Alarm Pheromone Responses Depend on Genotype, but Not on the Presence of Facultative Endosymbionts in the Pea Aphid Acyrthosiphon pisum
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Endosymbiont Treatments
2.2. Stimuli
2.3. Behavioural Tests
2.4. Electroantennogram
2.5. Data Analyses
2.5.1. Olfactometry
2.5.2. Electroantennogram
3. Results
3.1. Responses to EBF in the Olfactometer
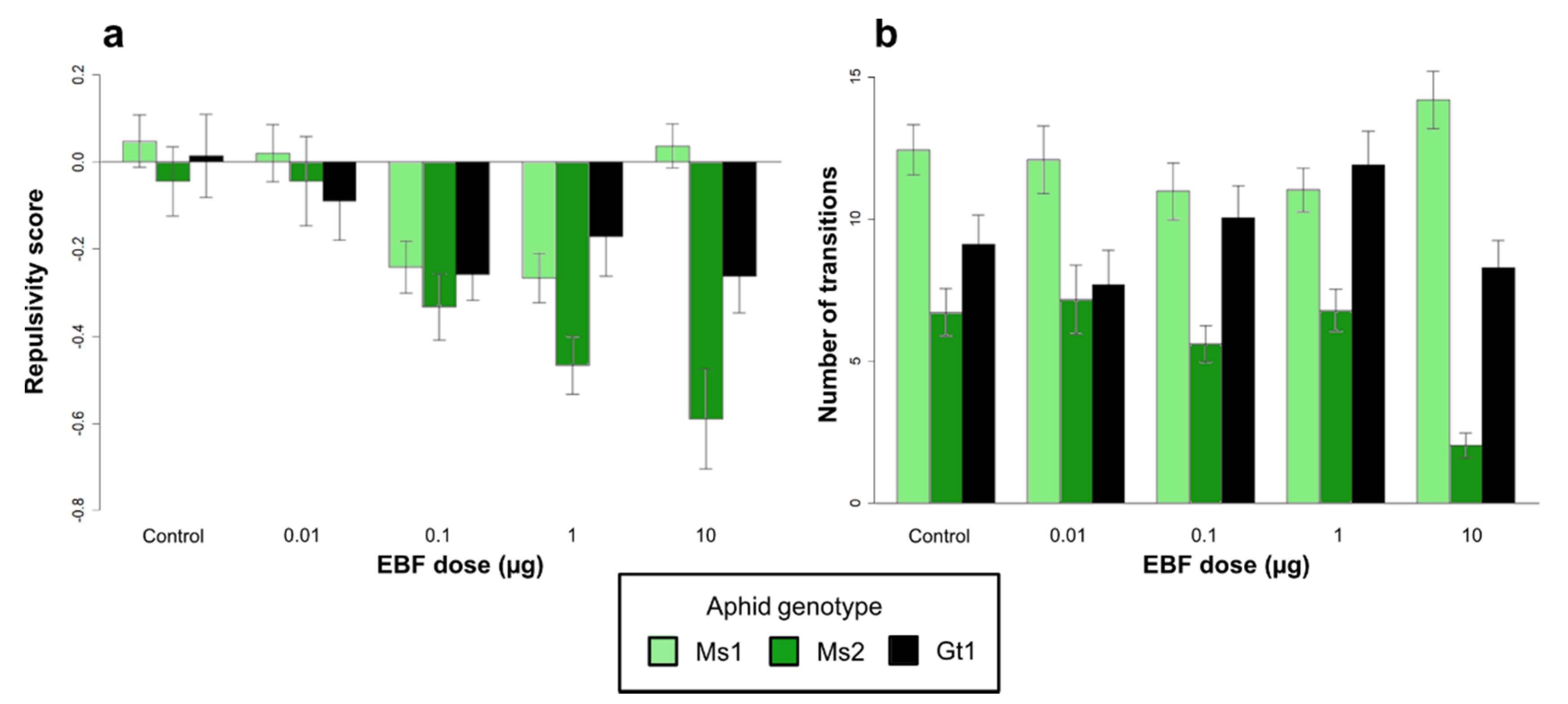
3.1.1. Effect of Aphid Genotypes on EBF Responses within the Olfactometer
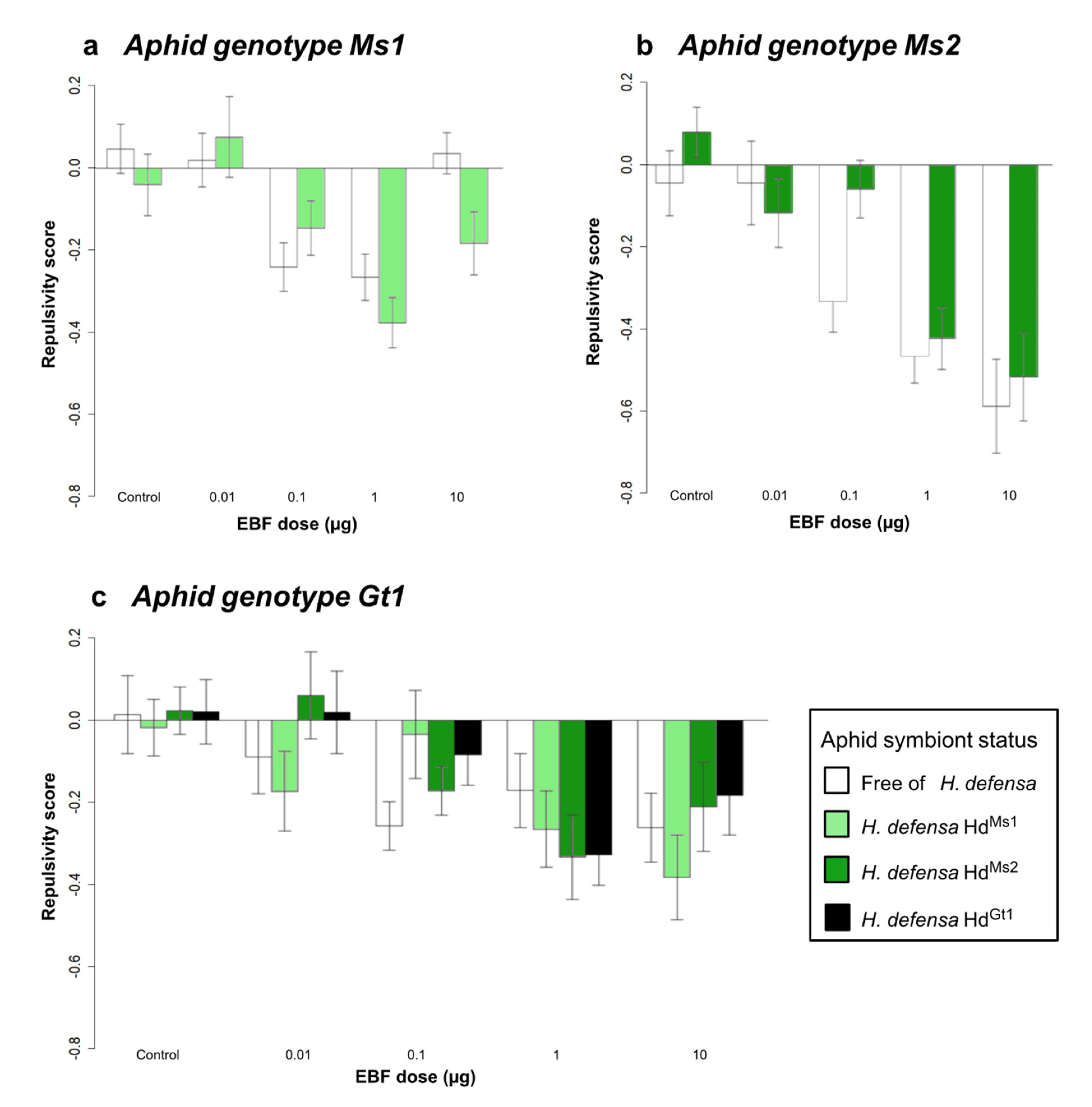
3.1.2. Effect of Symbiotic Status on EBF Responses within the Olfactometer
3.2. Effect of Genotype and Symbiotic Status on the Antennal Sensitivity to EBF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nault, L.R.; Edwards, L.J.; Styer, W.E. Aphid Alarm Pheromones: Secretion and Reception1. Environ. Entomol. 1973, 2, 101–105. [Google Scholar] [CrossRef]
- Kunert, G.; Belz, E.; Simon, J.-C.; Weisser, W.W.; Outreman, Y. Differences in defensive behaviour between host-adapted races of the pea aphid. Ecol. Entomol. 2010, 35, 147–154. [Google Scholar] [CrossRef]
- Vandermoten, S.; Mescher, M.C.; Francis, F.; Haubruge, E.; Verheggen, F.J. Aphid alarm pheromone: An overview of current knowledge on biosynthesis and functions. Insect Biochem. Mol. Biol. 2012, 42, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Aradottir, G.I.; Smart, L.E.; Martin, J.L.; Caulfield, J.C.; Doherty, A.; Sparks, C.A.; Woodcock, C.M.; Birkett, M.A.; Napier, J.A.; et al. The first crop plant genetically engineered to release an insect pheromone for defence. Sci. Rep. 2015, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, K.M.; Degnan, P.H.; Burke, G.R.; Moran, N.A. Facultative Symbionts in Aphids and the Horizontal Transfer of Ecologically Important Traits. Annu. Rev. Entomol. 2010, 55, 247–266. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Wang, B.; Grossi, G.; Falabella, P.; Liu, Y.; Yan, S.; Lu, J.; Xi, J.; Wang, G. Molecular Basis of Alarm Pheromone Detection in Aphids. Curr. Biol. 2017, 27, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Peccoud, J.; Simon, J.-C. The pea aphid complex as a model of ecological speciation. Ecol. Entomol. 2010, 35, 119–130. [Google Scholar] [CrossRef]
