Parasitoid Abundance and Community Composition in Desert Vineyards and Their Adjacent Natural Habitats
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Area and Sampling Design
2.2. Statistical Analyses
3. Results
3.1. Parasitoid Families
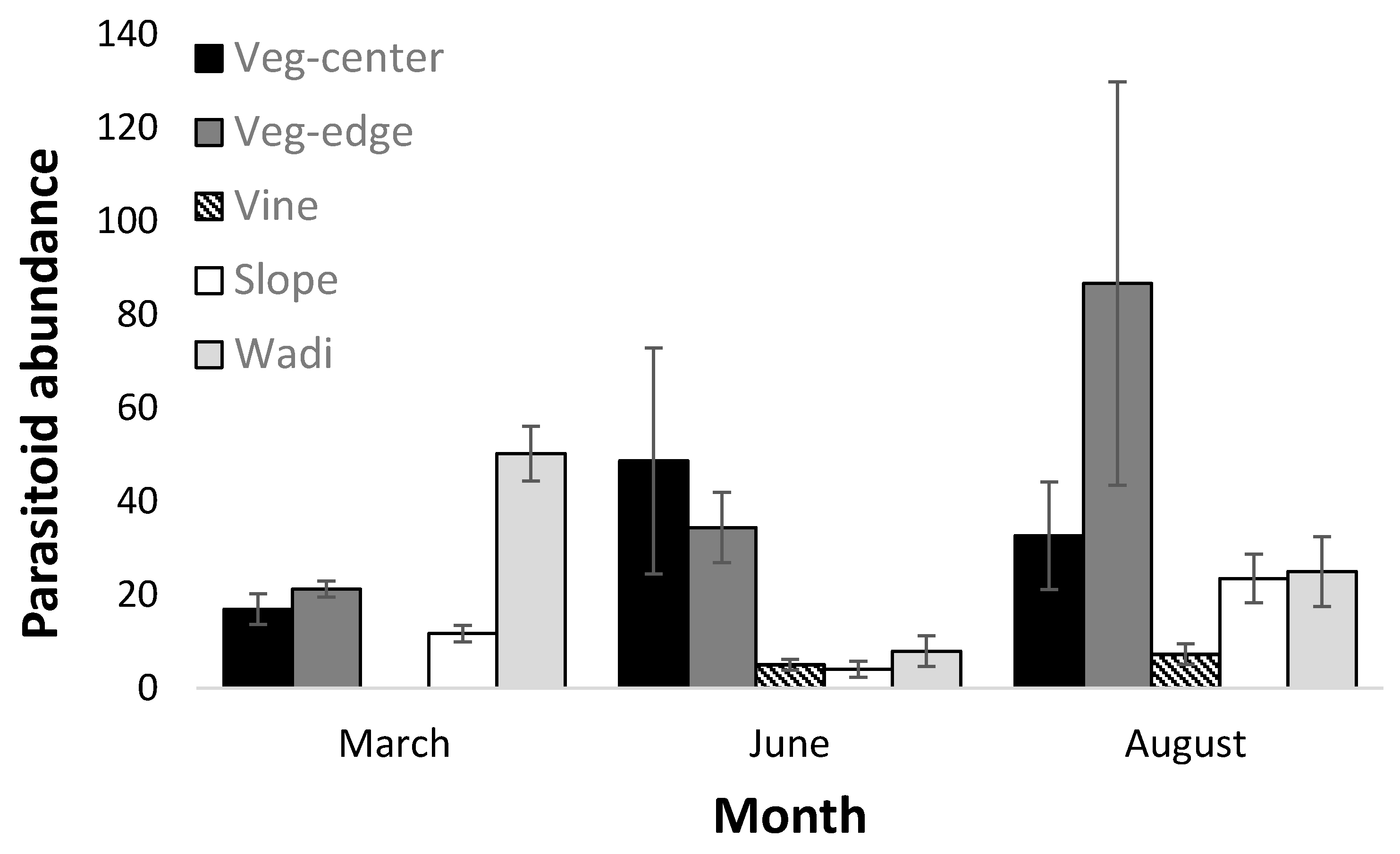
3.2. Parasitoid Abundance
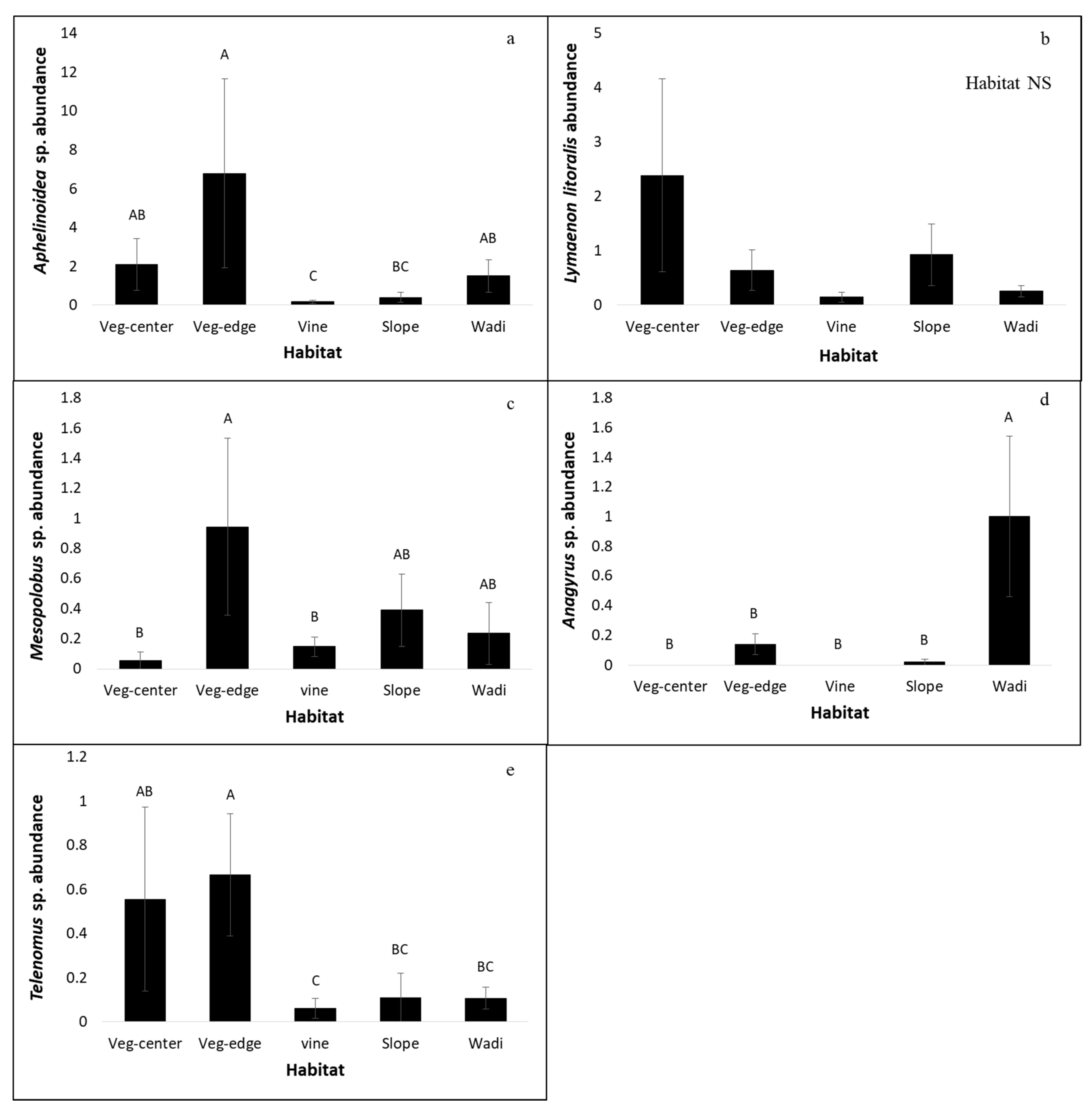
3.3. Dominant Species
3.4. Parasitoid Community Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barbosa, P. Conservation Biological Control; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef]
- Chaplin-Kramer, R.; O′Rourke, M.E.; Blitzer, E.J.; Kremen, C. A meta-analysis of crop pest and natural enemy response to landscape complexity. Ecol. Lett. 2011, 14, 922–932. [Google Scholar] [CrossRef]
- Rusch, A.; Chaplin-Kramer, R.; Gardiner, M.M.; Hawro, V.; Holland, J.; Landis, D.; Thies, C.; Tscharntke, T.; Weisser, W.W.; Winqvist, C.; et al. Agricultural landscape simplification reduces natural pest control: A quantitative synthesis. Agric. Ecosyst. Environ. 2016, 221, 198–204. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Tscharntke, T.; Rand, T.A.; Bianchi, F.J.J.A. The landscape context of trophic interactions: Insect spillover across the crop-noncrop interface. Ann. Zool. Fenn. 2005, 42, 421–432. [Google Scholar]
- Karp, D.S.; Chaplin-Kramer, R.; Meehan, T.D.; Martin, E.A.; DeClerck, F.; Grab, H.; Gratton, C.; Hunt, L.; Larsen, A.E.; Martinez-Salinas, A.; et al. Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc. Natl. Acad. Sci. USA 2018, 115, E7863–E7870. [Google Scholar] [CrossRef]
- Huang, J.P.; Ji, M.X.; Xie, Y.K.; Wang, S.S.; He, Y.L.; Ran, J.J. Global semi-arid climate change over last 60 years. Clim. Dyn. 2016, 46, 1131–1150. [Google Scholar] [CrossRef]
- Gavish-Regev, E.; Lubin, Y.; Coll, M. Migration patterns and functional groups of spiders in a desert agroecosystem. Ecol. Entomol. 2008, 33, 202–212. [Google Scholar] [CrossRef]
- Forbes, A.A.; Bagley, R.K.; Beer, M.A.; Hippee, A.C.; Widmayer, H.A. Quantifying the unquantifable: Why Hymenoptera, not Coleoptera, is the most speciose animal order. BMC Ecol. 2018, 21. [Google Scholar] [CrossRef]
- Kidd, N.A.C.; Jervis, M.A. Population dynamics. In Insects as Natural Enemies: A Practical Perspective; Jervis, M.A., Ed.; Springer: Dodrecht, The Netherlands, 2007. [Google Scholar]
