Performance of Two Trichogrammatid Species from Zambia on Fall Armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Population Establishment
2.2. Morphological Identification
2.3. Molecular Identification
2.4. Assessment of Biological Characteristics
2.5. Statistical Analysis
2.5.1. Phylogenetic Analysis
2.5.2. Bioassay Analysis
3. Results
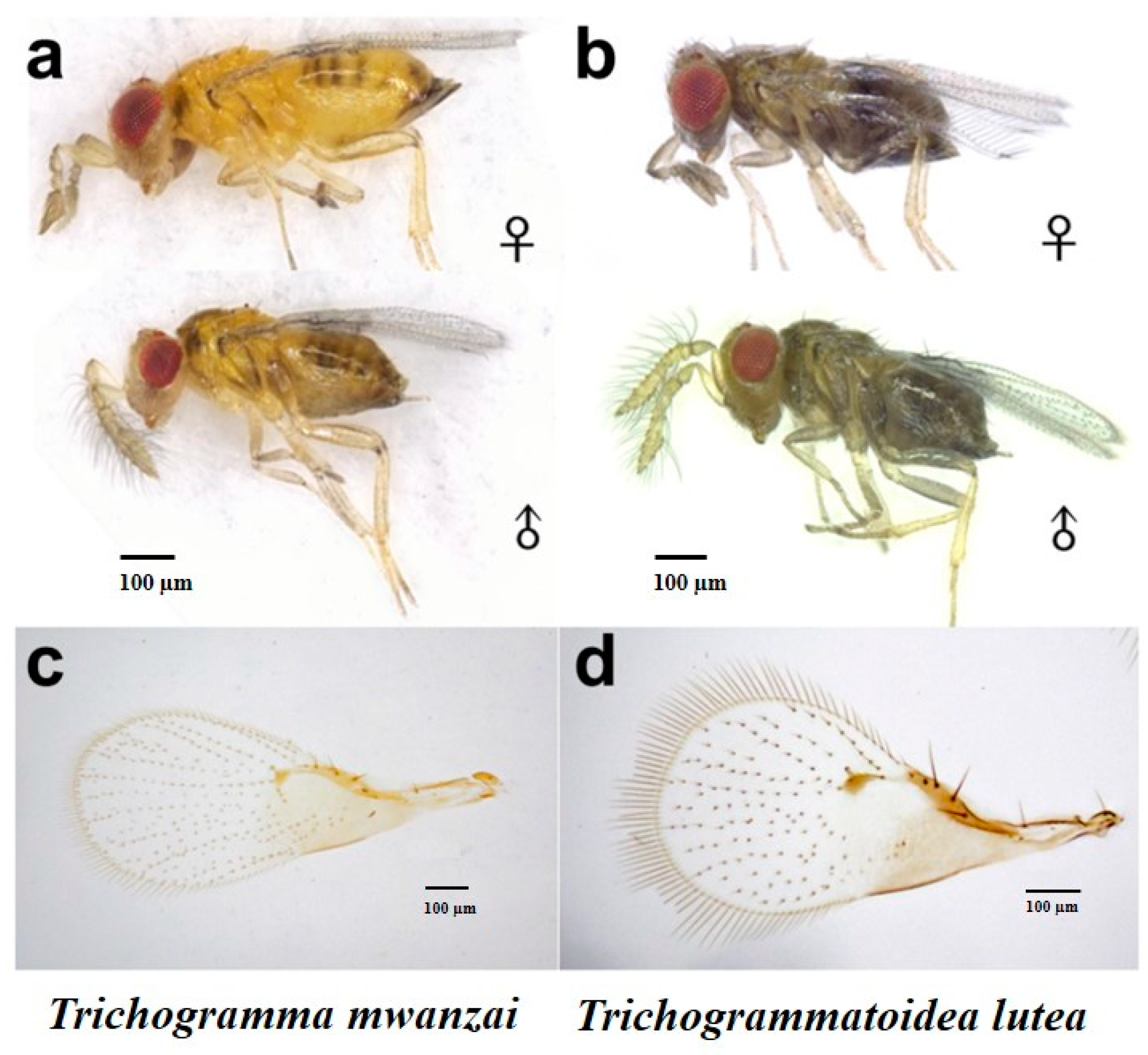
3.1. Morphological Identification
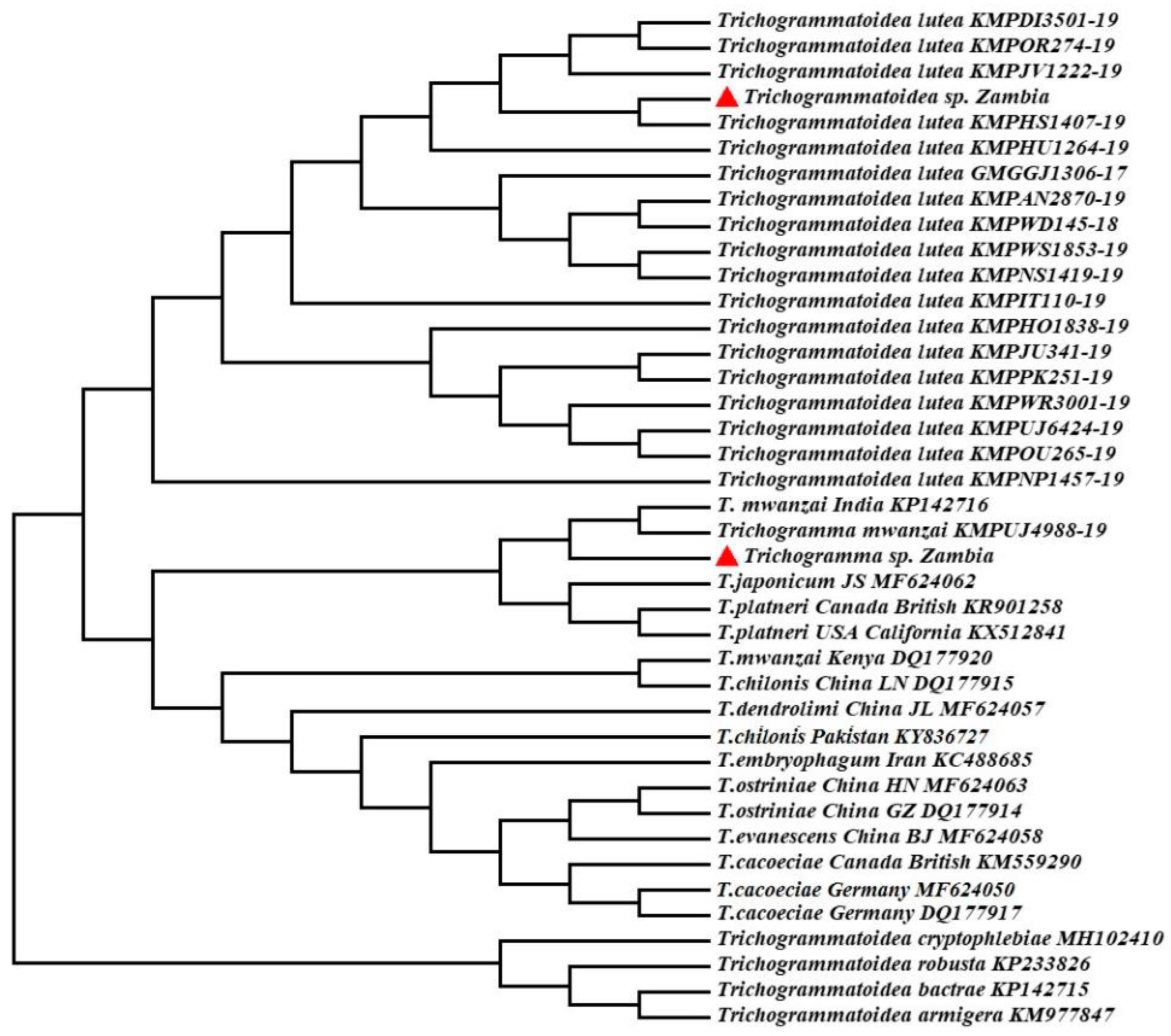
3.2. Molecular Biological Identification Based on COI
3.3. Performance on Host Eggs
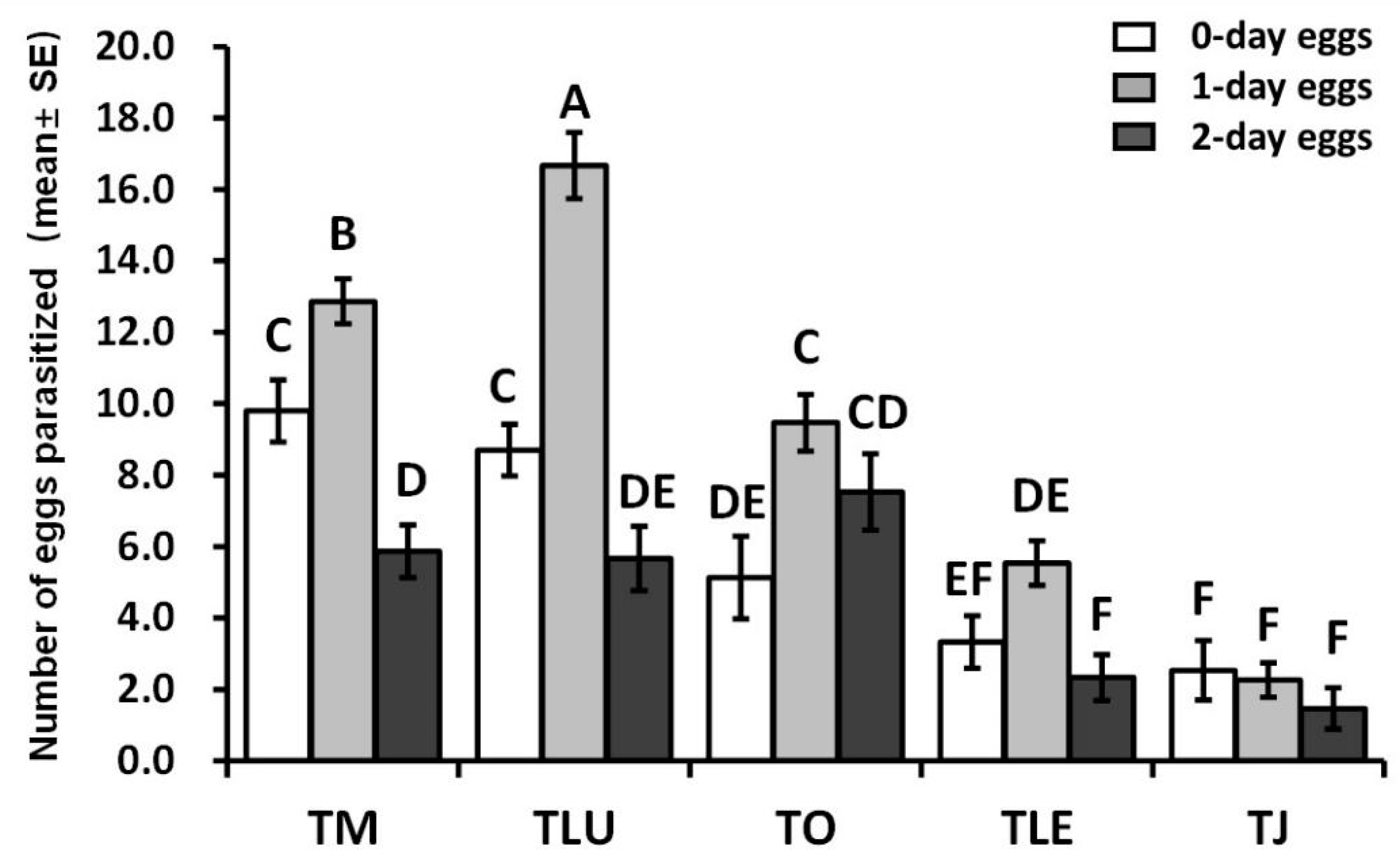
3.3.1. Parasitism of Trichogramma and Trichogrammatoidea Species on Different Ages of FAW Eggs
3.3.2. Development of Trichogramma and Trichogrammatoidea Species on FAW Eggs of Different Ages
3.3.3. Emergence and Sex Ratio of Trichogramma and Trichogrammatoidea Species Reared on FAW Eggs of Different Ages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sparks, A.N. A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 82. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in west and central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef]
