Differences in Gall Induction of Flower-like Galls on Haloxylon by Psyllids (Hemiptera: Aphalaridae), and the Emergence of Corresponding Parasitoids
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling Collections
2.2. Laboratory Rearing of the Galls
2.3. Identification of Gall Inducers and Parasitoids
2.4. Parasitization Indexes and Eclosion Rates of Parasitoids in Flower-like Galls of Haloxylon
2.5. Statistical Analysis
3. Results
3.1. The Occurrence of Flower-like Galls of Haloxylon
3.2. Species of Gall Inducers and Parasitoids of Flower-like Galls on Haloxylon
3.3. Parasitization Indexes of Parasitoids Recorded from Flower-like Galls on Haloxylon
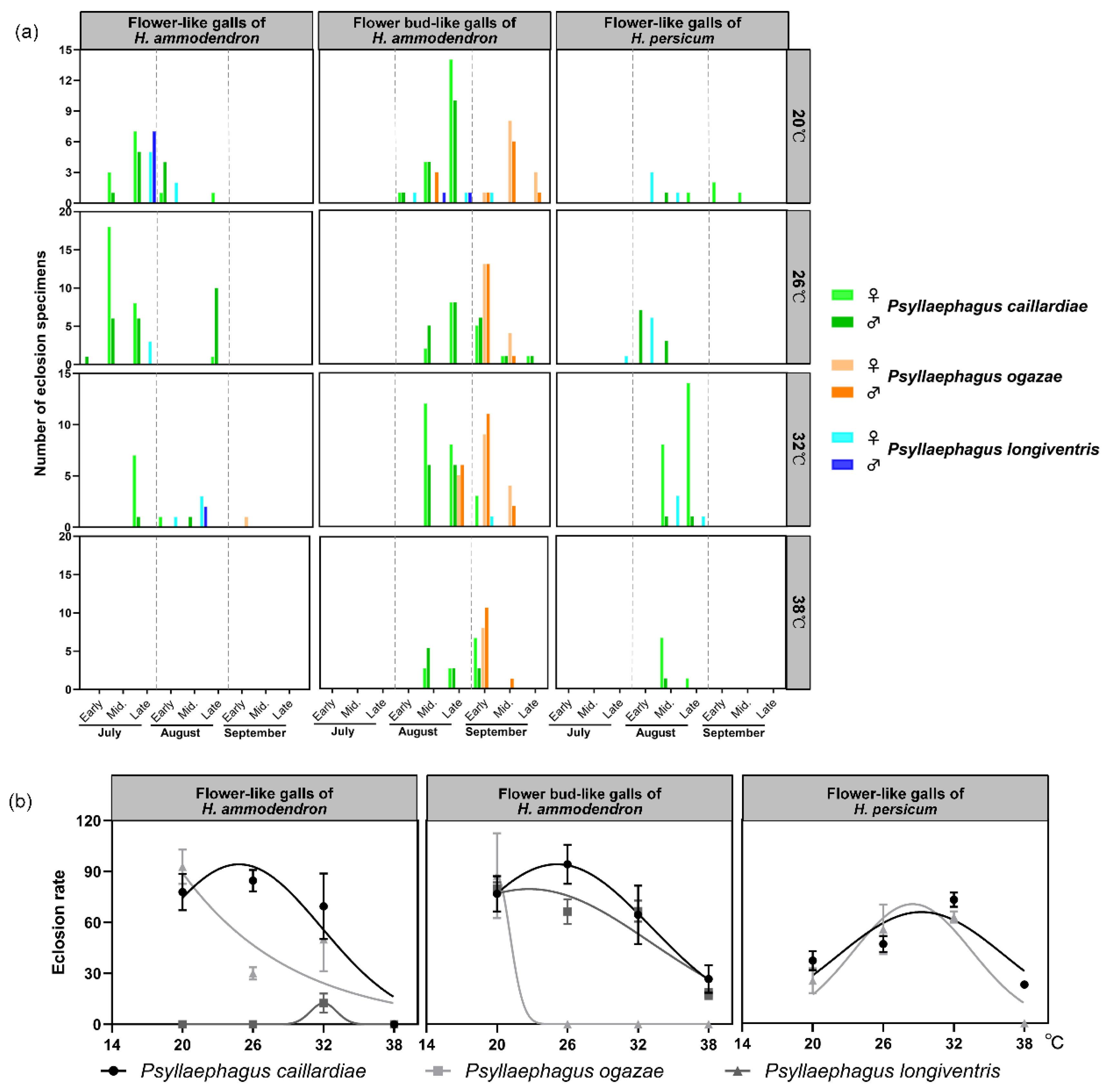
3.4. Effect of Temperature on the Emergence of Parasitoids from Flower-like Galls on Haloxylon
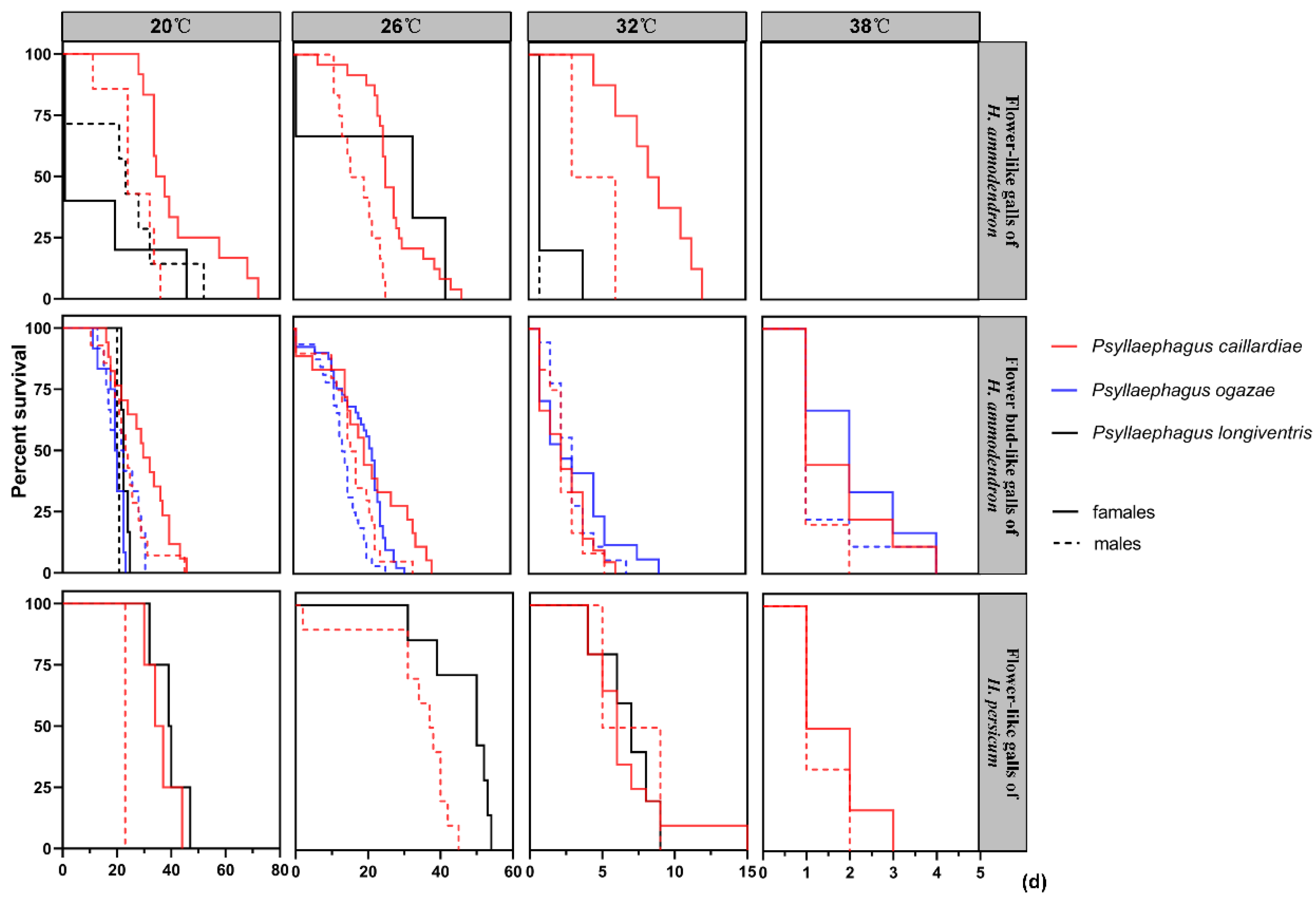
3.5. Effect of Temperature on the Lifespans of Adult Parasitoids Recovered from the Flower-like Galls on Haloxylon
3.6. Gall Inducers and Parasitoid Species Characterization
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Zheng, X.J.; Li, Y.; Xu, G.Q. Varying responses of two Haloxylon species to extreme drought and groundwater depth. Environ. Exp. Bot. 2019, 158, 63–72. [Google Scholar] [CrossRef]
- Buras, A.; Wucherer, W.; Zerbe, S.; Noviskiy, Z.; Muchitdinov, N.; Shimshikov, B.; Zverev, N.; Schmidt, S.; Wilmking, M.; Thevs, N. Allometric variability of Haloxylon species in Central Asia. For. Ecol. Manag. 2012, 274, 1–9. [Google Scholar] [CrossRef]
- Jia, Z.G.; Lu, Q.; Guo, B.G.; Zhao, M.; Lang, Y.Q. Progress in the study of psammophyte-Haloxylon. For. Res. 2004, 17, 125–132. [Google Scholar]
- Saji, A.; Sakkir, S.; Dhaheri, S.S.A. Galling insects associated with Haloxylon spp. (Bunge) in Abu Dhabi, United Arab Emirates. Int. J. Biodivers. Conserv. 2013, 5, 89–295. [Google Scholar] [CrossRef]
- Skuhrava, M.; Karimpour, Y.; Sadeghi, H.; Gol, A.; Joghataie, M. Gall midges (Diptera: Cecidomyiidae) of Iran: Annotated list and zoogeographical analysis. Acta Soc. Zool. Bohem. 2014, 78, 269–301. [Google Scholar]
- Slepyan, E.I. Specific characteristics of gall-forming and teratogenic processes on the assimilational shoots of Haloxylon aphyllum and H. persicum Chenopodiaceae Referat. Bot Zhur. 1958, 43, 1595–1607. [Google Scholar]
- Mohammadi, M. Phenological relation of the pests associated with saxaul plant in Abardeg area. Iran. J. Range Desert Res. 2003, 10, 17–38. [Google Scholar]
- Xue, X.F.; Zhang, J.P.; Li, F.L.; Hong, X.-Y. A new eriophyoid mite species (Acari: Eriophyidae) infesting Haloxylon ammodendron and H. persicum (Chenopodiaceae) in Xinjiang Uigur Autonomous Region, northwest China. Syst. Appl. Acarol 2012, 17, 202–209. [Google Scholar] [CrossRef]
