Temperature and Photoperiodic Response of Diapause Induction in Anastatus japonicus, an Egg Parasitoid of Stink Bugs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Experimental Conditions
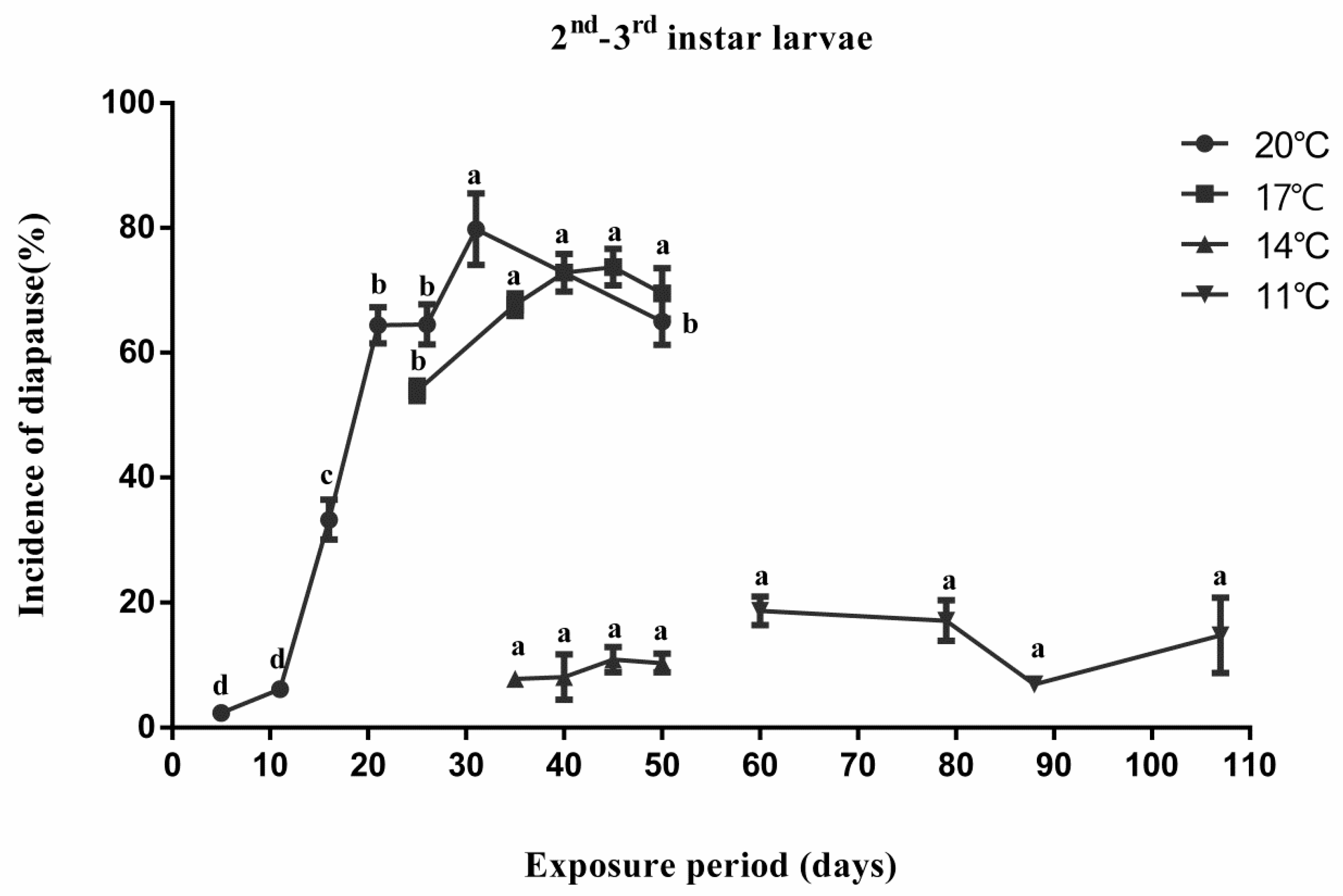
2.2. Effect of Temperature and Exposure Period on Diapause Induction
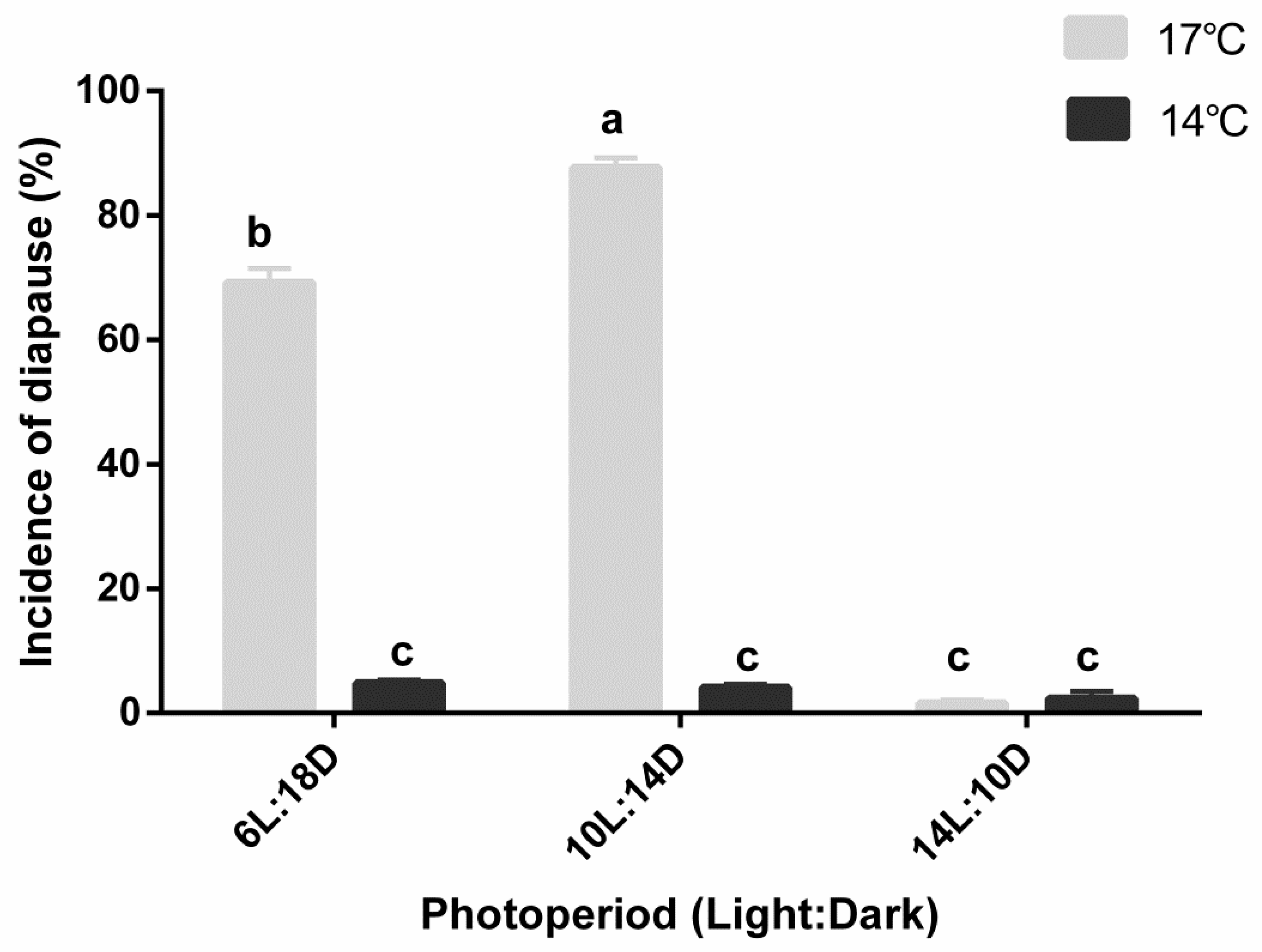
2.3. Effect of Photoperiod and Temperature on the Induction of Diapause
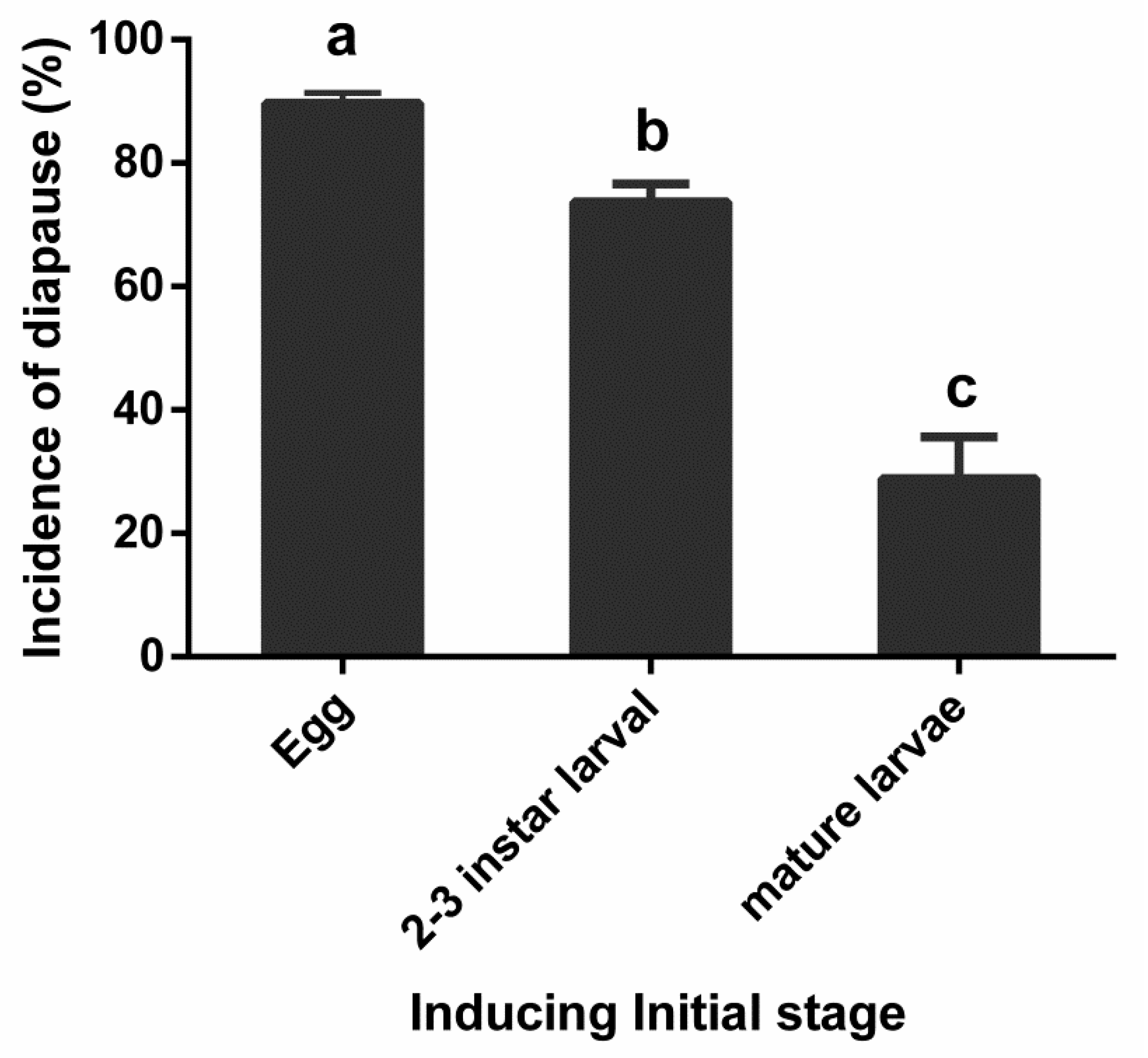
2.4. Effect of Inducing Initial Stage on Diapause Induction
2.5. Sensitive Stage to Diapause Induction
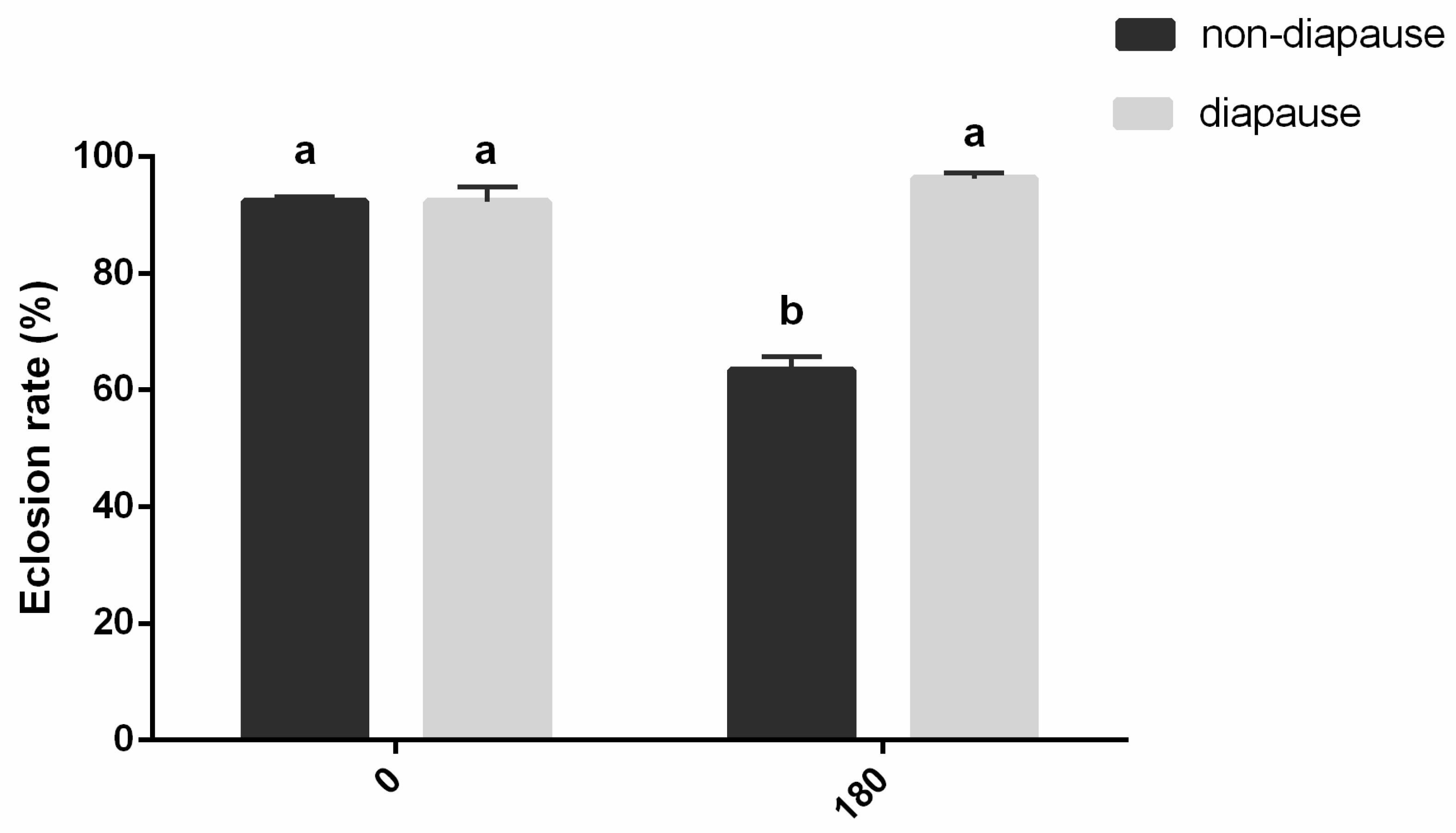
2.6. Effect of Chilling Period on Survival of Diapausing and Nondiapausing Mature Larvae of A. japonicus
2.7. Statistical Analyses
3. Results
3.1. Effect of Temperature and Exposure Period on Diapause Induction
3.2. Effect of Photoperiod and Temperature on Diapause Induction
3.3. Effect of Inducing Initial Stage on Diapause Induction
3.4. Sensitive Stage to Diapause Induction
3.5. Effect of Chilling Period on Survival of Diapausing and Nondiapausing Mature Larvae of A. japonicus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.L.; Chen, Q.; Guo, J.Q.; Li, J.; Wang, J.T.; Wen, M.; Zhao, H.B.; Ren, B.Z. Molecular basis of peripheral olfactory sensing during oviposition in the behavior of the parasitic wasp Anastatus japonicus. Insect. Biochem. Molec. 2017, 89, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.X.; Dong, Y.Z.; Lu, H. Progress in control techniques of litchi stink bug, Tessaratoma papillosa. Chin. J. Guangdong Agric. Sci. 2009, 6, 106–109. [Google Scholar]