- Casmuz, A.; Juarez, M.L.; Socias, M.G.; Murua, M.G.; Prieto, S.; Medina, S.; Willink, E.; Gastaminzaet, G. Review of the host plants of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Rev. Soc. Entomol. Arge. 2010, 69, 209–231. [Google Scholar]
- Montezano, D.G.; Sosa-Gómez, D.R.; Specht, A.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Hunt, T.E. Host Plants ofSpodoptera frugiperda(Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Sisay, B.; Simiyu, J.; Mendesil, E.; Likhayo, P.; Ayalew, G.; Mohamed, S.; Subramanian, S.; Tefera, T. Fall Armyworm, Spodoptera frugiperda Infestations in East Africa: Assessment of Damage and Parasitism. Insects 2019, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.H.; Silversides, R.H.; Lindquist, O.H. Migration flight by an aphid, rhopalosiphum maidis (Hemiptera: Aphididae), and a noctuid, Spodoptera Frugiperda (Lepidoptera: Noctuidae). Can. Entomol. 1975, 107, 567–576. [Google Scholar] [CrossRef]
- Westbrook, J.K.; Nagoshi, R.N.; Meagher, R.L.; Fleischer, S.J.; Jairam, S. Modeling seasonal migration of fall armyworm moths. Int. J. Biometeorol. 2004, 60, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.J. Migration and the life history strategy of the fall armyworm, Spodoptera frugiperda in the western hemisphere. Int. J. Trop. Insect Sci. 1987, 8, 543–549. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Huesing, J.E.; Eddy, R.; Peschke, V.M. Fall Armyworm in Africa: A Guide for Integrated Pest Management, 1st ed.; CIMMYT: CDMX, Mexico, 2018; pp. 11–106. [Google Scholar]
- Brévault, T.; Ndiaye, A.; Badiane, D.; Bal, A.B.; Sembène, M.; Silvie, P.; Haran, J. First records of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), in Senegal. Entomol. Gen. 2018, 37, 129–142. [Google Scholar] [CrossRef]
- CABI. Spodoptera Frugiperda. In Invasive Species Compendium; CAB International: Wallingford, UK, 2020; Available online: www.cabi.org/isc (accessed on 2 April 2020).