- Peccoud, J.; Ollivier, A.; Plantegenest, M.; Simon, J. A continuum of genetic divergence from sympatric host races to species in the pea aphid complex. Proc. Natl. Acad. Sci. USA 2009, 106, 7495–7500. [Google Scholar] [CrossRef] [Green Version]
- Peccoud, J.; Mahéo, F.; de la Huerta, M.; Laurence, C.; Simon, J.-C. Genetic characterisation of new host-specialised biotypes and novel associations with bacterial symbionts in the pea aphid complex. Insect Conserv. Divers. 2015, 8, 484–492. [Google Scholar] [CrossRef]
- Dion, E.; Zélé, F.; Simon, J.-C.; Outreman, Y. Rapid evolution of parasitoids when faced with the symbiont-mediated resistance of their hosts. J. Evol. Biol. 2011, 24, 741–750. [Google Scholar] [CrossRef]
- Polin, S.; Simon, J.-C.; Outreman, Y. An ecological cost associated with protective symbionts of aphids. Ecol. Evol. 2014, 4, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Sochard, C.; Leclair, M.; Simon, J.-C.; Outreman, Y. Host plant effects on the outcomes of defensive symbioses in the pea aphid complex. Evol. Ecol. 2019, 33, 651–669. [Google Scholar] [CrossRef]
- Sochard, C.; Bellec, L.; Simon, J.-C.; Outreman, Y. Influence of ‘protective’ symbionts throughout the different steps of an aphid-parasitoid interaction. Curr. Zool. 2020. [Google Scholar] [CrossRef]
- Sochard, C.; Le Floch, M.; Anton, S.; Outreman, Y.; Simon, J.-C. Little influence of gain and loss of symbionts on host plant selection in specialized pea aphid genotypes. Entomol. Gen. 2020. [Google Scholar] [CrossRef]
- Ben-Ari, M.; Outreman, Y.; Denis, G.; Le Gallic, J.-F.; Inbar, M.; Simon, J.-C. Differences in escape behavior between pea aphid biotypes reflect their host plants’ palatability to mammalian herbivores. Basic Appl. Ecol. 2019, 34, 108–117. [Google Scholar] [CrossRef]
- Frago, E.; Dicke, M.; Godfray, H.C.J. Insect symbionts as hidden players in insect–plant interactions. Trends Ecol. Evol. 2012, 27, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Sachs, J.L.; Skophammer, R.G.; Regus, J.U. Evolutionary transitions in bacterial symbiosis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 2), 10800–10807. [Google Scholar] [CrossRef] [Green Version]
- Feldhaar, H. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol. Entomol. 2011, 36, 533–543. [Google Scholar] [CrossRef]
- Douglas, A.E. The microbial dimension in insect nutritional ecology. Funct. Ecol. 2009, 23, 38–47. [Google Scholar] [CrossRef]