- Choudhary, J.S.; Prabhakar, C.S.; Das, B.; Kumar, S. Litchi stink bug (Tessaratoma javanica) outbreak in Jharkhand, India, on litchi. Phytoparasitica 2013, 41, 73–77. [Google Scholar] [CrossRef]
- Schulte, M.J.; Martin, K.; Sauerborn, J. Effects of azadirachtin injection in litchitrees (Litchi chinensis Sonn.) on the litchi stink bug (Tessaratoma papillosa Drury) in northern Thailand. J. Pest Sci. 2006, 79, 241. [Google Scholar] [CrossRef]
- Li, D.S.; Liao, C.Y.; Zhang, B.X.; Song, Z.W. Biological control of insect pests in litchi orchards in China. Biol. Control. 2014, 68, 23–36. [Google Scholar] [CrossRef]
- Zhao, C.; Xia, Y.; Guo, Y.; Li, M.; Zhang, B.X.; Li, D.S. The study on diapause induction in Anastatus japonicus Ashmead. Chin. J. Biol. Control 2019, 35, 282–287. [Google Scholar]
- Zhao, C.; Zhang, B.X.; Yuan, X.; Song, Z.W.; Liu, Z.X.; Li, D.S. Sixty Years’ Research on biological control of pests by Guangdong Academy of Agricultural Sciences: Achievements and prospects. Chin. J. Guangdong Agric. Sci. 2020, 47, 93–102. [Google Scholar]
- Denlinger, D.L. Regulation of diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef]
- Kostal, V. Eco-physiological phases of insect diapause. J. Insect. Physiol. 2006, 52, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Ragland, G.J.; Egan, S.P.; Feder, J.L.; Berlocher, S.H.; Hahn, D.A. Developmental trajectories of gene expression reveal candidates for diapause termination: A key life-history transition in the apple maggot fly Rhagoletis pomonella. J. Exp. Biol. 2011, 214, 3948–3960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Fan, H.; Wang, P.; Liu, Y.H. Expression Analysis Reveals the Association of Several Genes with Pupal Diapause in Bactrocera minax (Diptera: Tephritidae). Insects 2019, 10, 169. [Google Scholar] [CrossRef] [Green Version]