- Hajek, A.E.; Eilenberg, J. Natural Enemies: An Introduction to Biological Control; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Landis, D.A.; Haas, M.J. Influence of landscape structure on abundance and within-field distribution of European corn-borer (Lepidoptera, Pyralidae) larval parasitoids in Michigan. Environ. Entomol. 1992, 21, 409–416. [Google Scholar] [CrossRef]
- Kruess, A.; Tscharntke, T. Habitat fragmentation, species loss, and biological-control. Science 1994, 264, 1581–1584. [Google Scholar] [CrossRef]
- Murphy, B.C.; Rosenheim, J.A.; Dowell, R.V.; Granett, J. Habitat diversification tactic for improving biological control: Parasitism of the western grape leafhopper. Entomol. Exp. Appl. 1998, 87, 225–235. [Google Scholar] [CrossRef]
- Menalled, F.D.; Costamagna, A.C.; Marino, P.C.; Landis, D.A. Temporal variation in the response of parasitoids to agricultural landscape structure. Agric. Ecosyst. Environ. 2003, 96, 29–35. [Google Scholar] [CrossRef]
- Thomson, L.J.; Hoffmann, A.A. Vegetation increases the abundance of natural enemies in vineyards. Biol. Control. 2009, 49, 259–269. [Google Scholar] [CrossRef]
- Plecas, M.; Gagic, V.; Jankovic, M.; Petrovic-Obradovic, O.; Kavallieratos, N.G.; Tomanovic, Z.; Thies, C.; Tscharntke, T.; Cetkovic, A. Landscape composition and configuration influence cereal aphid-parasitoid-hyperparasitoid interactions and biological control differentially across years. Agric. Ecosyst. Environ. 2014, 183, 1–10. [Google Scholar] [CrossRef]
- Wilson, H.; Miles, A.F.; Daane, K.M.; Altieri, M.A. Landscape diversity and crop vigor influence biological control of the western grape leafhopper (E. elegantula Osborn) in vineyards. PLoS ONE 2015, 10, E0141752. [Google Scholar] [CrossRef]
- Danne, A.; Thomson, L.J.; Sharley, D.J.; Penfold, C.M.; Hoffmann, A.A. Effects of native grass cover crops on beneficial and pest invertebrates in Australian vineyards. Environ. Entomol. 2010, 39, 970–978. [Google Scholar] [CrossRef]
- Ashkenazi, E.; Avni, Y.; Avni, G. A comprehensive characterization of ancient desert agricultural systems in the Negev Highlands of Israel. J. Arid. Environ. 2012, 86, 55–64. [Google Scholar] [CrossRef]
- Erickson-Gini, T. Nabataean agriculture: Myth and reality. J. Arid. Environ. 2012, 86, 50–54. [Google Scholar] [CrossRef]
