Use of a Managed Solitary Bee to Pollinate Almonds: Population Sustainability and Increased Fruit Set
Abstract
:Simple Summary
Abstract
1. Introduction
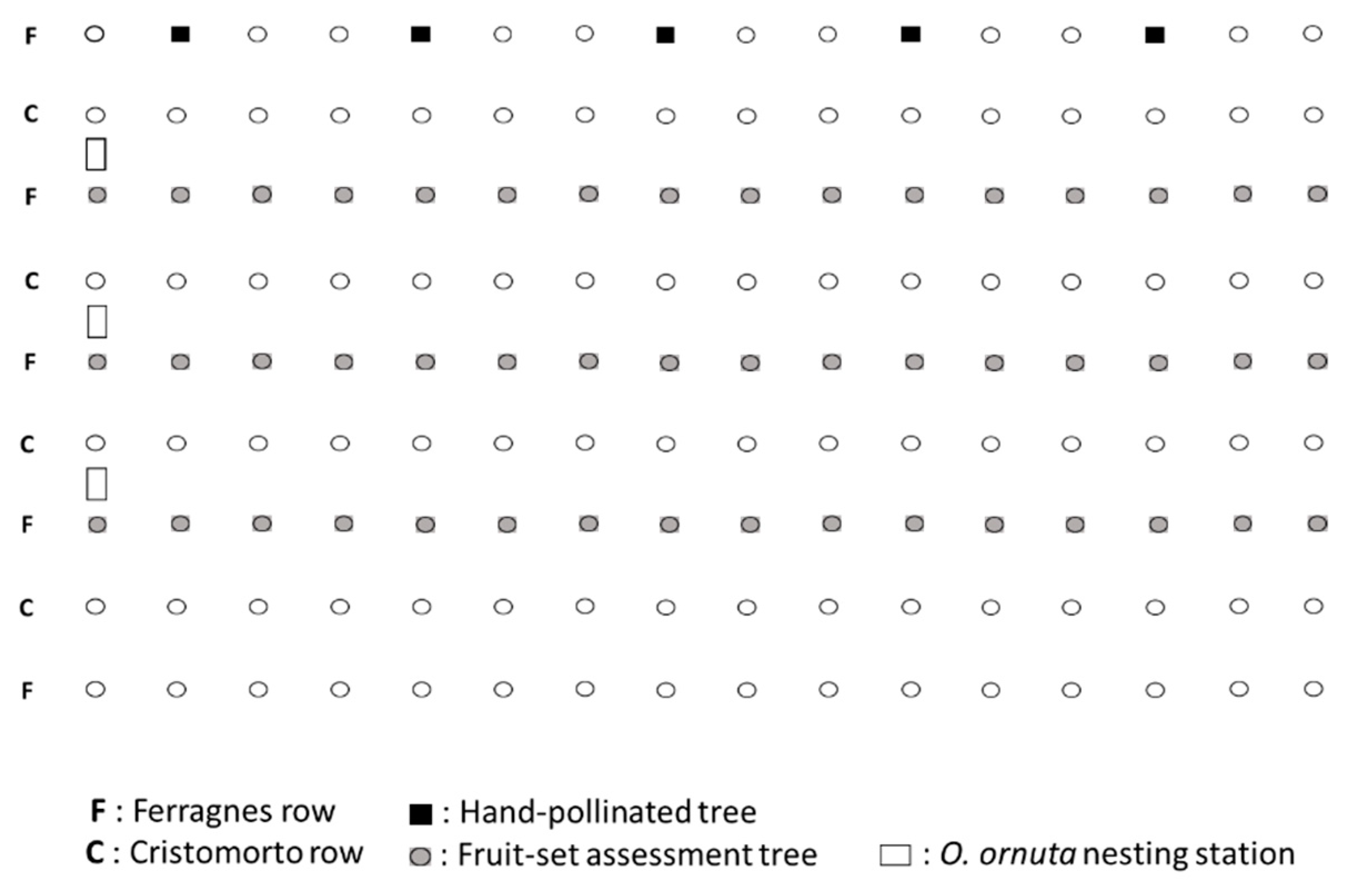
2. Materials and Methods
Statistical Analysis
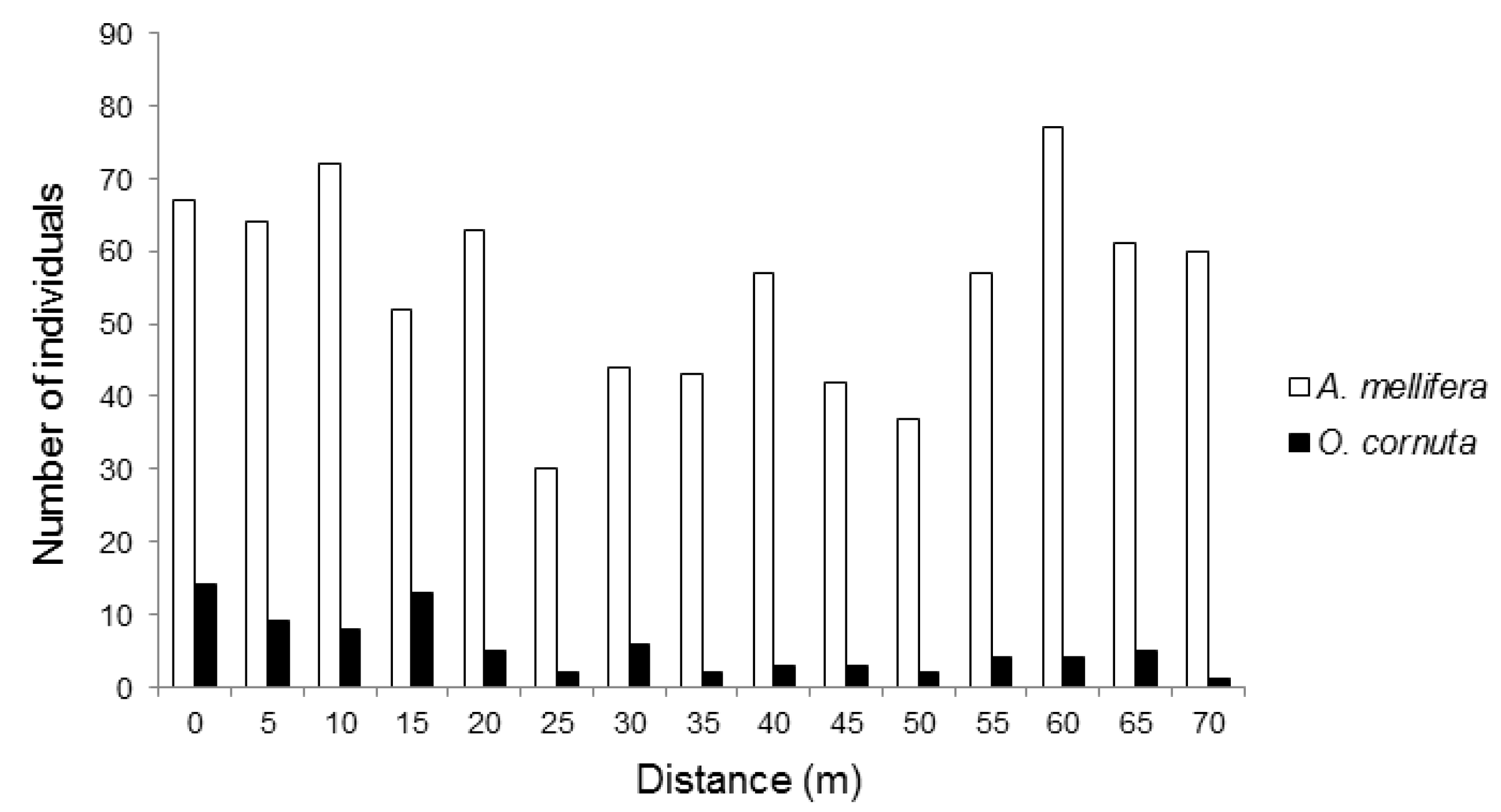
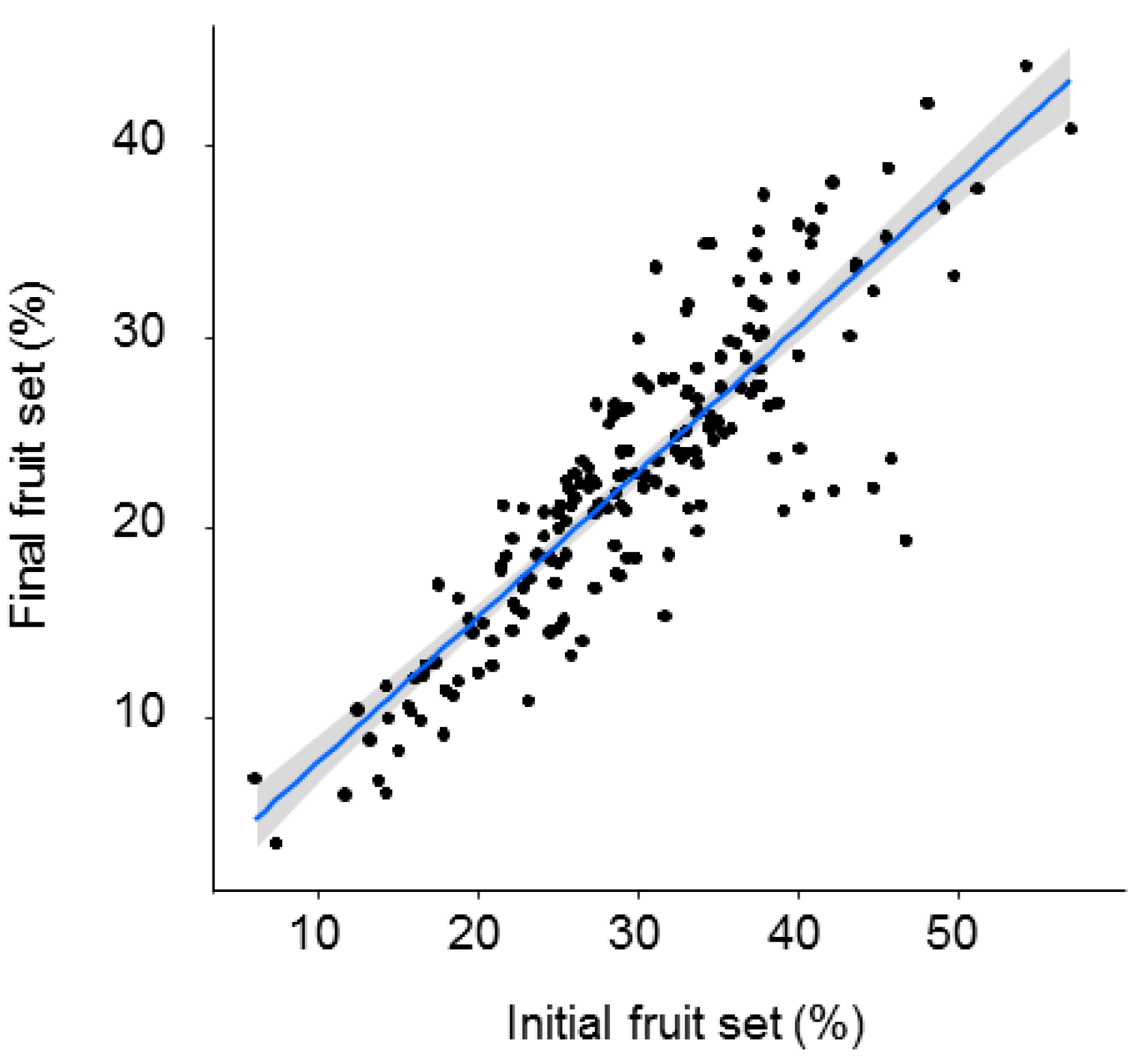
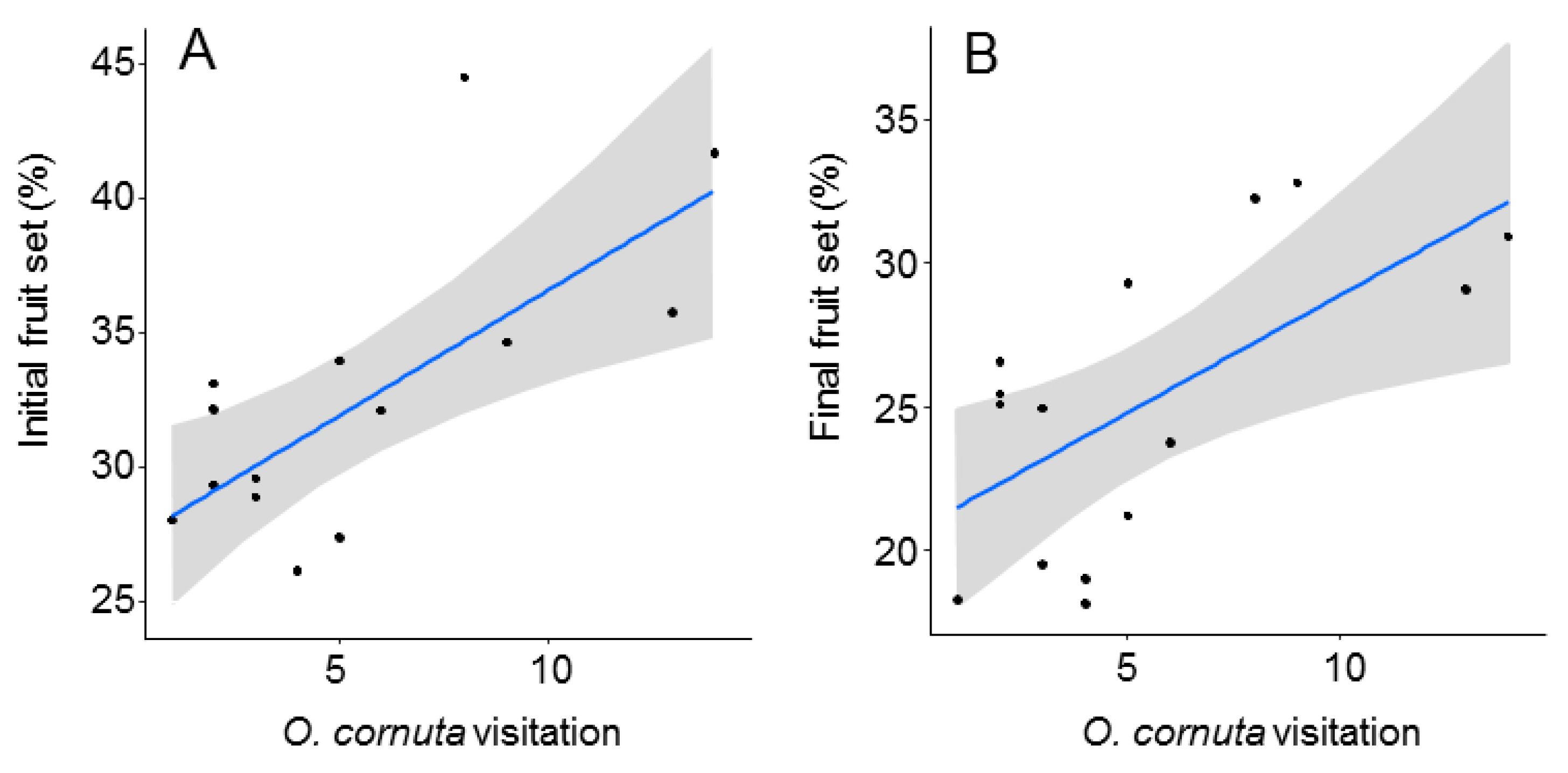
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
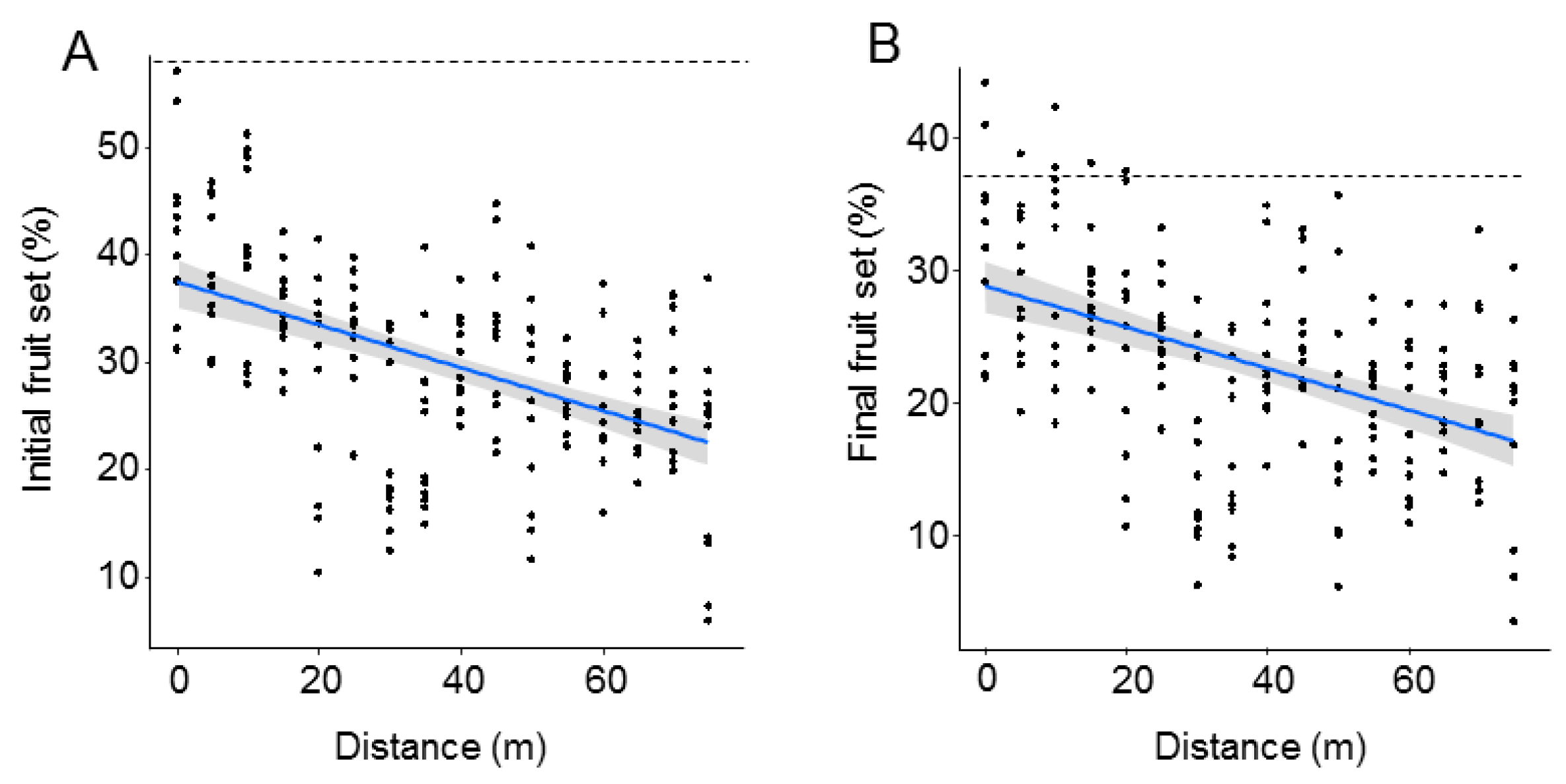
| (A) Initial Fruit Set | ||
|---|---|---|
| t-Value | p-Value | |
| (Intercept) | 32.03 | <0.0001 |
| Distance | −4.73 | <0.0001 |
| R2m: 0.24; R2c: 0.66 | ||
| (B) Final Fruit Set | ||
| t-Value | p-Value | |
| (Intercept) | 29.22 | <0.0001 |
| Distance | −4.31 | 0.0001 |
| R2m: 0.19; R2c: 0.59 | ||
References
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winfree, R.; Williams, N.M.; Gaines, H.; Ascher, J.S.; Kremen, C. Wild bee pollinators provide the majority of crop visitation across land-use gradients in New Jersey and Pennsylvania, USA. J. Appl. Ecol. 2008, 45, 793–802. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild Pollinators enhance fruit set of crops regardless of honey bee abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Hevia, V.; Bosch, J.; Azcárate, F.M.; Fernández, E.; Rodrigo, A.; Barril-Graells, H.; González, J.A. Bee diversity and abundance in a livestock drove road and its impact on pollination and seed set in adjacent sunflower fields. Agric. Ecosyst. Environ. 2016, 232, 336–344. [Google Scholar] [CrossRef]
- Park, M.G.; Raguso, R.A.; Losey, J.E.; Danforth, B.N. Per-visit pollinator performance and regional importance of wild Bombus and Andrena (Melandrena) compared to the managed honey bee in New York apple orchards. Apidologie 2016, 47, 145–160. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.-M.; Brittain, C.; Hendrix, S.D.; Thorp, R.; Williams, N.; Kremen, C. Wild pollination services to California almond rely on semi-natural habitat. J. Appl. Ecol. 2012, 49, 723–732. [Google Scholar] [CrossRef]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hass, A.L.; Kormann, U.G.; Tscharntke, T.; Clough, Y.; Bosem Baillod, A.; Sirami, C.; Fahrig, L.; Martin, J.-L.; Baudry, J.; Bertrand, C.; et al. Landscape configurational heterogeneity by small-scale agriculture, not crop diversity, maintains pollinators and plant reproduction in Western Europe. Proc. R. Soc. B 2018, 285, 20172242. [Google Scholar] [CrossRef] [PubMed]