- Firake, D.M.; Behere, G.T. Natural mortality of invasive fall armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) in maize agroecosystems of northeast India. Biol. Control 2020, 148, 104303. [Google Scholar] [CrossRef]
- Wu, Q.L.; Jiang, Y.Y.; Wu, K.M. Analysis of migration routes of the fall armyworm Spodoptera frugiperda (J.E. Smith) from Myanmar to China. Plant Prot. 2019, 45, 1–9. [Google Scholar] [CrossRef]
- Durocher-Granger, L.; Mfune, T.; Musesha, M.; Lowry, A.; Reynolds, K.; Buddie, A.; Cafà, G.; Offord, L.; Chipabika, G.; Dicke, M.; et al. Factors influencing the occurrence of fall armyworm parasitoids in Zambia. J. Pest Sci. 2021, 94, 1133–1146. [Google Scholar] [CrossRef]
- Harrison, R.D.; Thierfelder, C.; Baudron, F.; Chinwada, P.; Midega, C.; Schaffner, U.; Van Den Berg, J. Agro-ecological options for fall armyworm (Spodoptera frugiperda JE Smith) management: Providing low-cost, smallholder friendly solutions to an invasive pest. J. Environ. Manag. 2019, 243, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Nboyine, J.; Kusi, F.; Abudulai, M.; Badii, B.; Zakaria, M.; Adu, G.; Haruna, A.; Seidu, A.; Osei, V.; Alhassan, S.; et al. A new pest, Spodoptera frugiperda (J.E. Smith), in tropical Africa: Its seasonal dynamics and damage in maize fields in northern Ghana. Crop. Prot. 2020, 127, 104960. [Google Scholar] [CrossRef]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall armyworm: Impacts and implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef]
- Maiga, I.; Ndiaye, M.; Gagare, S.; Oumarou, G.; Oumarou, S. La Chenille D’automne Spodoptera Frugiperda, Nouveau Ravageur du Maïs en Afrique de l’Ouest, a Atteint le Niger; Centre Régional AGRHYMET: Niamey, Niger, 2017; Available online: http://www.reca-niger.org/IMG/pdf/Bulletin_special_Chenille.pdf (accessed on 26 March 2020).
- Hruska, J.A. Fall armyworm (Spodoptera frugiperda) management by smallholders. CAB Rev. 2019, 14, 1–11. [Google Scholar] [CrossRef]
- Weisenburger, D.D. Human health effects of agrichemical use. Hum. Pathol. 1993, 24, 571–576. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Varikou, K.; Garantonakis, N.; Birouraki, A. Exposure of Bombus terrestris L. to three different active ingredients and two application methods for olive pest control. Entomol. Gen. 2019, 39, 53–60. [Google Scholar] [CrossRef]
- Desneux, N.; Wajnberg, E.; Fauvergue, X.; Privet, S.; Kaiser, L. Oviposition behaviour and patch-time allocation in two aphid parasitoids exposed to deltamethrin residues. Entomol. Exp. Appl. 2004, 112, 227–235. [Google Scholar] [CrossRef]
- Taning, C.N.T.; Vanommeslaeghe, A.; Smagghe, G. With or without foraging for food, field-realistic concentrations of sulfoxaflor are equally toxic to bumblebees (Bombus terrestris). Entomol. Gen. 2019, 39, 151–155. [Google Scholar] [CrossRef]
- Menail, A.H.; Boutefnouchet-Bouchema, W.F.; Haddad, N.; Taning, C.N.T.; Smagghe, G.; Loucif-Ayad, W. Effects of thiamethoxam and spinosad on the survival and hypopharyngeal glands of the African honey bee (Apis mellifera intermissa). Entomol. Gen. 2020, 40, 207–215. [Google Scholar] [CrossRef]
- Richardson, E.B.; Troczka, B.J.; Gutbrod, O.; Davies, T.G.E.; Nauen, R. Diamide resistance: 10 years of lessons from lepidopteran pests. J. Pest Sci. 2020, 93, 911–928. [Google Scholar] [CrossRef]
- Kumela, T.; Simiyu, J.; Sisay, B.; Likhayo, P.; Mendesil, E.; Gohole, L.; Tefera, T. Farmers’ knowledge, perceptions, and management practices of the new invasive pest, fall armyworm (Spodoptera frugiperda) in Ethiopia and Kenya. Int. J. Pest Manag. 2019, 65, 1–9. [Google Scholar] [CrossRef]
- Molina-Ochoa, J.; Carpenter, J.E.; Heinrichs, E.; Foster, J.E. Parasitoids and parasites of spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas and Caribbean basin: An inventory. Fla. Entomol. 2003, 86, 254–289. [Google Scholar] [CrossRef]
- Dequech, S.T.B.; Camera, C.; Sturza, V.S.; Ribeiro, L.D.P.; Querino, R.B.; Poncio, S. Population fluctuation of Spodoptera frugiperda eggs and natural parasitism by Trichogramma in maize. Acta Sci. Agron. 2013, 35, 295–300. [Google Scholar] [CrossRef]
- Noyes, J.S. Universal Chalcidoidea Database. World Wide Web Electronic Publication. Available online: http://www.nhm.ac.uk/chalcidoids (accessed on 15 March 2021).