- Li, F.L.; Wu, X.h.; Wang, P.L.; Liang, H.J.; Wang, X.; Wang, L.J.; Zhang, J.P. The occurrence regularity of psyllid in Haloxylon spp and its influencing factors. Acta Ecol. Sin. 2012, 32, 2311–2319. [Google Scholar]
- Li, F.L.; Li, T.; Su, J.; Yang, S.; Wang, P.L.; Zhang, J.P. Temporal and spatial differences in gall induction on Haloxylon by Aceria haloxylonis (Acari: Eriophyidae) in the Gurbantunggut Desert. Syst. Appl. Acarol 2016, 21, 1670–1680. [Google Scholar] [CrossRef][Green Version]
- Sagar, D.; Balikai, R.A. Psyllid pests of horticultural and forage crops: Taxonomy, biology and their management. J. Exp. Zool. India 2013, 16, 1–18. [Google Scholar]
- Geiger, C.A.; Gutierrez, A.R. Ecology of Heteropsylla cubana (Homoptera: Psyllidae): Psyllid damage, tree phenology, thermal relations, and parasitism in the field. Environ. Entomol. 2000, 29, 76–86. [Google Scholar] [CrossRef]
- Jarausch, B.; Burckhardt, D.; Lauterer, P.; Jarausch, W. Psyllids (Hemiptera, Psylloidea) captured in commercial apple and stone fruit orchards in southwest Germany, eastern France and northwest Switzerland. Mitt. Schweiz. Entomol. Ges. 2009, 82, 205–215. [Google Scholar]
- Hoy, M.A.; Singh, R.; Rogers, M.E. Evaluations of a novel isolate of Isaria fumosorosea for control of the asian citrus psyllid, Diaphorina citri (hemiptera: Psyllidae). Fla. Entomol. 2010, 93, 24–32. [Google Scholar] [CrossRef]
- Shivankar, V.J.; Rao, C.N. Psyllids and their management. Pest Manag. Hortic. Ecosyst. 2010, 16, 1–4. [Google Scholar]
- Yana, W.; Tamesse, J.L.; Burckhardt, D. Jumping Plant-Lice of the Family Psyllidae Latreille (Hemiptera: Psylloidea) from the Center Region of Cameroon: Faunistics, Phenology and Host Plants. J. Entomol. 2010, 7, 1–18. [Google Scholar] [CrossRef]
- Spodek, M.; Burckhardt, D.; Protasov, A.; Mendel, Z. First record of two invasive eucalypt psyllids (Hemiptera: Psylloidea) in Israel. Phytoparasitica 2015, 43, 401–406. [Google Scholar] [CrossRef]
- Lashkari, M.; Burckhardt, D.; Manzari, S. First report of ten psyllid species (Hemiptera: Psylloidea) from Kerman province, Iran. J. Entomol. Res. 2016, 8, 223–235. [Google Scholar]
- Loginova, M.M.; Parfentiev, V.J. Species of the genus Caillardia Bergevin (Homoptera, Psyllidae) injurious to Haloxylon. Entomol. Obozr. 1956, 35, 377–396. [Google Scholar]
- Loginova, M.M. Review of the genus Caillardia Bergevin (Homoptera, Aphalaridae) with descriptions of new species. Tr. Zool. Inst. 1978, 71, 6–22. [Google Scholar]
- Spodek, M.; Burckhardt, D.; Freidberg, A. The Psylloidea (Hemiptera) of Israel. Zootaxa 2017, 4276, 301. [Google Scholar] [CrossRef]
- Sultanov, R.A.; Danilevich, O.K. Peculiarities of biology of two psyllids species of Caillardia Bergevin genus on the Haloxylon aphyllum (Minkw.) Iljin in southern Kyzylkum. Uzb. Biol. Zh. 1995, 4–5, 62–65. [Google Scholar]