- Sirami, C.; Gross, N.; Baillod, A.B.; Bertrand, C.; Carrié, R.; Hass, A.; Henckel, L.; Miguet, P.; Vuillot, C.; Alignier, A.; et al. Increasing crop heterogeneity enhances multitrophic diversity across agricultural regions. Proc. Natl. Acad. Sci. USA 2019, 116, 16442–16447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D.; et al. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Pitts-Singer, T.L.; Artz, D.R.; Peterson, S.S.; Boyle, N.K.; Wardell, G.I. Examination of a managed pollinator strategy for almond production using Apis mellifera (Hymenoptera: Apidae) and Osmia lignaria (Hymenoptera: Megachilidae). Environ. Entomol. 2018, 47, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Roquer-Beni, L.; Alins, G.; Arnan, X.; Boreux, V.; García, D.; Hambäck, P.A.; Happe, A.-K.; Klein, A.-M.; Miñarro, M.; Mody, K.; et al. Management-dependent effects of pollinator functional diversity on apple pollination services: A response-effect trait approach. (Unpublished; Manuscript in Preparation).
- McGregor, S.E. Insect Pollination of Cultivated Crop Plants; Agricultural Research Service: Washington, DC, USA, 1976; p. 411.
- Free, J.B. Insect Pollination of Crops, 2nd ed.; Academic Press: London, UK, 1993. [Google Scholar]
- Delaplane, K.S.; Mayer, D.R. Crop Pollination by Bees; CABI: Wallingford, UK, 2000. [Google Scholar]
- Vicens, N.; Bosch, J. Weather-dependent pollinator activity in an apple orchard, with special reference to Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae and Apidae). Environ. Entomol. 2000, 29, 413–420. [Google Scholar] [CrossRef]
- Visscher, P.K.; Seeley, T.D. Foraging strategy of honeybee colonies in temperate deciduous forest. Ecology 1982, 63, 1790–1801. [Google Scholar] [CrossRef]
- Beekman, M.; Ratnieks, F.L.W. Long-range foraging by the honey-bee, Apis mellifera L. Funct. Ecol. 2000, 14, 490–496. [Google Scholar] [CrossRef] [Green Version]
- Hagler, J.R.; Mueller, S.; Teuber, L.R.; Machtley, S.A.; Van Deynze, A. Foraging range of honey bees, Apis mellifera, in alfalfa seed production fields. J. Insect Sci. 2011, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch, J.; Blas, M. Foraging behaviour and pollinating efficiency of Osmia cornuta and Apis mellifera on almond (Hymenoptera, Megachilidae