- Pizzol, J.; Pintureau, B. Effect of photoperiod experienced by parents on diapause induction in Trichogramma cacoeciae. Entomol. Exp. Appl. 2008, 127, 72–77. [Google Scholar] [CrossRef]
- Reznik, S.Y.; Voinovich, N.D. Maternal regulation of Trichogramma embryophagum diapause photoperiodic sensitivity of adult females. BioControl 2011, 57, 158–162. [Google Scholar]
- Zhang, L.S.; Chen, H.Y.; Wang, M.Q.; Liu, C.X.; Zhang, Y.; Chen, C.F.; Wang, S.Y. Research Prospects in Diapause of Parasitoid Wasp. Chin. J. Biol. Control 2014, 30, 149–164. [Google Scholar]
- Zhang, L.S.; Chen, H.Y.; Zhang, J.; Li, Y.Y. Maternal effect of diapause in Aphidius gifuensis Ashmead. Chin. J. Appl. Entomol. 2014, 51, 35–44. [Google Scholar]
- Zhang, J.J.; Zhang, X.; Zang, L.S.; Du, W.M.; Hou, Y.Y.; Ruan, C.C.; Desneux, N. Advantages of diapause in Trichogramma dendrolimi mass production on eggs of the Chinese silkworm, Antheraea pernyi. Pest. Manag. Sci. 2018, 74, 959–965. [Google Scholar] [CrossRef]
- Huang, M.D.; Mai, X.H.; Wu, W.N.; Pu, Z.L. The bionomics of Anastatus sp. and its utilization for the control of lichi stink bug Tessaratoma papillosa Drury. Chin. J. Acta Entomol. Sin. 1974, 17, 362–375. [Google Scholar]
- Liu, C.C. A preliminary study of the biology of litchi stink bug, Tessaratoma papillosa Drury, and its control. Acta Phytophylac. Sin. 1965, 4, 329–340. [Google Scholar]