- Smith, S.M. Biological control with Trichogramma: Advances, success, and potential of their use. Annu. Rev. Entomol. 1996, 41, 375–406. [Google Scholar] [CrossRef]
- Huang, N.-X.; Jaworski, C.C.; Desneux, N.; Zhang, F.; Yang, P.-Y.; Wang, S. Long-term and large-scale releases of Trichogramma promote pesticide decrease in maize in northeastern China. Entomol. Gen. 2020, 40, 331–335. [Google Scholar] [CrossRef]
- Qu, Y.Y.; Chen, X.; Monticelli, L.S.; Zhang, F.; Desneux, N.; Huijie, D.; Wang, S. Parasitism performance of the parasitoid Trichogramma dendrolimi on the plum fruit moth Grapholitha funebrana. Entomol. Gen. 2020, 40, 385–395. [Google Scholar] [CrossRef]
- Zang, L.-S.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, present status, and perspectives. Annu. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef]
- Hou, Y.-Y.; Yang, X.; Zang, L.-S.; Zhang, C.; Monticelli, L.S.; Desneux, N. Effect of oriental armyworm mythimna separata egg age on the parasitism and host suitability for five Trichogramma species. J. Pest Sci. 2018, 91, 1181–1189. [Google Scholar] [CrossRef]
- Noyes, J.S. Collecting and preserving chalcid wasps (Hymenoptera: Chalcidoidea). J. Nat. Hist. 1982, 16, 315–334. [Google Scholar] [CrossRef]
- Schulten, G.G.M.; Feijen, H.R. Two new species of Trichogramma (Hymenoptera; Trichogrammatidae) from Malawi; egg parasitoids of diopsis macrophthalma Dalman (Diptera; Diopsidae). Entomol. Ber. Amsterdam. 1978, 38, 25–29. [Google Scholar]
- Schulten, G.G.M.; Feijen, H.R. A new species of Trichogramma (Hymenoptera: Trichogrammatidae) from Malawi, parasitizing eggs of Chilo diffusilineus (de Joannis). Entomol. Berichten. 1982, 42, 142–144. [Google Scholar]
- Lin, N.Q. Systematic Studies of Chinese Trichogrammatidae (Hymenoptera: Chalcidoidea); Fujian Science and Technology Publishing House: Fuzhou, China, 1994; p. 362. [Google Scholar]
- Nagaraja, H.; Nagarkatti, S. Redescriptions of some known species of Trichogramma (Hym., Trichogrammatidae), showing the im-portance of the male genitalia as a diagnostic character. Bull. Entomol. Res. 1971, 61, 13–31. [Google Scholar]
- Nagaraja, H. On some new species of Indian Trichogramma (Hymenoptera: Trichogrammatidae). Orient. Insects 1973, 7, 275–290. [Google Scholar] [CrossRef]
- Nagaraja, H. Studies on Trichogrammatoidea (Hymenoptera: Trichogrammatidae). Orient. Insects 1978, 12, 489–529. [Google Scholar] [CrossRef]
- Pinto, J.D.; Platner, G.R.; Oatman, E.R. Clarification of the Identity of Several Common Species of North American Trichogramma (Hymenoptera: Trichogrammatidae). Ann. Entomol. Soc. Am. 1978, 71, 169–180. [Google Scholar] [CrossRef]
- Pinto, J.D. Novel taxa of Trichogramma from the new world tropics and Australia (Hymenoptera: Trichogrammatidae). J. N. Y. Entomol. Soc. 1992, 100, 621–633. [Google Scholar]
- Pinto, J.D. Systematics of the North American species of Trichogramma Westwood (Hymenoptera: Trichogrammatidae). Mem. Entomol. Soc. Wash. 1999, 22, 1–287. [Google Scholar]
- Kumar, G.A.; Jalali, S.K.; Venkatesan, T.; Stouthamer, R.; Niranjana, P.; Lalitha, Y. Internal transcribed spacer-2 restriction fragment length polymorphism (ITS-2-RFLP) tool to differentiate some exotic and indigenous trichogrammatid egg parasitoids in India. Biol. Control 2009, 49, 207–213. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Wr, H.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome coxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 1994, 3, 294–299. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Bruce, Y.A.; Schulthess, F.; Mueke, J.; Gohole, L. Egg parasitoids of noctuid stemborers on maize in Kenya. Int. J. Biodivers. Sci. Manag. 2009, 5, 174–180. [Google Scholar] [CrossRef]
