]
- Henneman, M.L.; Dyreson, E.G.; Takabayashi, J.; Raguso, R.A. Response to Walnut Olfactory and Visual Cues by the Parasitic Wasp Diachasmimorpha juglandis. J. Chem. Ecol. 2002, 28, 2221–2244. [Google Scholar] [CrossRef]
- Aartsma, Y.; Bianchi, F.J.J.A.; van der Werf, W.; Poelman, E.H.; Dicke, M. Herbivore-Induced Plant Volatiles and Tritrophic Interactions across Spatial Scales. New Phytol. 2017, 216, 1054–1063. [Google Scholar] [CrossRef]
- Kroes, A.; van Loon, J.J.A.; Dicke, M. Density-Dependent Interference of Aphids with Caterpillar-Induced Defenses in Arabidopsis: Involvement of Phytohormones and Transcription Factors. Plant Cell Physiol. 2015, 56, 98–106. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Loughrin, J.H.; McCall, P.J.; Rose, U.S.R.; Lewis, W.J.; Tumlinson, J.H. How Caterpillar-Damaged Plants Protect Themselves by Attracting Parasitic Wasps. Proc. Acda. Natl. Sci. USA 1995, 92, 4169–4174. [Google Scholar] [CrossRef] [PubMed]
- Shiojiri, K.; Ozawa, R.; Kugimiya, S.; Uefune, M.; van Wijk, M.; Sabelis, M.W.; Takabayashi, J. Herbivore-Specific, Density-Dependent Induction of Plant Volatiles: Honest or “Cry Wolf” Signals? PLoS ONE 2010, 5, e12161. [Google Scholar] [CrossRef] [PubMed]
- Masson, C.; Mustaparta, H. Chemical Information Processing in the Olfactory System of Insects. Physiol. Rev. 1990, 70, 199–245. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Ortiz, V.; Sellés-Marchart, S.; Zubcoff-Vallejo, J.; Jander, G.; Casas, J.L. Changes in the Free Amino Acid Composition of Capsicum annuum (Pepper) Leaves in Response to Myzus persicae (Green Peach Aphid) Infestation. A Comparison with Water Stress. PLoS ONE 2018, 13, e0198093. [Google Scholar] [CrossRef]
- Cascone, P.; Gols, R.; Fatouros, N.E.; Ponzio, C.; Dicke, M.; Guerrieri, E. The Effect of Rearing History and Aphid Density on Volatile-Mediated Foraging Behaviour of Diaeretiella rapae. Ecol. Entomol. 2019, 44, 255–264. [Google Scholar] [CrossRef]
- Prado, E.; Tjallingii, W.F. Behavioral Evidence for Local Reduction of Aphid-Induced Resistance. J. Insect Sci. 2007, 7, 48. [Google Scholar] [CrossRef]
- Walling, L. Avoiding Effective Defenses: Strategies Employed by Phloem-Feeding Insects. Plant Physiol. 2008, 146, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Cotes, B.; Rännbäck, L.-M.; Björkman, M.; Norli, H.; Meyling, N.; Rämert, B.; Anderson, P. Habitat Selection of a Parasitoid Mediated by Volatiles Informing on Host and Intraguild Predator Densities. Oecologia 2015, 179, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Castelo, M.; Corley, J.; Desouhant, E. Conspecific Avoidance during Foraging in Venturia canescens (Hymenoptera: Ichneumonidae): The Roles of Host Presence and Conspecific Densities. J. Insect Behav. 2003, 16, 307–318. [Google Scholar] [CrossRef]
- Almohamad, R.; Hance, T. Encounters with Aphid Predators or Their Residues Impede Searching and Oviposition by the Aphid Parasitoid Aphidius ervi (Hymenoptera: Aphidiinae). Insect Sci. 2013, 21, 181–188. [Google Scholar] [CrossRef]
- Janssen, A.; Alphen, J.; Sabelis, M.; Bakker, K. Specificity of Odor Mediated Avoidance of Competition in Drosophila Parasitoids. Behav. Ecol. Sociobiol. 1995, 36, 229–235. [Google Scholar] [CrossRef]
- Xia, P.-L.; Xinglin, Y.; Li, Z.-T.; Feng, Y. The Impacts of Harmonia Axyridis Cues on Foraging Behavior of Aphidius Gifuensis to Myzus Persicae. J. Asia Pacific Entomol. 2021, 24, 278–284. [Google Scholar] [CrossRef]
- Muratori, F.B.; Damiens, D.; Hance, T.; Boivin, G. Bad Housekeeping: Why Do Aphids Leave Their Exuviae inside the Colony? BMC Evol. Biol. 2008, 8, 2008. [Google Scholar] [CrossRef]