- Pu, Z.L. Utilizing Eupelmid wasp Anastatus sp to control litchi stink bug Tessaratoma papillosa. In Selected Works of Pu Zhelong; Sun Yat-sen University, Guangdong Scientech Association, Eds.; Sun Yat-Sen University Press: Guangzhou, China, 1992; pp. 135–169. [Google Scholar]
- Stahl, J.M.; Babendreier, D.; Haye, T. Life history of Anastatus bifasciatus, a potential biological control agent of the brown marmorated stink bug in Europe. Biol. Control 2019, 129, 178–186. [Google Scholar] [CrossRef]
- Narasimham, A.U.; Sankaran, T. Biology of Anastatus umae (Hymenoptera: Eupelmidae), an oothecal parasite of Neostylopyga rhombifolia. Colemania 1982, 1, 135–140. [Google Scholar]
- Zhou, Z.J.; Zhang, W.M.; Yang, C.P. Study on the artificial breeding technology of Anastatus ramakrishnai (Mani). Chin. J. Sichuan For. Sci. Technol. 1987, 4, 30–34. [Google Scholar]
- Li, Y.Y.; Zhang, L.S.; Chen, H.Y.; Wang, W.; Zhang, J. Temperature and photoperiodic response of diapause induction in Aphidius gifuensis. Chin. J. Appl. Entomol. 2013, 50, 718–726. [Google Scholar]
- Christiansen-Weniger, P.; Hardie, J. Environmental and physiological factors for diapause induction and termination in the aphid parasitoid, Aphidius ervi (Hymenoptera: Aphidiidae). J. Insect Physiol. 1999, 45, 357–364. [Google Scholar] [CrossRef]
- Hou, Y.Y.; Xu, L.Z.; Wu, Y.; Wang, P.; Shi, J.J.; Zhai, B.P. Geographic variation of diapause and sensitive stages of photoperiodic response in Laodelphax striatellus Fallen (Hemiptera: Delphacidae). J. Insect Physiol. 2016, 16, 1–7. [Google Scholar]
- Tauber, M.J.; Tauber, C.A. Larval diapause in Chrysopa nigricornis: Sensitive stages, critical photoperiod, and termination (Neuroptera: Chrysopidae). Entomol. Exp. Appl. 1972, 15, 105–111. [Google Scholar] [CrossRef]