- Evenari, M.; Shanan, L.; Tadmor, N. The Negev: The Challenge of a Desert; Harvard University Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Daane, K.M.; Hogg, B.N.; Wilson, H.; Yokota, G.Y. Native grass ground covers provide multiple ecosystem services in Californian vineyards. J. Appl. Ecol. 2018, 55, 2473–2483. [Google Scholar] [CrossRef]
- Masner, L. Revisionary notes and keys to world genera of Scelionidae (Hymenoptera—Proctotrupoidea). Mem. Entomol Soc. Can. 1976, 108, 1–81. [Google Scholar] [CrossRef]
- Masner, L. Key to genera of Scelionidae of the Holarctic region, with descriptions of new genera and species (Hymenoptera, Proctotrupoidea). Mem. Entomol. Soc. Can. 1980, 112, 1–54. [Google Scholar] [CrossRef]
- Hayat, M. The Genera of Aphelinidae (Hymenoptera) of the World. Syst. Entomol. 1983, 8, 63–102. [Google Scholar] [CrossRef]
- Shaw, M.; Huddleston, T. Classification and Biology of Braconid Wasps. Handbooks for the Identification of British Insects; Royal Entomological Society of London: London, UK, 1991; Volume 7. [Google Scholar]
- Goulet, H.; Huber, J.T. Hymenoptera of the World: An Identification Guide to Families; Research Branch, Agriculture Canada: Ottawa, OA, Canada, 1993. [Google Scholar]
- Grissell, E.E. Torymidae. In Annotated keys to the Genera of Neartic Chalcidoidea (Hymenoptera); NRC Research Press: Ottawa, ON, Canada, 1997; pp. 709–725. [Google Scholar]
- Schauff, M.E.; LaSalle, J.; Coote, L.D. Eulophidae. In Annotated Keys to the Genera of Nearctic Chalcidoidea (Hymenoptera); Gibson, G.A.P., Huber, J.T., Woolley, J.B., Eds.; NRC Research Press: Ottawa, ON, Canada, 1997; pp. 327–429. [Google Scholar]
- Woolley, J.B. Aphelinidae. In Annotated keys to the Genera of Nearctic Chalcidoidea (Hymenoptera); Gibson, G.A.P., Huber, J.T., Woolley, J.B., Eds.; NRC Research Press: Ottawa, ON, Canada, 1997; pp. 134–150. [Google Scholar]
- Noyes, J.S. Universal Chalcidoidea Database. Available online: http://www.nhm.ac.uk/researchcuration/research/projects/chalcidoids (accessed on 20 January 2019).
- Pinto, J.D. A review of the new world genera of Trichogrammatidae (Hymenoptera). J. Hymenopt. Res. 2006, 15, 38–163. [Google Scholar]
- Ulrich, W. Body weight distributions of European Hymenoptera. Oikos 2006, 114, 518–528. [Google Scholar] [CrossRef]
- Huber, J.T.; Viggiani, G.; Jesu, R. Order Hymenoptera, Family Mymaridae. Arthropod Fauna UAE 2009, 2, 270–297. [Google Scholar]
