The Effect of Mirid Density on Volatile-Mediated Foraging Behaviour of Apolygus lucorum and Peristenus spretus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Cotton Plants
2.2. Cotton Plants Treatment
2.3. Behavioral Assays
2.4. Headspace Volatile Collection and Analysis
2.5. Data Analysis
3. Results
3.1. Behavioral Responses of Mirids and Parasitoids to Cotton Plants Volatiles
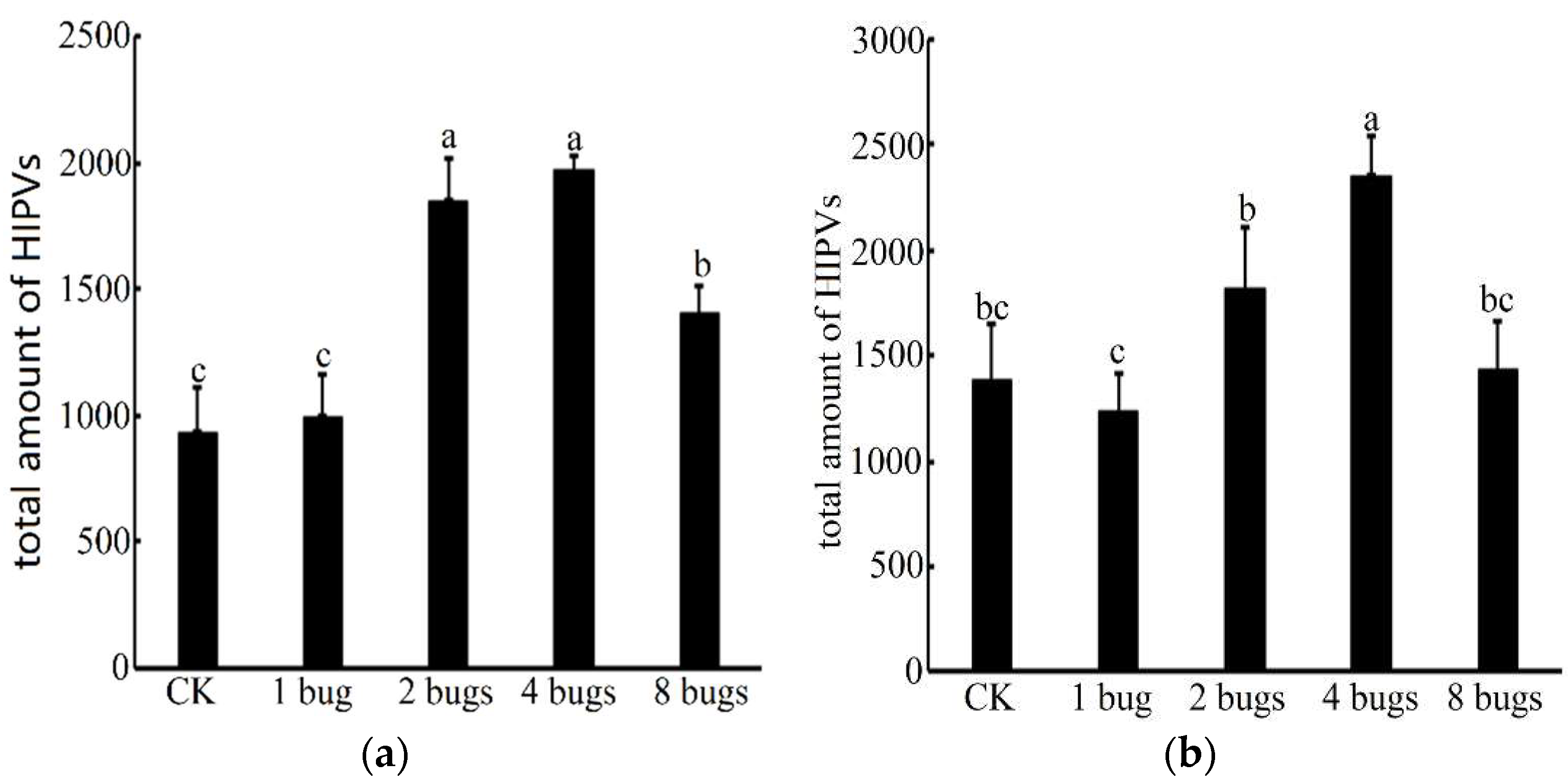
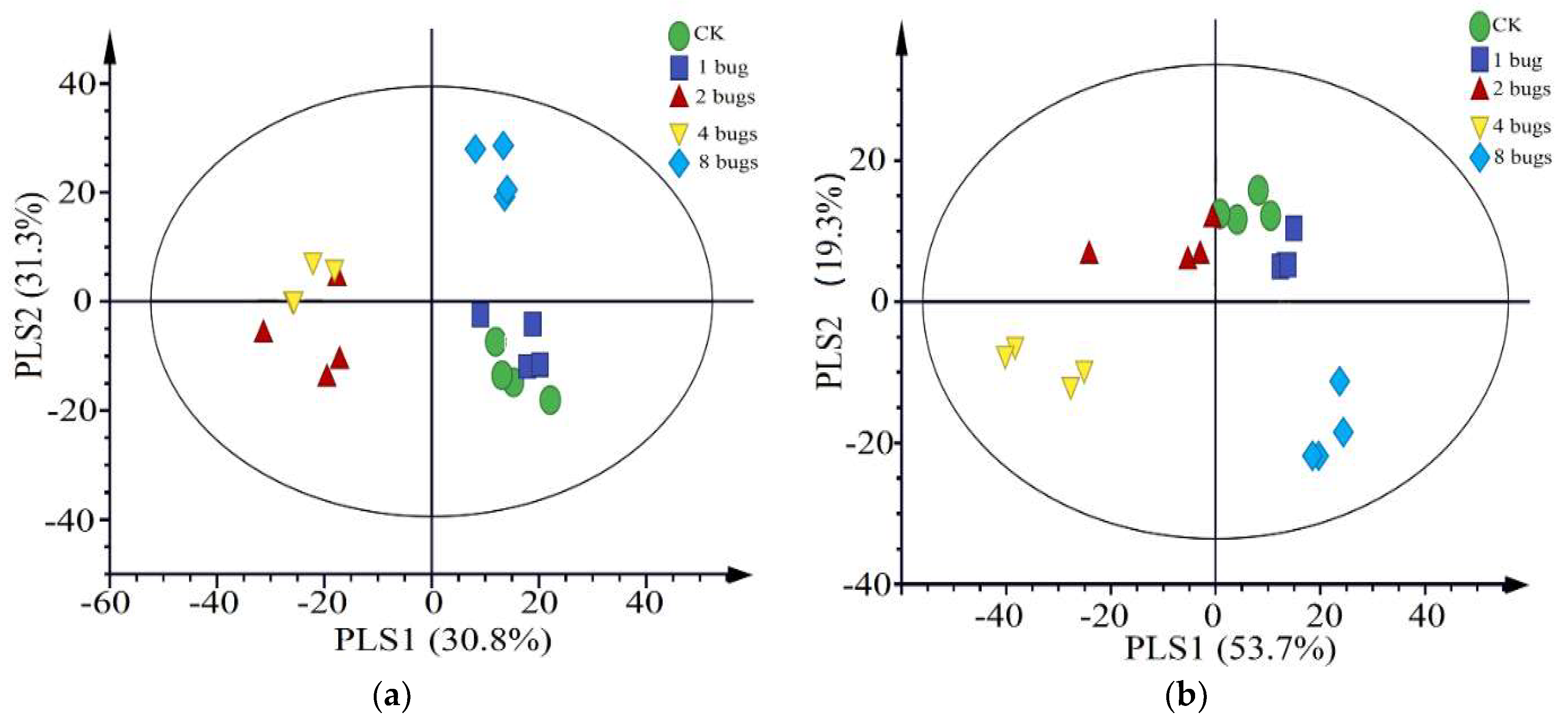
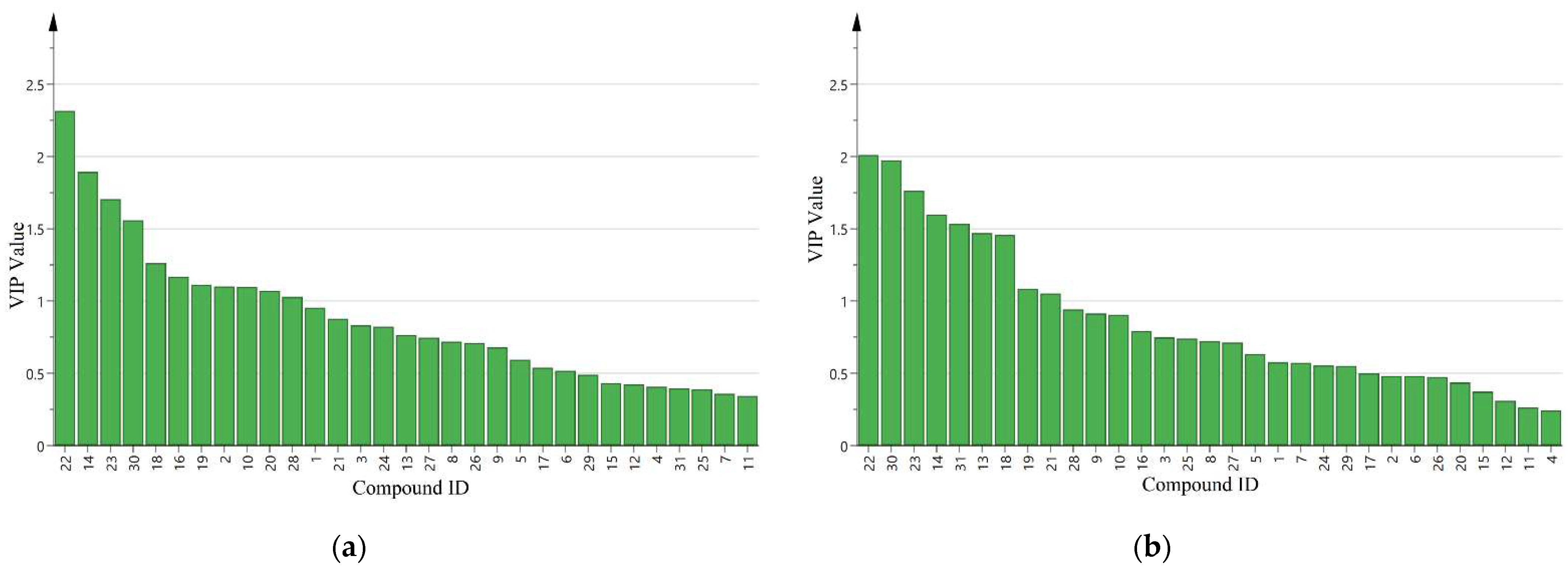
3.2. Volatile Cotton Emission Induced by Different Densities of A. lucorum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birkett, M.A.; Campbell, C.A.; Chamberlain, K.; Guerrieri, E.; Hick, A.J.; Martin, J.L.; Matthes, M.; Napier, J.A.; Pettersson, J.; Pickett, J.A.; et al. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 9329–9334. [Google Scholar] [CrossRef] [Green Version]
- Thaler, J.S.; Karban, R.; Ullman, D.E.; Boege, K.; Bostock, R.M. Cross-talk between jasmonate and salicylate plant defense pathways: Effects on several plant parasites. Oecologia 2002, 131, 227–235. [Google Scholar] [CrossRef]
- Dicke, M.; van Loon, J.J.; Soler, R. Chemical complexity of volatiles from plants induced by multiple attack. Nat. Chem. Biol. 2009, 5, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef]
- Hare, J.D. Ecological role of volatiles produced by plants in response to damage by herbivorous insects. Annu. Rev. Entomol. 2011, 56, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.; Crafts-Brandner, S.J.; Canas, L.A. Volatile emissions triggered by multiple herbivore damage: Beet armyworm and whitefly feeding on cotton plants. J. Chem. Ecol. 2003, 29, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- DeMoraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 2001, 410, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.I.; Kopke, S.; Kunert, M.; Volpe, V.; David, A.; Brand, P.; Dabrowska, P.; Maffei, M.E.; Boland, W. Effects of feeding Spodoptera littoralis on lima bean leaves: IV. Diurnal and nocturnal damage differentially initiate plant volatile emission. Plant Physiol. 