- Tauber, M.J.; Tauber, C.A.; Masaki, S. Seasonal Adaptations of Insects; Oxford University Press: New York, NY, USA, 1986. [Google Scholar]
- Li, Y.Y.; Wang, M.Z.; Gao, F.; Zhang, H.Z.; Chen, H.Y.; Wang, M.Q.; Liu, C.X.; Zhang, L.S. Exploiting diapause and cold tolerance to enhance the use of the green lacewing Chrysopa formosa for biological control. Biol Control 2018, 127, 116–126. [Google Scholar] [CrossRef]
- Leopold, R.A. Cold Storage of Insects for Integrated Pest Management, in Temperature Sensitivity in Insects and Application in Integrated Pest Management; Hallman., G.J., Denlinger, D.L., Eds.; Westview Press: Boulder, CO, USA, 1998; pp. 235–267. [Google Scholar]
- Pitcher, S.A.; Hoffmann, M.P.; Gardner, J.; Wright, M.G.; Kuhar, T.P. Cold storage of Trichogramma ostriniae reared on Sitotroga cerealella eggs. BioControl 2002, 47, 525–535. [Google Scholar] [CrossRef]
- Zhang, B.X.; Zheng, Y.; Song, Z.W.; Feng, X.X. Experiments and demonstration of releasing Anastatus fulloi Sheng&Wang for Tessaratoma papillosa Drury control. China Trop. Agric. 2020, 6, 57–62. [Google Scholar]
- Ma, C.S.; Chen, Y.W. Effects of constant temperature, exposure period, and age on diapaused induction in Trichogramma dendrolimi. Biol. Control 2006, 36, 267–273. [Google Scholar] [CrossRef]
- Laing, J.E.; Corrigan, J.E. Diapaused induction and post-diapaused emergence in Trichogramma minutum Riley (Hymenoptera: Trichogrammatidae): The role of host species, temperature and photoperiod. Can. Entomol. 1995, 127, 103–110. [Google Scholar] [CrossRef]