- Kalyebi, A.; Overholt, W.; Schulthess, F.; Mueke, J.; Sithanantham, S. The effect of temperature and humidity on the bionomics of six African egg parasitoids (Hymenoptera: Trichogrammatidae). Bull. Entomol. Res. 2006, 96, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Haile, A.T.; Hassan, S.A.; Sithanantham, S.; Ogol, C.K.P.O.; Baumgartner, J. Comparative life table analysis of Trichogramma bournieri Pintureau and Babault and Trichogramma sp. nr. mwanzai Schulten and Feijen (Hym., Trichogrammatidae) from Kenya. J. Appl. Entomol. 2002, 126, 287–292. [Google Scholar] [CrossRef]
- Bruce, Y.A. Host Suitability and Interspecific Competition among The West African Egg Parasitoid Telenomus isis and The Indigenous Egg Parasitoids in Kenya. Ph.D. Thesis, Kenyatta University, Nairobi, Kenya, 2008. [Google Scholar]
- Kfir, R. Parasitoids of the African stem borer, Busseola fusca (Lepidoptera: Noctuidae), in South Africa. Bull. Entomol. Res. 1995, 85, 369–377. [Google Scholar] [CrossRef]
- Laminou, S.A.; Ba, M.N.; Karimoune, L.; Doumma, A.; Muniappan, R. Parasitism of Locally Recruited Egg Parasitoids of the Fall Armyworm in Africa. Insects 2020, 11, 430. [Google Scholar] [CrossRef]
- Goulart, M.M.P.; Bueno, A.D.F.; Bueno, R.C.O.D.F.; Diniz, A.F. Host preference of the egg parasitoids Telenomus remus and Trichogramma pretiosum in laboratory. Rev. Bras. Entomol. 2011, 55, 129–133. [Google Scholar] [CrossRef]
- Wen, X.Y.; Xu, J.; Zang, L.S.; Shi, S.S. Parasitism and suitability of ascotis selenaria eggs for three Trichogramma species collected from soybean field. J. Environ. Entomol. 2015, 37, 1060–1063. [Google Scholar]
- Zhu, K.; Zhou, J.; Zhang, Z.; Zhang, C.; Hui, D. Parasitic efficacy and offspring fitness of Trichogramma pretiosum against Spodoptera frugiperda and Spodoptera litura at different egg ages. Plant Prot. 2019, 45, 54–59. [Google Scholar]
- Beserra, E.B.; Parra, J.R.P. Impact of the number of Spodoptera frugiperda egg layers on parasitism by Trichogramma atopovirilia. Sci. Agricola 2005, 62, 190–193. [Google Scholar] [CrossRef][Green Version]
- Bueno, V.H.P.; van Lenteren, J.C. The popularity of augmentative biological control in Latin America: History and state of affairs. In Proceedings of the First International Symposium on Biological Control of Arthropods, Honolulu, HI, USA, 14–18 January 2002; International Organization of Biological Control: Antibes, France, 2002; pp. 180–184. [Google Scholar]
- Figueiredo, M.; Lucia, T.; Cruz, I. Effect of Telenomus remus Nixon (Hymenoptera: Scelionidae) Density on Control of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae) Egg Masses upon Release in a Maize Field. Rev. Bras. Milho Sorgo 2002, 1, 12–19. [Google Scholar] [CrossRef]
- Cave, R.D. Biology, ecology and use in pest management of Telenomus remus. BioControl News Inf. 2000, 21, 21–26. [Google Scholar]
- Waage, J.K. Family planning in parasitoids: Adaptative patterns of progeny and sex allocation. In Insect Parasitoids. 13th Symposium of the Royal Entomological Society of London; Waage, J.K., Greathead, D.J., Eds.; Academic Press: London, UK, 1986; pp. 63–95. [Google Scholar]
- Calvin, D.D.; Knapp, M.C.; Welch, S.M.; Poston, F.L.; Elzinga, R.J. Impact of Environmental Factors on Trichogramma pretiosum Reared on Southwestern Corn Borer Eggs. Environ. Entomol. 1984, 13, 774–780. [Google Scholar] [CrossRef]