and Apidae). Appl. Entomol. Zool. 1994, 29, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vicens, N.; Bosch, J. Pollinating efficacy of Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae, Apidae) on ‘Red Delicious’ apple. Environ. Entomol. 2000, 29, 235–240. [Google Scholar] [CrossRef]
- Thomson, J.D.; Goodell, K. Pollen removal and deposition by honeybee and bumblebee visitors to apple and almond flowers. J. Appl. Ecol. 2001, 38, 1032–1044. [Google Scholar] [CrossRef]
- Monzón, V.H.; Bosch, J.; Retana, J. Foraging behavior and pollinating effectiveness of Osmia cornuta (Hymenoptera: Megachilidae) and Apis mellifera (Hymenoptera: Apidae) on “Comice” pear. Apidologie 2004, 35, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Eeraerts, M.; Vanderhaegen, R.; Smagghe, G.; Meeus, I. Pollination efficiency and foraging behaviour of honey bees and non-Apis bees to sweet cherry. Agric. For. Entomol. 2020, 22, 75–82. [Google Scholar] [CrossRef]
- Roquer-Beni, L.; Arnan, X.; Rodrigo, A.; Bosch, J. What Makes a Good Pollinator? Relationship between Pollinator Traits and Pollination Effectiveness in Apple Flowers. (Unpublished; Manuscript in Preparation).