2008, 146, 965–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martini, X.; Kuhns, E.H.; Hoyte, A.; Stelinski, L.L. Plant volatiles and density-dependent conspecific female odors are used by Asian citrus psyllid to evaluate host suitability on a spatial scale. Arthropod-Plant Interact. 2014, 8, 453–460. [Google Scholar] [CrossRef]
- Zhang, N.X.; VanWieringen, D.; Messelink, G.J.; Janssen, A. Herbivores avoid host plants previously exposed to their omnivorous predator Macrolophus pygmaeus. J. Pest Sci. 2019, 92, 737–745. [Google Scholar] [CrossRef] [Green Version]
- Brilli, F.; Ciccioli, P.; Frattoni, M.; Prestininzi, M.; Spanedda, A.F.; Loreto, F. Constitutive and herbivore-induced monoterpenes emitted by Populus x euroamericana leaves are key volatiles that orient Chrysomela populi beetles. Plant Cell Environ. 2009, 32, 542–552. [Google Scholar] [CrossRef]
- Arimura, G.; Kost, C.; Boland, W. Herbivore-induced, indirect plant defences. BBA-Mol. Cell Biol. Lipids 2005, 1734, 91–111. [Google Scholar] [CrossRef]
- Degenhardt, D.C.; Lincoln, D.E. Volatile emissions from an odorous plant in response to herbivory and methyl jasmonate exposure. J. Chem. Ecol. 2006, 32, 725–743. [Google Scholar] [CrossRef]
- Cai, X.M.; Sun, X.L.; Dong, W.X.; Wang, G.C.; Chen, Z.M. Herbivore species, infestation time, and herbivore density affect induced volatiles in tea plants. Chemoecology 2014, 24, 1–14. [Google Scholar] [CrossRef]
- Maffei, M.E.; Mithoefer, A.; Boland, W. Before gene expression: Early events in plant-insect interaction. Trends Plant Sci. 2007, 12, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Diezel, C.; VonDahl, C.C.; Gaquerel, E.; Baldwin, I.T. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol. 2009, 150, 1576–1586. [Google Scholar] [CrossRef] [Green Version]
- Loughrin, J.H.; Manukian, A.; Heath, R.R.; Turlings, T.C.; Tumlinson, J.H. Diurnal cycle of emission of induced volatile terpenoids by herbivore-injured cotton plant. Proc. Natl. Acad. Sci. USA 1994, 91, 18336–18340. [Google Scholar] [CrossRef] [Green Version]