- Lund, H.O. Some temperature and humidity relations of two races of Trichogramma minutum riley (Hym. Chalcididae) 1. Ann. Entomol. Soc. Am. 1934, 27, 324–340. [Google Scholar] [CrossRef]
- Pak, G.; Van Dalen, A.; Kaashoek, N.; Dijkman, H. Host egg chorion structure influencing host suitability for the egg parasitoid Trichogramma Westwood. J. Insect Physiol. 1990, 36, 869–875. [Google Scholar] [CrossRef]
- Vinson, S.B. Host selection by insect parasitoids. Annu. Rev. Entomol. 1976, 21, 109–133. [Google Scholar] [CrossRef]
- Pak, G.A. Behavioural variations among strains of Trichogramma spp: A review of the literature on host-age selection. J. Appl. Entomol. 1986, 101, 55–64. [Google Scholar] [CrossRef]
- Pizzol, J.; Desneux, N.; Wajnberg, E.; Thiéry, D. Parasitoid and host egg ages have independent impact on various biological traits in a Trichogramma species. J. Pest Sci. 2012, 85, 489–496. [Google Scholar] [CrossRef]
- Monje, J.C.; Zebitz, C.P.W.; Ohnesorge, B. Host and host age preference of Trichogramma galloi and T. pretiosum (Hymenoptera: Trichogrammatidae) reared on different hosts. J. Econ. Entomol. 1999, 92, 97–103. [Google Scholar] [CrossRef]
- Guo, X.; Di, N.; Chen, X.; Zhu, Z.; Zhang, F.; Tang, B.; Dai, H.; Li, J.; Guo, R.; Wang, S. Performance of Trichogramma pintoi when parasitizing eggs of the oriental fruit moth Grapholita molesta. Entomol. Gen. 2019, 39, 239–249. [Google Scholar] [CrossRef]
- Chabi-Olaye, A.; Fiaboe, M.K.; Schulthess, F. Host preference, suitability and thermal requirements of Lathromeris ovicidia Risbec (Hymenoptera: Trichogrammatidae) egg parasitoid of cereal stemborers in Africa. Biol. Control 2004, 30, 617–623. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Ren, B.-Z.; Yuan, X.-H.; Zang, L.-S.; Ruan, C.-C.; Sun, G.-Z.; Shao, X.-W. Effects of host-egg ages on host selection and suitability of four Chinese Trichogramma species, egg parasitoids of the rice striped stem borer, Chilo suppressalis. BioControl 2014, 59, 159–166. [Google Scholar] [CrossRef]
- Miura, K.; Kobayashi, M. Effects of host-egg age on the parasitism by Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae), an egg parasitoid of the diamondback moth. Appl. Entomol. Zool. 1998, 33, 219–222. [Google Scholar] [CrossRef]
- Safavi, M. Etude biologique et ecologique des hymenopteres parasites des oeufs des punaises de cereales. Entomophaga 1968, 13, 381–495. [Google Scholar] [CrossRef]
- Mattiacci, L.; Dicke, M. The parasitoidCotesia glomerata (Hymenoptera: Braconidae) discriminates between first and fifth larval instars of its hostPieris brassicae, on the basis of contact cues from frass, silk, and herbivore-damaged leaf tissue. J. Insect Behav. 1995, 8, 485–498. [Google Scholar] [CrossRef]
- Honda, J.Y.; Luck, R.F. Age and suitability of amorbia cuneana (Lepidoptera: Tortricidae) and Sabulodes aegrotata (Lepidoptera: Geometridae) Eggs for Trichogramma platneri (Hymenoptera: Trichogrammatidae). Biol. Control 2000, 18, 79–85. [Google Scholar] [CrossRef]
- Mansfield, S.; Mills, N.J. A comparison of methodologies for the assessment of host preferences of the gregarious egg parasitoid Trichogramma platneri. Biol. Control 2003, 29, 332–340. [Google Scholar] [CrossRef]
- Song, L.-W.; Wen, X.-Y.; Zang, L.-S.; Ruan, C.-C.; Shi, S.-S.; Shao, X.-W.; Zhang, F. Parasitism and suitability of different egg ages of the leguminivora GLYCINIVORELLA (Lepidoptera: Tortricidae) for three indigenous trichogramma Species. J. Econ. Entomol. 2015, 108, 933–939. [Google Scholar] [CrossRef]
- Hassan, S.A.; Guo, M.F. Selection of effective strains of egg parasites of the genus Trichogramma (Hym., Trichogrammatidae) to control the European corn borer Ostrinia nubilalis Hb. (Lep., Pyralidae). J. Appl. Entomol. 1991, 111, 335–341. [Google Scholar] [CrossRef]