- Haine, E.R. Symbiont-mediated protection. Proc. Biol. Sci. 2008, 275, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Engelstädter, J.; Hurst, G.D.D. The ecology and evolution of microbes that manipulate host reproduction. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 127–149. [Google Scholar] [CrossRef]
- Anderson, P.; Anton, S. Experience-based modulation of behavioural responses to plant volatiles and other sensory cues in insect herbivores. Plant. Cell Environ. 2014, 37, 1826–1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadenne, C.; Barrozo, R.B.; Anton, S. Plasticity in insect olfaction: To smell or not to smell? Annu. Rev. Entomol. 2016, 61, 317–333. [Google Scholar] [CrossRef] [PubMed]
- François, A.; Grebert, D.; Rhimi, M.; Mariadassou, M.; Naudon, L.; Rabot, S.; Meunier, N. Olfactory epithelium changes in germfree mice. Sci. Rep. 2016, 6, 24687. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.C.-N.; Wang, Q.-P.; Morimoto, J.; Senior, A.M.; Lihoreau, M.; Neely, G.G.; Simpson, S.J.; Ponton, F. Gut microbiota modifies olfactory-guided microbial preferences and foraging decisions in Drosophila. Curr. Biol. 2017, 1–13. [Google Scholar] [CrossRef]
- Gauthier, J.-P.; Outreman, Y.; Mieuzet, L.; Simon, J.-C. Bacterial communities associated with host-adapted populations of pea aphids revealed by deep sequencing of 16S ribosomal DNA. PLoS ONE 2015, 10, e0120664. [Google Scholar] [CrossRef]
- Tsuchida, T.; Koga, R.; Shibao, H.; Matsumoto, T.; Fukatsu, T. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 2002, 11, 2123–2135. [Google Scholar] [CrossRef]
- Simon, J.-C.; Carré, S.; Boutin, M.; Prunier-Leterme, N.; Sabater-Mun, B.; Latorre, A.; Bournoville, R. Host-based divergence in populations of the pea aphid: Insights from nuclear markers and the prevalence of facultative symbionts. Proc. R. Soc. Biol. Sci. R. Soc. 2003, 270, 1703–1712. [Google Scholar] [CrossRef] [Green Version]
- Leclair, M.; Pons, I.; Mahéo, F.; Morlière, S.; Simon, J.-C.; Outreman, Y. Diversity in symbiont consortia in the pea aphid complex is associated with large phenotypic variation in the insect host. Evol. Ecol. 2016, 30, 925–941. [Google Scholar] [CrossRef]
- Sochard, C.; Morlière, S.; Toussaint, G.; Outreman, Y.; Sugio, A.; Simon, J.-C. Examination of the success rate of secondary symbiont manipulation by microinjection methods in the pea aphid system. Entomol. Exp. Appl. 2020, 168, 174–183. [Google Scholar] [CrossRef]