- Pivnick, K.A. Diapause initiation and pupation site selection of the braconid parasitoid Microplitis mediator (Haliday): A case of manipulation of host behaviour. Can. Entomol. 1993, 125, 825–830. [Google Scholar] [CrossRef]
- Ryan, R.B. Maternal influence on diapause in a parasitic insect, Coeloides brunneri Vier (Hymenoptera: Braconidae). J. Insect Physiol. 1965, 11, 1331–1336. [Google Scholar] [CrossRef]
- Hou, Z.R. Study on the Lycorma delicatula and Egg Parasitoids. Master’s Thesis, Chinese Academy of Forestry, Beijing, China, June 2013. [Google Scholar]
- Hopper, K.R. Risk-spreading and bet-hedging in insect population biology. Annu. Rev. Entomol. 1999, 44, 535–560. [Google Scholar] [CrossRef]
- Hodek, I. Controversial aspects of diapause development. Euro. J. Entomol. 2002, 99, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.N.; Long, Z.R.; Zhang, Y.D.; Liang, T.T.; Zhu-Salzman, K. Effects of temperature, soil moisture and photoperiod on diapause termination and post-diapause development of the wheat blossom midge, Sitodiplosis mosellana (Géhin) (Diptera: Cecidomyiidae). J. Insect Physiol. 2017, 103, 78–85. [Google Scholar] [CrossRef]
- Lehmann, P.; Van Der Bijl, W.; Nylin, S.; Wheat, C.W.; Gotthard, K. Timing of diapause termination in relation to variation in winter climate. Physiol. Entomol. 2017, 42, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.J.; Zhang, X.; Du, W.M.; Ruan, C.C.; Zang, L.S.; Ren, B.Z. Effects of inducing initial stage, temperature and period on diapause induction and diapause termination of Trichogramma dendrolimi. Chin. J. Plant Prot. 2018, 45, 1308–1313. [Google Scholar]