- Lu, Q.G. Studies on the host preference of Trichogramma Sp. Nr. mwanzai [Hym: Trichogrammatidae]. Chin. J. Biol. Control 1991, 7, 108–110. [Google Scholar]
| Traits | Variance Source | Df | F | p |
|---|---|---|---|---|
| Number of eggs parasitized | Host age | 2 | 46.310 | <0.001 |
| Parasitoid species | 4 | 58.908 | <0.001 | |
| Parasitoid species × host age | 8 | 8.243 | <0.001 | |
| Error | 210 | - | - | |
| Percent adult emergence | Host age | 2 | 1.698 | 0.186 |
| Parasitoid species | 4 | 1.692 | 0.154 | |
| Parasitoid species × host age | 8 | 0.773 | 0.627 | |
| Error | 188 | - | - | |
| Developmental time | Host age | 2 | 3.479 | 0.033 |
| Parasitoid species | 4 | 4.433 | 0.002 | |
| Parasitoid species × host age | 8 | 11.447 | <0.001 | |
| Error | 188 | - | - | |
| Percent female progeny | Host age | 2 | 4.420 | <0.001 |
| Parasitoid species | 4 | 17.230 | 0.025 | |
| Parasitoid species × host age | 8 | 2.890 | 0.070 | |
| Error | 188 | - | - |
| Parameters | Species | No. of Egg Masses | Host Age (Days) | ||
|---|---|---|---|---|---|
| 0 | 1 | 2 | |||
| Developmental time (days) | T. mwanzai | 15 | 10.5 ± 0.1 b A | 9.5 ± 0.1 d B | 9.9 ± 0.1 b B |
| T. lutea | 15 | 11.4 ± 0.1 a A | 10.5 ± 0.1 bc B | 10.2 ± 0.1 b B | |
| T. ostriniae | 15 | 10.5 ± 0.2 b A | 10.2 ± 0.2 c A | 10.5 ± 0.2 b A | |
| T. leucaniae | 15 | 10.3 ± 0.2 b B | 11.6 ± 0.2 a A | 11.4 ± 0.2 a A | |
| T. japonicum | 15 | 10.6 ± 0.2 b B | 11.0±0.2a b AB | 11.7 ± 0.2 a A | |
| % Emergence | T. mwanzai | 15 | 98.3 ± 0.9 a A | 94.8 ± 2.0 a A | 93.2 ± 2.1 a A |
| T. lutea | 15 | 98.7 ± 0.9 a A | 99.4 ± 0.3 a A | 98.2 ± 1.4 a A | |
| T. ostriniae | 15 | 96.4 ± 1.8 a A | 93.3 ± 2.6 a A | 97.5 ± 1.2 a A | |
| T. leucaniae | 15 | 96.7 ± 2.6 a A | 96.3 ± 2.4 a A | 97.1 ± 1.9 a A | |
| T. japonicum | 15 | 99.1 ± 0.9 a A | 90.2 ± 6.9 a A | 85.7 ± 14.3 a A | |
| % Female progeny | T. mwanzai | 15 | 85.1 ± 2.2 a A | 82.3 ± 2.8 a A | 60.1 ± 3.8 a B |
| T. lutea | 15 | 82.2 ± 3.7 a A | 79.7 ± 2.7a A | 50.8 ± 1.6 b B | |
| T. ostriniae | 15 | 82.5 ± 5.6 a A | 77.2 ± 3.6 a A | 69.9 ± 4.1 a A | |
| T. leucaniae | 15 | 82.7 ± 5.9 a A | 77.6 ± 5.0 a A | 70.8 ± 7.1 a A | |
| T. japonicum | 15 | 63.6 ± 13.2 a A | 49.6 ± 9.4 b A | 64.4 ± 11.8 a A | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.-W.; Hu, H.-Y.; Nkunika, P.O.Y.; Dai, P.; Xu, W.; Bao, H.-P.; Desneux, N.; Zang, L.-S. Performance of Two Trichogrammatid Species from Zambia on Fall Armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Insects 2021, 12, 859. https://doi.org/10.3390/insects12100859
Sun J-W, Hu H-Y, Nkunika POY, Dai P, Xu W, Bao H-P, Desneux N, Zang L-S. Performance of Two Trichogrammatid Species from Zambia on Fall Armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Insects. 2021; 12(10):859. https://doi.org/10.3390/insects12100859
Chicago/Turabian StyleSun, Jia-Wei, Hong-Ying Hu, Phillip O. Y. Nkunika, Peng Dai, Wei Xu, He-Ping Bao, Nicolas Desneux, and Lian-Sheng Zang. 2021. "Performance of Two Trichogrammatid Species from Zambia on Fall Armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae)" Insects 12, no. 10: 859. https://doi.org/10.3390/insects12100859
APA StyleSun, J.-W., Hu, H.-Y., Nkunika, P. O. Y., Dai, P., Xu, W., Bao, H.-P., Desneux, N., & Zang, L.-S. (2021). Performance of Two Trichogrammatid Species from Zambia on Fall Armyworm, Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae). Insects, 12(10), 859. https://doi.org/10.3390/insects12100859