- Pricop, E. Identification key to European genera of the Mymaridae (Hymenoptera:Chalcidoidea), with additional notes. ELBA Bioflux 2013, 569–581. [Google Scholar]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- McArdle, B.H.; Anderson, M.J. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 2001, 82, 290–297. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package Version 2.5-2; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Triapitsyn, S.V. Review of the palearctic Aphelinoidea (Hymenoptera: Trichogrammatidae), with focus on the species described by Ś. Nowicki. Isr. J. Entomol. 2018, 48, 33–81. [Google Scholar]
- Triapitsyn, S.V.; Huber, J.T.; Logarzo, G.A.; Berezovskiy, V.V.; Aquino, D.A. Review of Gonatocerus (Hymenoptera: Mymaridae) in the neotropical region, with description of eleven new species. Zootaxa 2010, 2456, 1–243. [Google Scholar] [CrossRef]
- Yefremova, Z.A. An annotated checklist of the Eulophidae (excl. Tetrastichinae) (Hymenoptera: Chalcidoidea) of Israel. Zootaxa 2015, 3957, 1–36. [Google Scholar] [CrossRef]
- Aishan, Z.; Triapitsyn, S.V.; Hu, H.Y. Review of Tumidiclava Girault (Hymenoptera: Trichogrammatidae) from Xingjiang, China, with description of two new species and taxonomic notes on other Holarctic taxa. Zootaxa 2015, 3949, 393–407. [Google Scholar] [CrossRef]
- Noma, T.; Brewer, M.J. Seasonal abundance of resident parasitoids and predatory flies and corresponding soybean aphid densities, with comments on classical biological control of soybean aphid in the Midwest. J. Econ. Entomol. 2008, 101, 278–287. [Google Scholar] [CrossRef]
- Yang, F.; Xu, L.; Wu, Y.K.; Wang, Q.; Yao, Z.W.; Zikic, V.; Tomanovic, Z.; Ferrer-Suay, M.; Selfa, J.; Pujade-Villar, J.; et al. Species composition and seasonal dynamics of aphid parasitoids and hyperparasitoids in wheat fields in northern China. Sci. Rep. 2017, 7, 13989. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Jervis, M.A. Does floral nectar improve biological control by parasitoids. In Plant.-Provided Food and Plant.-Carnivore Mutualism; Waeckers, F., van Rijn, P.C.J., Bruin, J., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 267–304. [Google Scholar]
- Kishinevsky, M.; Cohen, N.; Chiel, E.; Wajnberg, E.; Keasar, T. Sugar feeding of parasitoids in an agroecosystem: Effects of community composition, habitat and vegetation. Insect Conserv. Diver. 2018, 11, 50–57. [Google Scholar] [CrossRef]