- Mattiacci, L.; Rocca, B.A.; Scascighini, N.; D’Alessandro, M.; Hern, A.; Dorn, S. Systemically induced plant volatiles emitted at the time of “danger”. J. Chem. Ecol. 2001, 27, 2233–2252. [Google Scholar] [CrossRef]
- Shiojiri, K.; Ozawa, R.; Kugimiya, S.; Uefune, M.; VanWijk, M.; Sabelis, M.W.; Takabayashi, J. Herbivore-specific, density-dependent induction of plant volatiles: Honest or “cry wolf’” signals? PLoS ONE 2010, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xiu, C.L.; Xu, B.; Pan, H.S.; Zhang, W.; Yang, Y.Z.; Lu, Y.H. Volatiles from Sophora japonica flowers attract Harmonia axyridis adults (Coleoptera: Coccinellidae). J. Integr. Agric. 2019, 18, 873–883. [Google Scholar] [CrossRef] [Green Version]
- Kappers, I.F.; Hoogerbrugge, H.; Bouwmeester, H.J.; Dicke, M. Variation in herbivory-induced volatiles among cucumber (Cucumis sativus L.) varieties has consequences for the attraction of carnivorous natural enemies. J. Chem. Ecol. 2011, 37, 150–160. [Google Scholar] [CrossRef] [Green Version]
- Ingegno, B.L.; Pansa, M.G.; Tavella, L. Plant preference in the zoophytophagous generalist predator Macrolophus pygmaeus (Heteroptera: Miridae). Biol. Control 2011, 58, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Benelli, G.; Revadi, S.; Carpita, A.; Giunti, G.; Raspi, A.; Anfora, G.; Canale, A. Behavioral and electrophysiological responses of the parasitic wasp Psyttalia concolor (Szepligeti) (Hymenoptera: Braconidae) to Ceratitis capitata-induced fruit volatiles. Biol. Control 2013, 64, 116–124. [Google Scholar] [CrossRef]
- Lu, Y.H.; Wu, K.M.; Jiang, Y.Y.; Xia, B.; Li, P.; Feng, H.Q.; Wyckhuys, K.A.; Guo, Y.Y. Mirid bug outbreaks in multiple crops correlated with wide-scale adoption of Bt cotton in China. Science 2010, 328, 1151–1154. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.P.; Li, H.M.; Lu, Y.H.; Zhang, F.; Haye, T.; Kuhlmann, U.; Wu, K.M. Functional response and mutual interference of Peristenus spretus (Hymenoptera: Braconidae), a parasitoid of Apolygus lucorum (Heteroptera: Miridae). Biocontrol Sci. Technol. 2014, 24, 247–256. [Google Scholar] [CrossRef]