- Wu, S.H.; Kostromytska, O.S.; Xue, F.S.; Koppenhöfer, A.M. Chilling effect on termination of reproductive diapause in Listronotus maculicollis (Coleoptera: Curculionidae). J. Insect Physiol. 2018, 104, 25–32. [Google Scholar] [CrossRef]
| Groups | Egg | 1st–2nd Instar Larvae | 2nd–3rd Instar Larvae | Matur Larvae | Diapause |
|---|---|---|---|---|---|
| a | S | L | L | L | 4.41% |
| b | L | S | L | L | 7.50% |
| c | L | L | S | L | 56.13% |
| d | L | L | L | S | 32.17% |
| e | L | L | S | S | 61.81% |
| f | L | S | S | L | 84.68% |
| g | S | S | S | L | 90% |
| h | S | L | S | L | 65.32% |
| i | S | S | L | L | 26.49% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Guo, Y.; Liu, Z.; Xia, Y.; Li, Y.; Song, Z.; Zhang, B.; Li, D. Temperature and Photoperiodic Response of Diapause Induction in Anastatus japonicus, an Egg Parasitoid of Stink Bugs. Insects 2021, 12, 872. https://doi.org/10.3390/insects12100872
Zhao C, Guo Y, Liu Z, Xia Y, Li Y, Song Z, Zhang B, Li D. Temperature and Photoperiodic Response of Diapause Induction in Anastatus japonicus, an Egg Parasitoid of Stink Bugs. Insects. 2021; 12(10):872. https://doi.org/10.3390/insects12100872
Chicago/Turabian StyleZhao, Can, Yi Guo, Zixin Liu, Yue Xia, Yuyan Li, Ziwei Song, Baoxin Zhang, and Dunsong Li. 2021. "Temperature and Photoperiodic Response of Diapause Induction in Anastatus japonicus, an Egg Parasitoid of Stink Bugs" Insects 12, no. 10: 872. https://doi.org/10.3390/insects12100872
APA StyleZhao, C., Guo, Y., Liu, Z., Xia, Y., Li, Y., Song, Z., Zhang, B., & Li, D. (2021). Temperature and Photoperiodic Response of Diapause Induction in Anastatus japonicus, an Egg Parasitoid of Stink Bugs. Insects, 12(10), 872. https://doi.org/10.3390/insects12100872