- Kaplin, V.G. Complexes of Arthropoda animals inhabiting galls of Haloxylon Psyllidae of genus Caillardia Bergev. in eastern Kara-Kum. Izv. Akad. Nauk Turkm. SSR Ser. Biol. Nauk 1981, 20–27. [Google Scholar]
- Passos, L.C.; Soares, M.A.; Collares, L.J.; Malagoli, I.; Desneux, N.; Carvalho, G.A. Lethal, sublethal and transgenerational effects of insecticides on Macrolophus basicornis, predator of Tuta absoluta. Entomol. Gen. 2018, 38, 127–143. [Google Scholar] [CrossRef]
- Soares, M.A.; Campos, M.R.; Passos, L.C.; Carvalho, G.A.; Haro, M.M.; Lavoir, A.V.; Biondi, A.; Zappala, L.; Desneux, N. Botanical insecticide and natural enemies: A potential combination for pest management against Tuta absoluta. J. Pest Sci. 2019, 92, 1445. [Google Scholar] [CrossRef]
- Dhawan, A.K.; Singh, S.; Kumar, S. Integrated Pest Management (IPM) helps reduce pesticide load in cotton. J. Agric. Sci. Technol. 2009, 11, 599–611. [Google Scholar]
- Daniel, C.; Grunder, J. Integrated management of european cherry fruit fly Rhagoletis cerasi (L.): Situation in Switzerland and Europe. Insects 2012, 3, 956–988. [Google Scholar] [CrossRef]
- Chen, H.Y.; Li, H.L.; Pang, H.; Zhu, C.D.; Zhang, Y.Z. Investigating the Parasitoid community associated with the invasive mealybug Phenacoccus solenopsis in Southern China. Insects 2021, 12, 290. [Google Scholar] [CrossRef]
- Cao, Y.; Ren, J.; Cui, Z.; Chen, W. The development of IPM in our country and pests cortrol. Syst. Sci. Compr. Stud. Agric. 2002, 18, 69–70. [Google Scholar]
- Paparella, F.; Ferracini, C.; Portaluri, A.; Manzo, A.; Alma, A. Biological control of the chestnut gall wasp with T. sinensis: A mathematical model. Ecol. Model. 2016, 338, 17–36. [Google Scholar] [CrossRef]
- Kos, K.; Kriston, E.; Melika, G. Invasive chestnut gall wasp Dryocosmus kuriphilus (Hymenoptera: Cynipidae), its native parasitoid community and association with oak gall wasps in Slovenia. Eur. J. Entomol. 2015, 112, 698–704. [Google Scholar] [CrossRef]
- Nieves-Aldrey, J.L.; Gil-Tapetado, D.; Gavira, O.; Boyero, J.R.; Polidori, C.; Lombardero, M.J.; Blanco, D.; del Castillo, C.R.; Rodriguez-Rojo, P.; Vela, J.M.; et al. Torymus sinensis Kamijo, a biocontrol agent against the invasive chestnut gall wasp Dryocosmus kuriphilus Yasumatsu in Spain: Its natural dispersal from France and first data on establishment after experimental releases. For. Syst. 2019, 28, 3. [Google Scholar] [CrossRef]
- Bonsignore, C.P.; Bernardo, U. Effects of environmental parameters on the chestnut gall wasp and its complex of indigenous parasitoids. Sci. Nat. 2018, 105, 20. [Google Scholar] [CrossRef]
- Bhede, B.V.; Bhosle, B.B.; Shinde, S.T.; Sharma, O.P. Ecofriendly integrated pest management in pigeonpea. J. Entomol. Res. 2014, 38, 259–263. [Google Scholar]