- Muratori, F.; Le Ralec, A.; Lognay, G.; Hance, T. Epicuticular Factors Involved in Host Recognition for the Aphid Parasitoid Aphidius Rhopalosiphi. J. Chem. Ecol. 2006, 32, 579–593. [Google Scholar] [CrossRef]
- Du, Y.J.; Poppy, G.M.; Powell, W. Relative Importance of Semiochemicals from First and Second Trophic Levels in Host Foraging Behavior of Aphidius ervi. J. Chem. Ecol. 1996, 22, 1591–1605. [Google Scholar] [CrossRef]
- Guerrieri, E.; Poppy, G.M.; Powell, W.; Rao, R.; Pennacchio, F. Plant-to-Plant Communication Mediating in Flight Orientation of Aphidius ervi. J. Chem. Ecol. 2002, 28, 1703–1715. [Google Scholar] [CrossRef]
- Guerrieri, E.; Poppy, G.M.; Powell, W.; Tremblay, E.; Pennacchio, F. Induction and Systemic Release of Herbivore-Induced Plant Volatiles Mediating in Flight Orientation of Aphidius ervi. J. Chem. Ecol. 1999, 25, 1247–1261. [Google Scholar] [CrossRef]
- Sasso, R.; Iodice, L.; Digilio, M.C.; Carretta, A.; Ariati, L.; Guerrieri, E. Host-Locating Response by the Aphid Parasitoid Aphidius ervi to Tomato Plant Volatiles. J. Plant Interact. 2007, 2, 175–183. [Google Scholar] [CrossRef]
- Du, Y.J.; Poppy, G.M.; Powell, W.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Identification of Semiochemicals Released during Aphid Feeding That Attract Parasitoid Aphidius ervi. J. Chem. Ecol. 1998, 24, 1355–1368. [Google Scholar] [CrossRef]
- Taborsky, M. Sample Size in the Study of Behaviour. Ethology 2010, 116, 185–202. [Google Scholar] [CrossRef]
- Champely, S. Pwr: Basic Functions for Power Analysis. 2020. Available online: https://github.com/heliosdrm/pwr (accessed on 18 September 2021).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- R. Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; ISBN 3-900051-07-0. [Google Scholar]
- Guerrieri, E.; Pennacchio, F.; Tremblay, E. Flight Behavior of the Aphid Parasitoid Aphidius ervi (Hymenoptera, Braconidae) in Response to Plant and Host Volatiles. Eur. J. Entomol. 1993, 90, 415–421. [Google Scholar]
- Lucas-Barbosa, D.; Poelman, E.H.; Aartsma, Y.; Snoeren, T.A.L.; van Loon, J.J.A.; Dicke, M. Caught between Parasitoids and Predators—Survival of a Specialist Herbivore on Leaves and Flowers of Mustard Plants. J. Chem. Ecol. 2014, 40, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-L.; Liu, T.-X. Aphid-Induced Plant Volatiles Affect the Attractiveness of Tomato Plants to Bemisia tabaci and Associated Natural Enemies. Entomol. Exp. Appl. 2014, 151, 259–269. [Google Scholar] [CrossRef]
- Gagic, V.; Petrović-Obradović, O.; Fründ, J.; Kavallieratos, N.G.; Athanassiou, C.G.; Starý, P.; Tomanović, Ž. The Effects of Aphid Traits on Parasitoid Host Use and Specialist Advantage. PLoS ONE 2016, 11, e0157674. [Google Scholar] [CrossRef]
- Jaroasik, V.; Lapchin, L. An Experimental Investigation of Patterns of Parasitism at Three Spatial Scales in an Aphid-Parasitoid System (Hymenoptera: Aphidiidae). Eur. J. Endocrinol. 2001, 98, 295–299. [Google Scholar] [CrossRef]
- Hopper, K. Risk-Spreading and Bet-Hedging in Insect Population Biology. Ann. Rev. Entomol. 1999, 44, 535–560. [Google Scholar] [CrossRef]
- Seger, J.; Brockman, H.J. What is bethedging? In Oxford Surveys in Evolutionary Biology; Harvey, P.H., Partridge, L., Eds.; Oxford University Press: Oxford, UK, 1987; Volume 4. [Google Scholar]
- Ives, A.R.; Settle, W.H. The Failure of a Parasitoid to Persist with a Superabundant Host: The Importance of the Numerical Response. Oikos 1996, 75, 269–278. [Google Scholar] [CrossRef]