- Sandström, J. Performance of pea aphid (Acyrthosiphon pisum clones on host plants and synthetic diets mimicking the same plants phloem amino acid composition. J. Comput. Neurosci. 1994, 40, 1051–1057. [Google Scholar] [CrossRef]
- Thöming, G.; Norli, H.R.; Saucke, H.; Knudsen, G.K. Pea plant volatiles guide host location behaviour in the pea moth. Arthropod-Plant Interact. 2014, 8, 109–122. [Google Scholar] [CrossRef]
- Pettersson, J. An aphid sex attractant. Insect Syst. Evol. 1970, 1, 63–73. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-37027-9. [Google Scholar]
- Duvaux, L.; Geissmann, Q.; Gharbi, K.; Zhou, J.-J.; Ferrari, J.; Smadja, C.M.; Butlin, R.K. Dynamics of copy number variation in host races of the pea aphid. Mol. Biol. Evol. 2015, 32, 63–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyres, I.; Duvaux, L.; Gharbi, K.; Tucker, R.; Hopkins, D.; Simon, J.-C.; Ferrari, J.; Smadja, C.M.; Butlin, R.K. Targeted re-sequencing confirms the importance of chemosensory genes in aphid host race differentiation. Mol. Ecol. 2016, 26, 43–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyres, I.; Jaquiéry, J.; Sugio, A.; Duvaux, L.; Gharbi, K.; Zhou, J.-J.; Legeai, F.; Nelson, M.; Simon, J.-C.; Smadja, C.M.; et al. Differential gene expression according to race and host plant in the pea aphid. Mol. Ecol. 2016, 25, 4197–4215. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Nielsen, J.E.; Cunningham, J.P.; McGraw, E.A. Wolbachia infection alters olfactory-cued locomotion in Drosophila spp. Appl. Environ. Microbiol. 2008, 74, 3943–3948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Y.; Wang, Y. Infection of Wolbachia may improve the olfactory response of Drosophila. Chin. Sci. Bull. 2009, 54, 1369–1375. [Google Scholar] [CrossRef] [Green Version]


| Line Name | Aphid Genotype | Aphid Biotype | Secondary Symbiont Strain |
|---|---|---|---|
| Ms1-Hd- | Ms1 | Medicago sativa | No secondary symbiont |
| Ms1-HdMs1 | Hamiltonella defensa from Ms1 | ||
| Ms2-Hd- | Ms2 | Medicago sativa | No secondary symbiont |
| Ms2-HdMs2 | H. defensa from Ms2 | ||
| Gt1-Hd- | Gt1 | Genista tinctoria | No secondary symbiont |
| Gt1-HdGt1 | H. defensa from Gt1 | ||
| Gt1-Hd Ms1 | H. defensa from Ms1 | ||