- Triapitsyn, V.A. Parasitic Hymenoptera of the family Encyrtidae of Palaearctics. Opredelitelipo Faune SSSR Izdavaemiye Zoologiya 1989, 158, 251–263. [Google Scholar]
- Triapitsyn, S.V. A new species of the genus Aprostocetus westwood, 1833 (Hymenoptera, Eulophidae: Tetrastichinae) collected by E. S. Sugonjaev in galls on saxauls in Uzbekistan. Entomol. Obozr. 2015, 94, 455–458. [Google Scholar] [CrossRef]
- Noyes, J.S.; Hayat, M. A review of the genera of Indo-Pacific Encyrtidae (Hymenoptera: Chalcidoidea). Bull. Br. Mus. Entomol. 1984, 48, 131–395. [Google Scholar]
- Noyes, J.S.; Woolley, J.B.; Zolnerowic, G. Encyrtidae. In Annotated Keys to the Genera of Nearctic Chalcidoidea (Hymenoptera); Gibson, G.A.P., Huber, J.T., Woolley, J.B., Eds.; NRC Research Press: Ottawa, ON, Canada, 1997. [Google Scholar]
- Muhetaier, N.; Chen, G.-H.; Peng, J.; Gao, B.; Zhang, X.D.; Xi, O.Y.; Aishan, J.; Hu, H.Y. DNA barcoding of Anagrus dmitrievi (Hymenoptera, Mymaridae). Chin. J. Appl. Entomol. 2021, 58, 335–346. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, X.; Feng, G.; Hu, H.; Niu, L.; Hebert, P.D.N.; Huang, D. COI and ITS2 sequences delimit species, reveal cryptic taxa and host specificity of fig-associated Sycophila (Hymenoptera, Eurytomidae). Mol. Ecol. Resour. 2010, 10, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Rugman-Jones, P.F.; Hoddle, M.S.; Stouthamer, R. Nuclear-Mitochondrial barcoding exposes the global pest western flower thrips (Thysanoptera: Thripidae) as two sympatric cryptic species in its native California. J. Econ. Entomol. 2010, 103, 877. [Google Scholar] [CrossRef] [PubMed]
- Triapitsyn, S.V.; Rugman-Jones, P.F.; Tretiakov, P.S.; Daane, K.M.; Wilson, H. Reassessment of molecular and morphological variation within the Anagrus atomus species complex (Hymenoptera: Mymaridae): Egg parasitoids of leafhoppers (Hemiptera: Cicadellidae) in Europe and North America. J. Nat. Hist. 2020, 54, 1735–1758. [Google Scholar] [CrossRef]
- Morse, J.G.; Rugman-Jones, P.F.; Woolley, J.B.; Heraty, J.M.; Triapitsyn, S.V.; Hofshi, R.; Stouthamer, R. Armored scales and their parasitoids on commercial avocados grown in California or imported from Mexico. J. Econ. Entomol. 2016, 109, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Colazza, S.; Bin, F. Efficiency of Trissolcus basalis (Hymenoptera: Scelionidae) as an egg parasitoid of Nezara viridula (Heteroptera: Pentatomidae) in Central Italy. Environ. Entomol. 1995, 24, 1703–1707. [Google Scholar] [CrossRef]
- Virla, E.G.; Van Nieuwenhove, G.A.; Palottini, F.; Triapitsyn, S.V.; Logarzo, G.A. Spatial and seasonal distribution of egg parasitoids of the sharpshooter Tapajosa rubromarginata (Hemiptera: Cicadellidae: Proconiini) on feral Johnson grass and commercial citrus host in Argentina. Biol. Control 2019, 132, 81–88. [Google Scholar] [CrossRef]