- Bosch, J.; Kemp, W.P. Developing and establishing bee species as crop pollinators: The example of Osmia spp. (Hymenoptera: Megachilidae) and fruit trees. Bull. Entomol. Res. 2002, 92, 3–16. [Google Scholar] [PubMed]
- Sédivy, C.; Dorn, S. Towards a sustainable management of bees of the subgenus Osmia (Megachilidae; Osmia) as fruit tree pollinators. Apidologie 2014, 45, 88–105. [Google Scholar] [CrossRef] [Green Version]
- Vicens, N.; Bosch, J. Nest site orientation and relocation of populations of the orchard pollinator Osmia cornuta (Hymenoptera: Megachilidae). Environ. Entomol. 29, 69–75. [CrossRef]
- Guédot, C.; Bosch, J.; Kemp, W.P. Effect of three-dimension and color contrast on nest localization performance of two solitary bees (Hymenoptera: Megachilidae). J. Kansas Entomol. Soc. 2007, 80, 90–104. [Google Scholar] [CrossRef]
- Biddinger, D.; Joshi, N.; Rajotte, E.; Halbrendt, N. An immunomarking method to determine the foraging patterns of Osmia cornifrons and resulting fruit set in a cherry orchard. Apidologie 2013, 44, 738–749. [Google Scholar] [CrossRef] [Green Version]
- Torchio, P.F. Use of Osmia lignaria Say (Hymenoptera: Apoidea: Megachilidae) as a pollinator in an apple and prune orchard. J. Kansas Entomol. Soc. 1976, 49, 475–482. [Google Scholar]
- Torchio, P.F. Field experiments with Osmia lignaria propinqua Cresson as a pollinator in almond orchards: II, 1976 studies (Hymenoptera: Megachilidae). J. Kansas Entomol. Soc. 1981, 54, 824–836. [Google Scholar]
- Maeta, Y. Comparative studies on the biology of the bees of the genus Osmia in Japan, with special reference to their management for pollination of crops (Hymenoptera, Megachilidae). Bull. Tohoku Natl. Agric. Exp. Stn. 1978, 57, 1–221. (In Japanese) [Google Scholar]
- Márquez, J.; Bosch, J.; Vicens, N. Pollens collected by wild and managed populations of the potential orchard pollinator Osmia cornuta (Latr.) (Hym., Megachilidae). J. Appl. Entomol. 1994, 117, 353–359. [Google Scholar] [CrossRef]
- Maccagnani, B.; Ladurner, E.; Santi, F.; Burgio, G. Osmia cornuta (Hymenoptera, Megachilidae) as a pollinator of pear (Pyrus communis): Fruit- and seed-set. Apidologie 2003, 34, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Garratt, M.P.D.; Breeze, T.D.; Boreux, V.; Fountain, M.T.; McKerchar, M.; Webber, S.M.; Coston, D.J.; Jenner, N.; Dean, R.; Westbury, D.B.; et al. Apple pollination: Demand depends on variety and supply depends on pollinator identity. PLoS ONE 2016, 11, e0153889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch, J.; Kemp, W.P.; Trostle, G.E. Bee population returns and cherry yields in an orchard pollinated with Osmia lignaria (Hymenoptera: Megachilidae). J. Econ. Entomol. 2006, 99, 408–413. [Google Scholar] [CrossRef]
- Sheffield, C.S. Pollination, seed set and fruit quality in apple: Studies with Osmia lignaria (Hymenoptera: Megachilidae) in the Annapolis Valley, Nova Scotia, Canada. J. Pollinat. Ecol. 2014, 12. [Google Scholar] [CrossRef]
- Velthuis, H.H.W.; van Doorn, A. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 2006, 37, 421–451. [Google Scholar] [CrossRef] [Green Version]
- van der Steen, J.J.M. Indoor rearing of the solitary bee Osmia rufa L. Proc. Exp. Appl. Entomol. NEV Amst. 1997, 8, 81–84. [Google Scholar]
- Ladurner, E.; Maccagnani, B.; Tesoriero, D.; Nepi, M.; Felicioli, A. Laboratory rearing of Osmia cornuta Latreille (Hymenoptera Megachilidae) on artificial diet. Boll. Dell’ist. Entomol. Univ. Bologna 1999, 53, 133–146. [Google Scholar]
- Bosch, J.; Vicens, N. Body size as an estimator of production costs in a solitary bee. Ecol. Entomol. 2002, 27, 129–137. [Google Scholar] [CrossRef]
- Kester, D.E.; Griggs, W.H. Fruit setting in the almond: The effect of cross-pollinating various percentages of flowers. Proc. Am. Soc. Hortic. Sci. 1959, 74, 206–213. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Inference: A Practical Information-Theoretic Approach, 1st ed.; Springer: New York, NY, USA, 2002. [Google Scholar] [CrossRef] [Green Version]
- Barton, K. ‘MuMIn’: Multi-Model Inference. R Package Version 1.43.17. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 11 January 2021).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. ‘nlme’: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-151. Available online: https://CRAN.R-project.org/package=nlme (accessed on 11 January 2021).