- Lu, Y.H.; Qiu, F.; Feng, H.Q.; Li, H.B.; Yang, Z.C.; Wyckhuys, K.A.; Wu, K.M. Species composition and seasonal abundance of pestiferous plant bugs (Hemiptera: Miridae) on Bt Cotton in China. Crop Prot. 2008, 27, 465–472. [Google Scholar] [CrossRef]
- Luo, S.P.; Zhang, F.; Wu, K.M. Effect of temperature on the reproductive biology of Peristenus spretus (Hymenoptera: Braconidae), a biological control agent of the plant bug Apolygus lucorum (Hemiptera: Miridae). Biocontrol Sci. Technol. 2015, 25, 1410–1425. [Google Scholar] [CrossRef]
- Loivamaeki, M.; Mumm, R.; Dicke, M.; Schnitzler, J.P. Isoprene interferes with the attraction of bodyguards by herbaceous plants. Proc. Natl. Acad. Sci. USA 2008, 105, 17430–17435. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, G.R.; Stone, N. Method for identification of spectral targets in discrete frequency infrared spectroscopy for clinical diagnostics. Appl. Spectrosc. 2015, 69, 1066–1073. [Google Scholar] [CrossRef]
- Tzin, V.; Hojo, Y.; Strickler, S.R.; Bartsch, L.J.; Archer, C.M.; Ahern, K.R.; Zhou, S.; Christensen, S.A.; Galis, I.; Mueller, L.A.; et al. Rapid defense responses in maize leaves induced by Spodoptera exigua caterpillar feeding. J. Exp. Bot. 2017, 68, 4709–4723. [Google Scholar] [CrossRef]
- Anderson, P.; Jonsson, M.; Morte, U. Variation in damage to cotton affecting larval feeding preference of Spodoptera littoralis. Entomol. Exp. Appl. 2001, 101, 191–198. [Google Scholar] [CrossRef]
- Girling, R.D.; Stewart, J.A.; Dherbecourt, J.; Staley, J.T.; Wright, D.J.; Poppy, G.M. Parasitoids select plants more heavily infested with their caterpillar hosts: A new approach to aid interpretation of plant headspace volatiles. Proc. R. Soc. B Biol. Sci. 2011, 278, 2646–2653. [Google Scholar] [CrossRef] [Green Version]
- Maeda, T.; Takabayashi, J. Production of herbivore-induced plant volatiles and their attractiveness to Phytoseius persimilis (Acari: Phytoseiidae) with changes of Tetranychus urticae (Acari: Tetranychidae) density on a plant. Appl. Entomol. Zool. 2001, 36, 47–52. [Google Scholar] [CrossRef]