- Burckhardt, D.; Ouvrard, D.; Queiroz, D.; Percy, D. Psyllid host-plants (Hemiptera: Psylloidea): Resolving a semantic problem. Fla. Entomol. 2014, 97, 242–246. [Google Scholar] [CrossRef]
- Hodkinson, I.D. The biology and ecology of the gall-forming Psylloidea (Homoptera). In Biology of Gall Insects; Ananthakrishnan, T.N., Ed.; Oxford University Press: London, UK, 1984. [Google Scholar]
- Yang, M.M.; Raman, A. Diversity, richness, and patterns of radiation among gall-inducing psyllids (Hemiptera: Psylloidea) in the orient and eastern Palearctic. Orient. Insects 2007, 41, 55–65. [Google Scholar] [CrossRef]
- Yang, M.M.; Liao, L.H.; Lou, M.F.; Chen, W.C.; Huang, S.S.; Tung, G.S.; Weng, Y.C.; Shen, C.C. Diversity, Biology, and Nutritional Adaptation of Psyllids and their Galls in Taiwan. In Galling Arthropods and Their Associates; Ozaki, K., Yukawa, J., Ohgushi, T., Price, P.W., Eds.; Springer: Tokyo, Japan, 2006. [Google Scholar]
- Sardon-Gutierrez, S.; Gil-Tapetado, D.; Gomez, J.F.; Nieves-Aldrey, J.L. Ecological niche modelling of species of the rose gall wasp Diplolepis (Hymenoptera: Cynipidae) on the Iberian Peninsula. Eur. J. Entomol. 2021, 118, 31–45. [Google Scholar] [CrossRef]
- Cho, G.; Malenovský, I.; Burckhardt, D.; Inoue, H.; Lee, S. DNA barcoding of pear psyllids (Hemiptera: Psylloidea: Psyllidae), a tale of continued misidentifications. Bull. Entomol. Res. 2020, 110, 521–534. [Google Scholar] [CrossRef]
- Purcell, M.F.; Thornhill, A.H.; Wallenius, T.C.; Yeates, D.K.; Rowell, D.M. Plant host relationships of three lineages of the gall-inducing fly Fergusonina Malloch (Diptera: Fergusoninidae) on Eucalyptus L’Hérit. Arthropod-Plant Interact. 2017, 12, 133–145. [Google Scholar] [CrossRef]
- Moraglio, S.T.; Tortorici, F.; Pansa, M.G.; Castelli, G.; Pontini, M.; Scovero, S.; Visentin, S.; Tavella, L. A 3-year survey on parasitism of Halyomorpha halys by egg parasitoids in northern Italy. J. Pest Sci. 2020, 93, 183–194. [Google Scholar] [CrossRef]
- Stone, G.N.; Schonrogge, K. The adaptive significance of insect gall morphology. Trends Ecol. Evol. 2003, 18, 512–522. [Google Scholar] [CrossRef]
- Hernández-López, M.; Hernández-Ortiz, V.; Castillo-Campos, G.; Fernandes, G.W. Size matters: Larger galls produced by Eutreta xanthochaeta (Diptera: Tephritidae) on Lippia myriocephala (Verbenaceae) predict lower rates of parasitic wasps. Arthropod-Plant Interact. 2021, 15, 615–625. [Google Scholar] [CrossRef]
- Joseph, M.B.; Gentles, M.; Pearse, I.S. The parasitoid community of Andricus quercuscalifornicus and its association with gall size, phenology, and location. Biodivers. Conserv. 2011, 20, 203–216. [Google Scholar] [CrossRef]