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.0.1; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Melin, A.; Rouget, M.; Midgley, J.J.; Donadson, J.S. Pollination ecosystem services in South African agricultural systems. S. Afr. J. Sci. 2014, 110, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sgolastra, F.; Arnan, X.; Pitts-Singer, T.L.; Maini, S.; Kemp, W.P.; Bosch, J. Pre-wintering conditions and post-winter performance in a solitary bee: Does diapause impose an energetic cost on reproductive success? Ecol. Entomol. 2016, 41, 201–210. [Google Scholar] [CrossRef]
- Felicioli, A. Le osmie. In Api e Impollinazione; Pinzauti, M., Ed.; Giunta Regionale Toscana: Firenze, Italy, 2000; pp. 159–188. [Google Scholar]
- Krunić, M.; Stanisaviljević, L.Ž. The Biology of European Orchard bee Osmia Cornuta (Latr.) (Hymenoptera: Megachilidae); Faculty of Biology University of Belgrade: Belgrade, Serbia, 2006. [Google Scholar]
- Bosch, J.; Kemp, W.P. How to Manage the Blue Orchard Bee: As an Orchard Pollinator; Sustainable Agriculture Network: Beltsville, MD, USA, 2001. [Google Scholar]
- Bosch, J.; Vicens, N. Relationship between body size, provisioning rate, longevity and reproductive success in females of the solitary bee Osmia cornuta. Behav. Ecol. Sociobiol. 2006, 60, 26–33. [Google Scholar] [CrossRef]
- Boyle, N.K.; Pitts-Singer, T.L. The effect of nest box distribution on sustainable propagation of Osmia lignaria (Hymenoptera: Megachilidae) in commercial tart cherry orchards. J. Insect Sci. 2016, 17, 41. [Google Scholar] [CrossRef] [Green Version]
- Bosch, J. Osmia cornuta Latr. (Hymenoptera, Megachilidae) as a potential pollinator in almond orchards: Releasing methods and nest-hole length. J. Appl. Entomol. 1994, 117, 151–157. [Google Scholar] [CrossRef]
- Bosch, J. Improvement of field management of Osmia cornuta Latr. for almond pollination (Hymenoptera, Megachilidae). Apidologie 1994, 25, 71–83. [Google Scholar] [CrossRef] [Green Version]
- Bosch, J. Parasitism in wild and managed populations of the almond pollinator Osmia cornuta Latr. (Hymenoptera, Megachilidae). J. Apicult. Res. 1992, 31, 77–82. [Google Scholar] [CrossRef]
- Bosch, J. Comparison of nesting materials for the orchard pollinator Osmia cornuta Latr. (Hymenoptera, Megachilidae). Entomol. Gen. 1995, 19, 285–289. [Google Scholar] [CrossRef]
- Torchio, P.F. Field experiments with the pollinator species, Osmia lignaria propinqua Cresson (Hymenoptera: Megachilidae) in apple orchards: III, 1977 studies. J. Kansas Entomol. Soc. 1984, 57, 517–521. [Google Scholar]
- Torchio, P.F. Field experiments with the pollinator species, Osmia lignaria propinqua Cresson in apple orchards: V, 1979–1980, methods of introducing bees, nesting success, seed counts, fruit yields (Hymenoptera: Megachilidae). J. Kansas Entomol. Soc. 1985, 58, 448–464. [Google Scholar]
- Pitts-Singer, T.L. Olfactory response of megachilid bees, Osmia lignaria, Megachile rotundata, and M. pugnata, to individual cues from old nest cavities. Environ. Entomol. 2007, 36, 402–408. [Google Scholar] [CrossRef]