- Uefune, M.; Nakashima, Y.; Tagashira, E.; Takabayashi, J.; Takagi, M. Response of Wollastoniella rotunda (Hemiptera: Anthocoridae) to volatiles from eggplants infested with its prey Thrips palmi and Tetranychus kanzawai: Prey species and density effects. Biol. Control 2010, 54, 19–22. [Google Scholar] [CrossRef]
- Bawin, T.; DeBacker, L.; Dujeu, D.; Legrand, P.; Megido, R.C.; Francis, F.; Verheggen, F.J. Infestation level influences oviposition site selection in the tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae). Insects 2014, 5, 877–884. [Google Scholar] [CrossRef]
- DeBacker, L.; Megido, R.C.; Fauconnier, M.L.; Brostaux, Y.; Francis, F.; Verheggen, F. Tuta absoluta-induced plant volatiles: Attractiveness towards the generalist predator Macrolophus pygmaeus. Arthropod-Plant Interact. 2015, 9, 465–476. [Google Scholar] [CrossRef]
- Turlings, T.C.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, G.; Huang, X.; Guo, H.; Su, X.; Han, L.; Zhang, Y.; Qi, Z.; Xiao, Y.; Cheng, H. Overexpression of the caryophyllene synthase gene GhTPS1 in cotton negatively affects multiple pests while attracting parasitoids. Pest Manag. Sci. 2020, 76, 1722–1730. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Kou, J.F.; Jing, W.X.; Han, X.Q.; Liu, D.F.; Ghasemzadeh, S.; Sun, P.; Shi, W.P.; Zhang, Y.J. Transcriptomic and metabolomic reprogramming in cotton after Apolygus lucorum feeding implicated in enhancing recruitment of the parasitoid Peristenus spretus. J. Pest. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Radhika, V.; Kost, C.; Bartram, S.; Heil, M.; Boland, W. Testing the optimal defence hypothesis for two indirect defences: Extrafloral nectar and volatile organic compounds. Planta 2008, 228, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Lou, Y.R.; Tzin, V.; Jander, G. Alteration of plant primary metabolism in response to insect herbivory. Plant Physiol. 2015, 169, 1488–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponzio, C.; Papazian, S.; Albrectsen, B.R.; Dicke, M.; Gols, R. Dual herbivore attack and herbivore density affect metabolic profiles of Brassica nigra leaves. Plant Cell Environ. 2017, 40, 1356–1367. [Google Scholar] [CrossRef] [Green Version]