- Laszlo, Z.; Tothmeresz, B. The enemy hypothesis: Correlates of gall morphology with parasitoid attack rates in two closely related rose cynipid galls. Bull. Entomol. Res. 2013, 103, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Yao, Z.; Ren, Y.; Jiang, Z.; Gu, Y.; Wang, B.; Zhang, H. Study on the Structure Characteristics and Growth and Development of Leafy Galls in Haloxylon ammodendron. Acta Bot. Boreali-Occident. Sin. 2017, 37, 1749–1755. [Google Scholar]
- Tang, X.; Zhang, Y.; Hu, H. Seven new record species of Psyllaephagus (Hymenoptera: Encyrtidae) from China. Entomotaxonomia 2016, 38, 63–78. [Google Scholar] [CrossRef]
- Wang, J.L.; Lao, G.F.; Li, Y.W.; Yang, M.; Mo, Z.Q.; Dan, X.M. Effects of temperature and host species on the life cycle of Cryptocaryon irritans. Aquaculture 2018, 485, 49–52. [Google Scholar] [CrossRef]
- Gopko, M.; Mironova, E.; Pasternak, A.; Mikheev, V.; Taskinen, J. Parasite transmission in aquatic ecosystems under temperature change: Effects of host activity and elimination of parasite larvae by filter-feeders. Oikos 2020, 129, 1531–1540. [Google Scholar] [CrossRef]
- Bari, M.N.; Jahan, M.; Islam, K.S. Effects of Temperature on the life table parameters of Trichogramma zahiri (Hymenoptera: Trichogrammatidae), an egg parasitoid of Dicladispa armigera (Chrysomelidae: Coleoptera). Environ. Entomol. 2015, 44, 368–378. [Google Scholar] [CrossRef]
- Dang Hoa, T.; Khac, P.; Ueno, T.; Takagi, M. Effects of temperature and host on the immature development of the parasitoid Neochrysocharis okazakii (Hymenoptera: Eulophidae). J.-Fac. Agric. Kyushu Univ. 2012, 57, 133–137. [Google Scholar]
- Quacchia, A.; Moriya, S.; Bosio, G.; Scapin, I.; Alma, A. Rearing, release and settlement prospect in Italy of Torymus sinensis, the biological control agent of the chestnut gall wasp Dryocosmus kuriphilus. BioControl 2008, 53, 829–839. [Google Scholar] [CrossRef]
- Sangtongpraow, B.; Charernsom, K. Biological traits of Quadrastichus mendeli (Hymenoptera, Eulophidae), parasitoid of the eucalyptus gall wasp Leptocybe invasa (Hymenoptera, Eulophidae) in Thailand. Parasite 2019, 26, 8. [Google Scholar] [CrossRef] [PubMed]

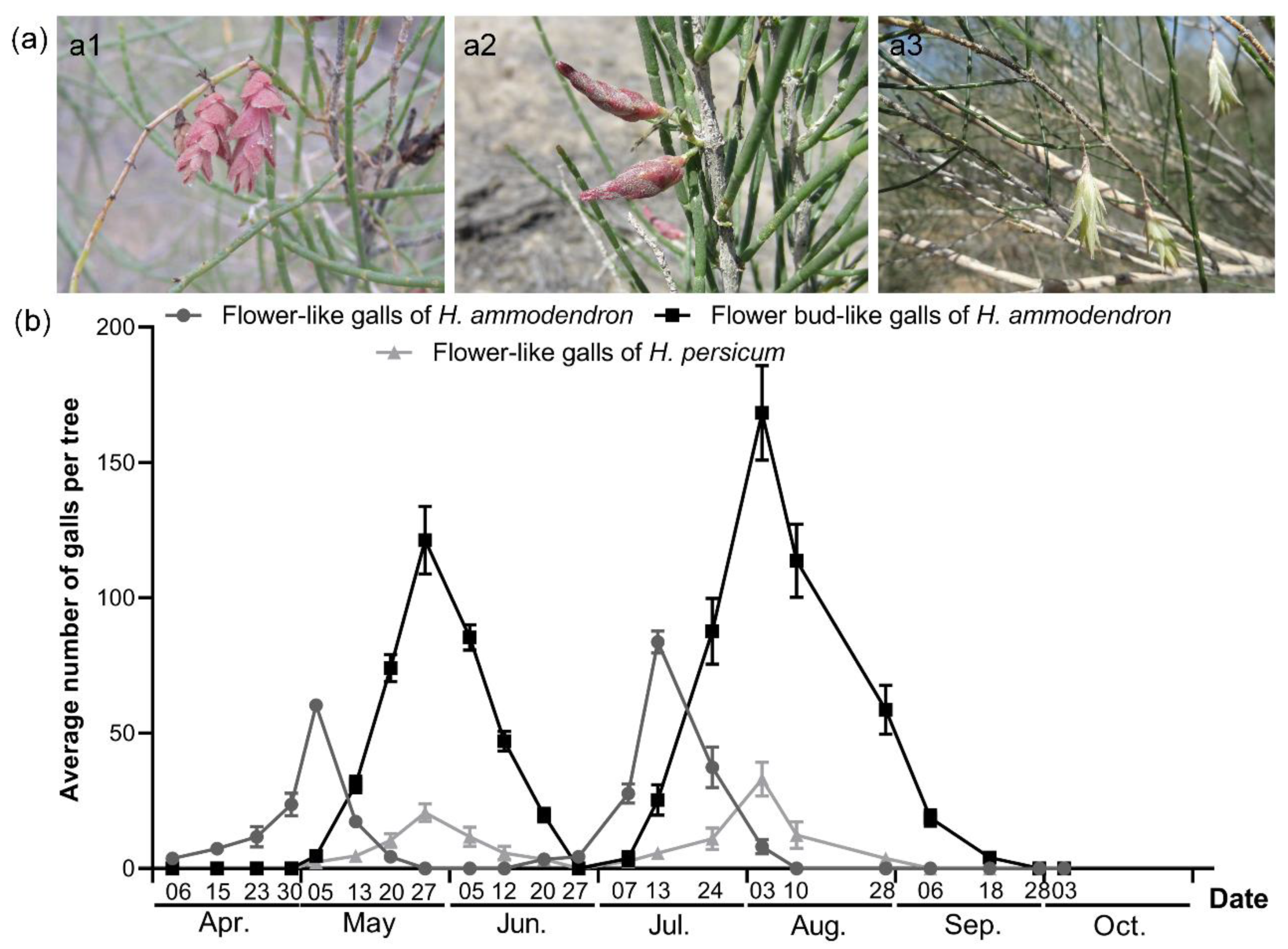
| Study Site | Coordinates | Altitude (m) | Habitat Type | Sampling Areas (m3) | Main Vegetation | Gall Collected |
|---|---|---|---|---|---|---|
| S1: Beishawo Desert of Fukang city in Xinjiang | E 87°53′6″ N 44°22′26″ | 374 | Saline-alkali desertification | 100 | Haloxylon Halimodendron Alhagi Salsola Tamarix | Flower-like galls of H. ammodendron, Flower bud-like galls of H. ammodendron |
| S2: Fukang National Field Scientific Observation and Research Station for Desert Ecosystems in Xinjiang | E 87°55′45″ N 44°22′0″ | 408 | Saline-alkali desertification | 100 | Haloxylon Halimodendron Salsola Tamarix | Flower-like galls of H. persicum |