- Yang, S.; Xu, R.; Yang, S.Y.; Kuang, R.P. Olfactory Responses of Aphidius gifuensis to Odors of Host Plants and Aphid-Plant Complexes. Insect Sci. 2009, 16, 503–510. [Google Scholar] [CrossRef]
- Ismail, M.; Albittar, L. Mortality Factors Affecting Immature Stages of Codling Moth, Cydia pomonella (Lepidoptera: Tortricidae), and the Impact of Parasitoid Complex. Biocontrol. Sci. Technol. 2016, 26, 72–85. [Google Scholar] [CrossRef]
- Albittar, L.; Ismail, M.; Bragard, C.; Hance, T. Host Plants and Aphid Hosts Influence the Selection Behaviour of Three Aphid Parasitoids (Hymenoptera: Braconidae: Aphidiinae). Eur. J. Entomol. 2016, 113, 516–522. [Google Scholar] [CrossRef][Green Version]
- Pareja, M.; Mohib, A.; Birkett, M.; Dufour, S.; Glinwood, R. Multivariate Statistics Coupled to Generalized Linear Models Reveal Complex Use of Chemical Cues by a Parasitoid. Anim. Behav. 2009, 77, 901–909. [Google Scholar] [CrossRef]
- Price, P.W. Inversely Density-Dependent Parasitism: The Role of Plant Refuges for Hosts. J. Anim. Ecol. 1988, 57, 89–96. [Google Scholar] [CrossRef]
- Lessells, C.M. Parasitoid Foraging: Should Parasitism Be Density Dependent? J. Anim. Ecol. 1985, 54, 27–41. [Google Scholar] [CrossRef]
- Aartsma, Y.; Leroy, B.; Werf, W.; Dicke, M.; Poelman, E.; Bianchi, F. Intraspecific Variation in Herbivore-Induced Plant Volatiles Influences the Spatial Range of Plant-Parasitoid Interactions. Oikos 2019, 128, 77–86. [Google Scholar] [CrossRef]
- Pareja, M.; Moraes, M.C.B.; Clark, S.J.; Birkett, M.A.; Powell, W. Response of the Aphid Parasitoid Aphidius Funebris to Volatiles from Undamaged and Aphid-Infested Centaurea Nigra. J. Chem. Ecol. 2007, 33, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Birkett, M.; Campbell, C.; Chamberlain, K.; Guerrieri, E.; Hick, A.; Martin, J.; Matthes, M.; Napier, J.; Pettersson, J.; Pickett, J.; et al. New Roles for Cis-Jasmone as an Insect Semiochemical and in Plant Defense. Proc. Acad. Natl. Sci. USA 2000, 97, 9329–9334. [Google Scholar] [CrossRef]
- Sun, Y.-L.; Dong, J.; Huang, L.-Q.; Wang, C.-Z. The Cotton Bollworm Endoparasitoid Campoletis Chlorideae Is Attracted by Cis-Jasmone or Cis-3-Hexenyl Acetate but Not by Their Mixtures. Arthropod-Plant Interact. 2020, 14, 169–179. [Google Scholar] [CrossRef]
- Baluška, F.; Ninkovic, V. Plant Communication from an Ecological Perspective, Signaling and Communication in Plants; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Frago, E.; Mala, M.; Weldegergis, B.T.; Yang, C.; McLean, A.; Godfray, H.C.J.; Gols, R.; Dicke, M. Symbionts Protect Aphids from Parasitic Wasps by Attenuating Herbivore-Induced Plant Volatiles. Nat. Commun. 2017, 8, 1860. [Google Scholar] [CrossRef]
- Vosteen, I.; Weisser, W.; Kunert, G. Is There Any Evidence That Aphid Alarm Pheromones Work as Prey and Host Finding Kairomones for Natural Enemies? Ecol. Entomol. 2016, 41, 1–12. [Google Scholar] [CrossRef]
- Dall, S.R.X.; Giraldeau, L.-A.; Olsson, O.; McNamara, J.M.; Stephens, D.W. Information and Its Use by Animals in Evolutionary Ecology. Trends Ecol. Evol. 2005, 20, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ito, E.; Yamada, Y.Y. Presence of a Conspecific Increases Superparasitism but Not Infanticide under Self- and Conspecific Superparasitism in a Semisolitary Parasitoid, Echthrodelphax fairchildii (Hymenoptera: Dryinidae). Entomol. Sci. 2016, 19, 25–33. [Google Scholar] [CrossRef]