- Vicens, N.; Bosch, J.; Blas, M. Análisis de los nidos de algunas Osmia (Hymenoptera, Megachilidae) nidificantes en cavidades preestablecidas. Orsis 1993, 8, 41–52. [Google Scholar]
- Bosch, J.; Vicens, N. Sex allocation in the solitary bee Osmia cornuta: Do females behave in agreement with Fisher’s theory? Behav. Ecol. Sociobiol. 2005, 59, 124–132. [Google Scholar] [CrossRef]
- Tepedino, V.J.; Torchio, P.F. The influence of nest-hole selection on sex ratio and progeny size in Osmia lignaria propinqua (Hymenoptera: Megachilidae). Ann. Entomol. Soc. Am. 1989, 82, 355–360. [Google Scholar] [CrossRef]
- Seidelmann, K.; Bienasch, A.; Pröhl, F. The impact of nest tube dimensions on reproduction parameters in a cavity nesting solitary bee, Osmia bicornis (Hymenoptera: Megachili-dae). Apidologie 2016, 47, 114–122. [Google Scholar] [CrossRef] [Green Version]
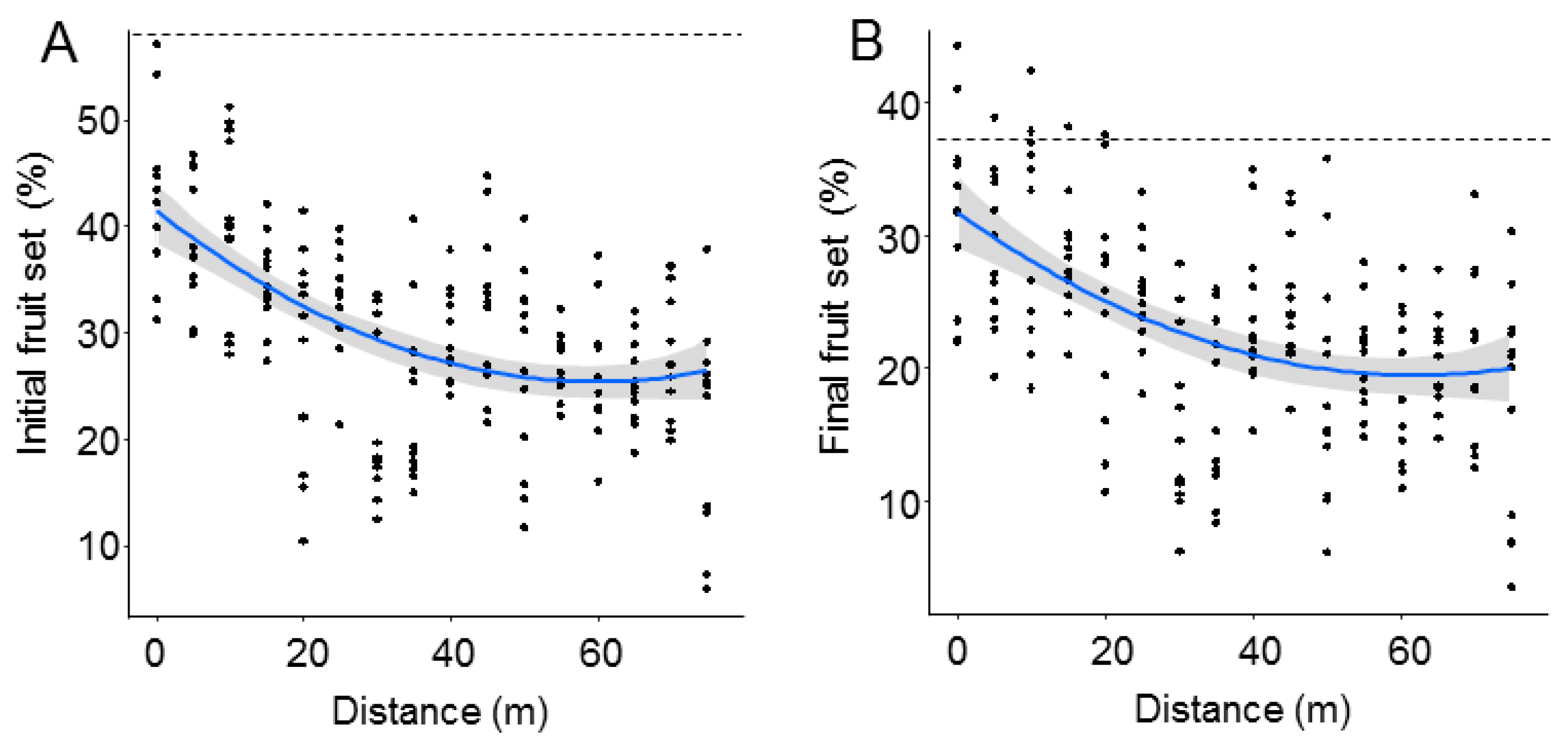
| A. Initial Fruit Set | B. Final Fruit Set | |||
|---|---|---|---|---|
| t-Value | p-Value | t-Value | p-Value | |
| (Intercept) | 25.27 | <0.0001 | 22.63 | <0.0001 |
| Distance | −3.33 | <0.0001 | −2.85 | 0.0001 |
| Distance2 | 2.12 | 0.0395 | 1.75 | 0.0857 |
| R2m: 0.28; R2c: 0.67 | R2m: 0.22; R2c: 0.59 | |||
| (A) Initial Fruit Set. | |||||
|---|---|---|---|---|---|
| Estimate | SE | Adjusted SE | z Value | Pr(>|z|) | |
| (Intercept) | 0.5545 | 0.028 | 0.031 | 17.62 | <2e−16 |
| O. cornuta | 0.0099 | 0.003 | 0.003 | 3.08 | 0.002 |
| A. mellifera | −0.0004 | 0.001 | 0.001 | 0.39 | 0.689 |
| Adjusted R2: 0.44 | |||||
| (B) Final Fruit Set | |||||
| Estimate | SE | Adjusted SE | z Value | Pr(>|z|) | |
| (Intercept) | 0.489 | 0.043 | 0.046 | 10.63 | <2e−16 |
| O. cornuta | 0.009 | 0.003 | 0.003 | 2.70 | 0.007 |
| A. mellifera | −0.001 | 0.001 | 0.001 | 1.17 | 0.241 |
| Adjusted R2: 0.36 | |||||
| This Study | Other Studies 7 | |
|---|---|---|
| Winter mortality 1 | 8.1% | 5.5 ± 0.7 (n = 8) |
| Female establishment 2 | 54.4% | 50.7 ± 5.1 (n = 14) |
| Fecundity 3 | 9.2 | 9.8 ± 0.9 (n = 10) |
| Progeny sex ratio 4 | 2.6 | 2.5 ± 0.18 (n = 12) |
| Developmental mortality 5 | 5.3% | 8.5 ± 0.9 (n = 15) |
| Parasitism 6 | 1.8% | 1.6 ± 0.4 (n = 17) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosch, J.; Osorio-Canadas, S.; Sgolastra, F.; Vicens, N. Use of a Managed Solitary Bee to Pollinate Almonds: Population Sustainability and Increased Fruit Set. Insects 2021, 12, 56. https://doi.org/10.3390/insects12010056
Bosch J, Osorio-Canadas S, Sgolastra F, Vicens N. Use of a Managed Solitary Bee to Pollinate Almonds: Population Sustainability and Increased Fruit Set. Insects. 2021; 12(1):56. https://doi.org/10.3390/insects12010056
Chicago/Turabian StyleBosch, Jordi, Sergio Osorio-Canadas, Fabio Sgolastra, and Narcís Vicens. 2021. "Use of a Managed Solitary Bee to Pollinate Almonds: Population Sustainability and Increased Fruit Set" Insects 12, no. 1: 56. https://doi.org/10.3390/insects12010056
APA StyleBosch, J., Osorio-Canadas, S., Sgolastra, F., & Vicens, N. (2021). Use of a Managed Solitary Bee to Pollinate Almonds: Population Sustainability and Increased Fruit Set. Insects, 12(1), 56. https://doi.org/10.3390/insects12010056