- Pasquale, C.; Rieta, G.; Ninae, F.; Camille, P.; Icke, M.D.; Guerrier, E.M. The effect of rearing history and aphid density on volatile-mediated foraging behaviour of Diaeretiella rapa. Ecol. Entomol. 2019, 44, 255–264. [Google Scholar]
- Zalucki, M.P.; Clarke, A.R.; Malcolm, S.B. Ecology and behavior of first instar larval lepidoptera. Annu. Rev. Entomol. 2002, 47, 361–393. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Tena, A.; Xiu, C.; Liu, B.; Lu, Y.; Desneux, N. Floral feeding increases diet breadth in a polyphagous mirid. J. Pest. Sci. 2019, 92, 1089–1100. [Google Scholar] [CrossRef]
- Lou, Y.G.; Ma, B.; Cheng, J.A. Attraction of the parasitoid Anagrus nilaparvatae to rice volatiles induced by the rice brown planthopper Nilaparvata lugens. J. Chem. Ecol. 2005, 31, 2357–2372. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Su, H.; Zhang, S.; Jing, T.; Liu, Z.; Yang, Y. The Effect of Mirid Density on Volatile-Mediated Foraging Behaviour of Apolygus lucorum and Peristenus spretus. Insects 2021, 12, 870. https://doi.org/10.3390/insects12100870
Chen H, Su H, Zhang S, Jing T, Liu Z, Yang Y. The Effect of Mirid Density on Volatile-Mediated Foraging Behaviour of Apolygus lucorum and Peristenus spretus. Insects. 2021; 12(10):870. https://doi.org/10.3390/insects12100870
Chicago/Turabian StyleChen, Han, Honghua Su, Shuai Zhang, Tianxing Jing, Zhe Liu, and Yizhong Yang. 2021. "The Effect of Mirid Density on Volatile-Mediated Foraging Behaviour of Apolygus lucorum and Peristenus spretus" Insects 12, no. 10: 870. https://doi.org/10.3390/insects12100870
APA StyleChen, H., Su, H., Zhang, S., Jing, T., Liu, Z., & Yang, Y. (2021). The Effect of Mirid Density on Volatile-Mediated Foraging Behaviour of Apolygus lucorum and Peristenus spretus. Insects, 12(10), 870. https://doi.org/10.3390/insects12100870