- Tamò, C.; Roelfstra, L.; Guillaume, S.; Turlings, T. Odour-Mediated Long-Range Avoidance of Interspecific Competition by a Solitary Endoparasitoid: A Time-Saving Foraging Strategy. J. Anim. Ecol. 2006, 75, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- McBrien, H.; Mackauer, M. Decision to Superparasitize Based on Larval Survival: Competition between Aphid Parasitoids Aphidius ervi and Aphidius smithi. Entomol. Exp. Appl. 1991, 59, 145–150. [Google Scholar] [CrossRef]
- Van Lenteren, J.C. Host discrimination by parasitoids. In Semiochemicals: Their Role in Pest Control; Wiley and Sons: New York, NY, USA, 1981; pp. 153–179. [Google Scholar]
- Le Lann, C.; Outreman, Y.; Alphen, J.; van Baaren, J. First in, Last out: Asymmetric Competition Influences Patch Exploitation of a Parasitoid. Behav. Ecol. 2011, 22, 101–107. [Google Scholar] [CrossRef]

| Time Taken in Seconds by Parasitoid Females to Make a Decision | ||
|---|---|---|
| Individual Density | Aphid-Infested Plants | Non-Infested Plants |
| 10 | 36.22 s (49.16) | 30.85 s (48.82) |
| 30 | 37.69 s (18.23) | 16.34 s (42.20) |
| 50 | 40.26 s (43.53) | 17.14 s (16.83) |
| 100 | 23.03 s (16.61) | 45.28 s (78.61) |
| 200 | 29.56 s (42.75) | 38.30 s (29.65) |
| The Rate of Choice Hesitation of Parasitoid Females between the Two Choices | ||
|---|---|---|
| Individual Density | From Aphid-Infested to Non-Infested Plants | From Non-Infested to Aphid-Infested Plants |
| 10 | 0.10 ± 0.05 | 0.20 ± 0.07 |
| 30 | 0.07 ± 0.05 | 0.20 ± 0.07 |
| 50 | 0.07 ± 0.05 | 0.10 ± 0.05 |
| 100 | 0.03 ± 0.03 | 0.03 ± 0.03 |
| 200 | 0.23 ± 0.07 | 0.03 ± 0.03 |
| VOCs ng/μL | Non-Infested | Aphid Infested | Statistical Analysis | |
|---|---|---|---|---|
| 0 Individuals | 50 Individuals | 100 Individuals | ||
| 6-methyl-5-hepten-2-one | 1.03 (0.95) b | 3.81 (0.64) a | 0.35 (0.25) b | KW test: χ2 = 9.26, df = 2, p = 0.009 |
| cis-jasmone | 1.84 (0.68) a | 3.25 (1.25) a | 0.32 (0.04) b | KW test: χ2 = 8.89, df = 2, p = 0.01 |
| cis-3-hexenol | 0.77 (1.15) a | 2.61 (2.46) a | 0.11 (only one record) | MW U test: W = 6.5, p = 0.77 |
| cis-3-hexenyl-acetate | 1.88 (0.47) a | 3.17 (1.42) a | 0 | MW U test: W = 4, p = 0.67 |
| linalool | 0.46 (0.03) a | 1.37 (0.37) a | 0 | MW U test: W = 4, p = 0.33 |
| E-2-hexenal | 0.87 | 1.43 | 0 | No test |
| Total | 5.22 (1.40) b | 12.49 (2.92) a | 0.53 (0.62) c | KW test: χ2 = 9.84, df = 2, p = 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, M.; Zanolli, P.; Muratori, F.; Hance, T. Aphids Facing Their Parasitoids: A First Look at How Chemical Signals May Make Higher Densities of the Pea Aphid Acyrthosiphon pisum Less Attractive to the Parasitoid Aphidius ervi. Insects 2021, 12, 878. https://doi.org/10.3390/insects12100878
Ismail M, Zanolli P, Muratori F, Hance T. Aphids Facing Their Parasitoids: A First Look at How Chemical Signals May Make Higher Densities of the Pea Aphid Acyrthosiphon pisum Less Attractive to the Parasitoid Aphidius ervi. Insects. 2021; 12(10):878. https://doi.org/10.3390/insects12100878
Chicago/Turabian StyleIsmail, Mohannad, Penelope Zanolli, Frédéric Muratori, and Thierry Hance. 2021. "Aphids Facing Their Parasitoids: A First Look at How Chemical Signals May Make Higher Densities of the Pea Aphid Acyrthosiphon pisum Less Attractive to the Parasitoid Aphidius ervi" Insects 12, no. 10: 878. https://doi.org/10.3390/insects12100878
APA StyleIsmail, M., Zanolli, P., Muratori, F., & Hance, T. (2021). Aphids Facing Their Parasitoids: A First Look at How Chemical Signals May Make Higher Densities of the Pea Aphid Acyrthosiphon pisum Less Attractive to the Parasitoid Aphidius ervi. Insects, 12(10), 878. https://doi.org/10.3390/insects12100878