| Type of Galls | Gall Inducers (Number of Adults) | Parasitoids (Number of Adults and Sex Ratio) | Generation of Gall Inducers | Overwintering Mode of Gall Inducers | Host Stage Attacked (Instar) | Habit of Parasitoids | Deworming State (Host) | Deworming State (Parasitoid) | Identifier |
|---|---|---|---|---|---|---|---|---|---|
| Flower-like galls of H. ammodendron | C.anabasidis (146) C. azurea (58) | P. caillardiae (♀47♂35) P. ogazae (♀1♂0) P. longiventris (♀14♂9) | 2 | Adults in the dead grass or the bark of the H. ammodendron | Nymph (III, IV) | Primary, solitary, endoparasitoid | Nymph | Adult | S.V. Triapitsyn and H.-Y. Hu |
| Flower bud-like galls of H. ammodendron | C. robusta (12) C. nana (189) | P. caillardiae (♀68♂56) P. ogazae (♀53♂54) P. longiventris (♀4♂2) | 2 | Adults in the dead grass or the bark of the H. ammodendron | Nymph (III, IV) | Primary, solitary, endoparasitoid | Nymph | Adult | S.V. Triapitsyn and H.-Y. Hu |
| Flower-like galls of H. persicum | C. notata (161) | P. caillardiae (♀32♂14) P. longiventris (♀15♂0) | 2 | Adults in the dead grass or the bark of the H. persicum | Nymph (IV) | Primary, solitary, endoparasitoid | Nymph | Adult | S.V. Triapitsyn and H.-Y. Hu |
| Temperature | Date | Psyllaephagus caillardiae | Psyllaephagus ogazae | Psyllaephagus longiventris | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DE | EE | PI | RI | DE | EE | PI | RI | DE | EE | PI | RI | ||
| Flower-like galls of H. ammodendron | 07/July/2018 | 23.38 | 96.67 | 18.81 | 4.61(F) | / | / | / | / | 6.25 | 50.00 | 1.76 | 0.60(S) |
| 13/July/2018 | 54.29 | 74.69 | 40.87 | 22.89(VF) | 0.72 | 25.00 | 0.48 | 0.01(S) | 12.14 | 82.64 | 10.03 | 1.34(F) | |
| 24/July/2018 | 35.19 | 95.83 | 34.33 | 12.08(VF) | 7.41 | 100 | 5.97 | 0.44(S) | 11.11 | 100.00 | 8.96 | 1.00(S) | |
| 03/August/2018 | 16.67 | 100.00 | 16.67 | 2.78(F) | 16.67 | 100.00 | 16.67 | 2.78(F) | / | / | / | / | |
| Total | 32.38 | 91.80 | 27.67 | 10.59(VF) | 8.27 | 75.00 | 7.71 | 1.08(F) | 9.83 | 77.55 | 6.92 | 0.98(S) | |
| Flower bud-like galls of H. ammodendron | 24/July/2018 | 66.67 | 100.00 | 66.67 | 44.44(VF) | / | / | / | / | 33.33 | 100.00 | 33.33 | 11.11(VF) |
| 03/August/2018 | 1.67 | 16.67 | 7.14 | 0.48(S) | 1.67 | 16.67 | 3.13 | 0.21(S) | 4.17 | 25.00 | 7.81 | 1.30(F) | |
| 10/August/2018 | 32.58 | 75.76 | 26.38 | 9.21(F) | 28.57 | 57.61 | 20.08 | 7.3(F) | / | / | / | / | |
| 18/August/2018 | 58.97 | 100.00 | 59.80 | 36.54(VF) | 16.67 | 75.00 | 16.49 | 3.80 F) | 1.28 | 25.00 | 1.28 | 0.07(R) | |
| 28/August/2018 | 23.89 | 100.00 | 24.16 | 6.37(F) | 44.97 | 100.00 | 44.97 | 21.49(VF) | 0.89 | 25.00 | 0.89 | 0.03(R) | |