| Gt1-HdMs2 | H. defensa from Ms2 |
| Variables | |||||||
|---|---|---|---|---|---|---|---|
| Repulsivity Score | Number of Transitions | ||||||
| Covariates | F | Df | p-Value | χ² | Df | p-Value | |
| Effect of aphid genotype | Aphid genotype (1) | 0.34 | 2 | 0.707 | 1345.37 | 2 | <0.001 |
| EBF dose (2) | 2.21 | 4 | 0.066 | 9.82 | 4 | 0.043 | |
| (1) × (2) | 2.72 | 8 | 0.006 | 55.78 | 8 | <0.001 | |
| Effect of aphid symbiotic status | Genotype Ms1 | ||||||
| Symbiotic status (1) | 1.47 | 1 | 0.225 | 0.01 | 1 | 0.928 | |
| EBF dose (2) | 9.64 | 4 | <0.001 | 8.35 | 4 | 0.079 | |
| (1) × (2) | 1.77 | 4 | 0.134 | 7.59 | 4 | 0.107 | |
| Genotype Ms2 | |||||||
| Symbiotic status (1) | 2.63 | 1 | 0.105 | 2.05 | 1 | 0.152 | |
| EBF dose (2) | 15.79 | 4 | <0.001 | 45.62 | 4 | <0.001 | |
| (1) × (2) | 1.09 | 4 | 0.361 | 15.58 | 4 | 0.003 | |
| Genotype Gt1 | |||||||
| Symbiotic status (1) | 0.50 | 3 | 0.681 | 2.87 | 3 | 0.411 | |
| EBF dose (2) | 8.12 | 4 | <0.001 | 6.76 | 4 | 0.148 | |
| (1) × (2) | 0.98 | 12 | 0.464 | 46.38 | 12 | <0.001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badji, C.A.; Sol-Mochkovitch, Z.; Fallais, C.; Sochard, C.; Simon, J.-C.; Outreman, Y.; Anton, S. Alarm Pheromone Responses Depend on Genotype, but Not on the Presence of Facultative Endosymbionts in the Pea Aphid Acyrthosiphon pisum. Insects 2021, 12, 43. https://doi.org/10.3390/insects12010043
Badji CA, Sol-Mochkovitch Z, Fallais C, Sochard C, Simon J-C, Outreman Y, Anton S. Alarm Pheromone Responses Depend on Genotype, but Not on the Presence of Facultative Endosymbionts in the Pea Aphid Acyrthosiphon pisum. Insects. 2021; 12(1):43. https://doi.org/10.3390/insects12010043
Chicago/Turabian StyleBadji, Cesar Auguste, Zoé Sol-Mochkovitch, Charlotte Fallais, Corentin Sochard, Jean-Christophe Simon, Yannick Outreman, and Sylvia Anton. 2021. "Alarm Pheromone Responses Depend on Genotype, but Not on the Presence of Facultative Endosymbionts in the Pea Aphid Acyrthosiphon pisum" Insects 12, no. 1: 43. https://doi.org/10.3390/insects12010043
APA StyleBadji, C. A., Sol-Mochkovitch, Z., Fallais, C., Sochard, C., Simon, J.-C., Outreman, Y., & Anton, S. (2021). Alarm Pheromone Responses Depend on Genotype, but Not on the Presence of Facultative Endosymbionts in the Pea Aphid Acyrthosiphon pisum. Insects, 12(1), 43. https://doi.org/10.3390/insects12010043