| Total | 36.76 | 78.49 | 36.83 | 19.41(VF) | 22.97 | 62.32 | 21.17 | 8.21(F) | 9.92 | 43.75 | 10.83 | 3.13 (F) | |
| Flower-like gall of H. persicum | 24/July/2018 | 20.00 | 100.00 | 20.00 | 0.04(R) | / | / | / | / | 11.67 | 100.00 | 11.67 | 0.01(R) |
| 03/August/2018 | 25.73 | 100.00 | 25.73 | 0.07(R) | / | / | / | / | 10.54 | 100.00 | 10.54 | 0.01(R) | |
| 11/August/2018 | 54.63 | 98.53 | 55.47 | 0.30(S) | / | / | / | / | 7.41 | 50.00 | 6.67 | 0.01(R) | |
| Total | 33.45 | 99.51 | 33.73 | 0.14(S) | / | / | / | / | 9.87 | 83.33 | 9.63 | 0.01(R) | |
| Species | No. Identified by Molecular Methods | Sex | Associated Host | CO1 GenBank Accession No. | 28S GenBank Accession No. |
|---|---|---|---|---|---|
| P. caillardiae | 3 | 1♂2♀ | Flower-like galls H. ammodendron Flower bud-like galls of H. ammodendron | MZ436006 MZ438311 | MZ436078 MZ469911 MZ469912 |
| P. ogazae | 4 | 2♂2♀ | Flower bud-like galls of H. ammodendron | / | MZ436075 MZ469916 MZ469909 MZ469905 |
| P. longiventris | 3 | 1♂2♀ | Flower-like galls of H. persicum | / | MZ436079 MZ469917 |
| C. anabasidis | 3 | 2♂1♀ | Flower-like galls H. ammodendron | MZ436072 MZ437083 | MZ436073 MZ469908 MZ469903 |
| C. azurea | 3 | 2♂1♀ | Flower-like galls H. ammodendron | MZ436003 MZ437085 MZ438310 | MZ436076 MZ469907 |
| C. robusta | 4 | 1♂3♀ | Flower bud-like galls of H. ammodendron | MZ436005 | MZ436077 MZ469915 MZ469904 |
| C. nana | 3 | 2♂1♀ | Flower bud-like galls of H. ammodendron | / | MZ436074 MZ469914 MZ469910 |
| C. notate | 5 | 3♂2♀ | Flower-like galls of H. persicum | MZ436004 | MZ436080 MZ469906 MZ469913 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Jiang, L.-L.; Guo, J.; Zhang, D.-K.; Hu, H.-Y. Differences in Gall Induction of Flower-like Galls on Haloxylon by Psyllids (Hemiptera: Aphalaridae), and the Emergence of Corresponding Parasitoids. Insects 2021, 12, 861. https://doi.org/10.3390/insects12100861
Zhao Q, Jiang L-L, Guo J, Zhang D-K, Hu H-Y. Differences in Gall Induction of Flower-like Galls on Haloxylon by Psyllids (Hemiptera: Aphalaridae), and the Emergence of Corresponding Parasitoids. Insects. 2021; 12(10):861. https://doi.org/10.3390/insects12100861
Chicago/Turabian StyleZhao, Qian, Ling-Ling Jiang, Jie Guo, Dong-Kang Zhang, and Hong-Ying Hu. 2021. "Differences in Gall Induction of Flower-like Galls on Haloxylon by Psyllids (Hemiptera: Aphalaridae), and the Emergence of Corresponding Parasitoids" Insects 12, no. 10: 861. https://doi.org/10.3390/insects12100861
APA StyleZhao, Q., Jiang, L.-L., Guo, J., Zhang, D.-K., & Hu, H.-Y. (2021). Differences in Gall Induction of Flower-like Galls on Haloxylon by Psyllids (Hemiptera: Aphalaridae), and the Emergence of Corresponding Parasitoids. Insects, 12(10), 861. https://doi.org/10.3390/insects12100861