- Kishinevsky, M.; Keasar, T.; Bar-Massada, A. Parasitoid abundance on plants: Effects of host abundance, plant species, and plant flowering state. Arthropod-Plant Interact. 2017, 11, 155–161. [Google Scholar] [CrossRef]
- Kishinevsky, M.; Keasar, T.; Harari, A.R.; Chiel, E. A comparison of naturally growing vegetation vs. border-planted companion plants for sustaining parasitoids in pomegranate orchards. Agric. Ecosyst. Environ. 2017, 246, 117–123. [Google Scholar] [CrossRef]
- Shapira, I.; Rosenfeld, A.; Rothschild, A.; Ackerman, M.; Eshel, G.; Keasar, T. Herbaceous vegetation enhancement increases biodiversity in a wine-producing vineyard in Israel, promoting shifts in agricultural practices in other vineyards. Conserv. Evid. 2017, 14, 10–15. [Google Scholar]
- Nguyen, H.D.D.; Nansen, C. Edge-biased distributions of insects. A review. Agron. Sustain. Dev. 2018, 38, 11. [Google Scholar] [CrossRef]
- Bayoun, I.M.; Walker, G.P.; Triapitsyn, S.V. Parasitization of beet leafhopper eggs, Circulifer tenellus, in California. J. Appl. Entomol. 2008, 132, 412–424. [Google Scholar] [CrossRef]
- Vu, Q.; Ramal, A.F.; Villegas, J.M.; Jamoralin, A.; Bernal, C.C.; Pasang, J.M.; Horgan, F.G. Enhancing the parasitism of insect herbivores through diversification of habitat in Philippine rice fields. Paddy Water Environ. 2018, 16, 379–390. [Google Scholar] [CrossRef]
- Chong, J.H.; Oetting, R.D. Host stage selection of the mealybug parasitoid Anagyrus spec. nov near sinope. Entomol. Exp. Appl. 2006, 121, 39–50. [Google Scholar] [CrossRef]
- Taekul, C.; Valerio, A.A.; Austin, A.D.; Klompen, H.; Johnson, N.F. Molecular phylogeny of telenomine egg parasitoids (Hymenoptera: P latygastridae sl.: T elenominae): Evolution of host shifts and implications for classification. Syst. Entomol. 2014, 39, 24–35. [Google Scholar] [CrossRef]
- Foba, C.N.; Salifu, D.; Lagat, Z.O.; Gitonga, L.M.; Akutse, K.S.; Fiaboe, K.K.M. Liriomyza leafminer (Diptera: Agromyzidae) parasitoid complex in different agroecological zones, seasons, and host plants in Kenya. Environ. Entomol. 2016, 45, 357–366. [Google Scholar] [CrossRef]
- Shapira, I.; Gavish-Regev, E.; Sharon, R.; Harari, A.R.; Kishinevsky, M.; Keasar, T. Habitat use by crop pests and natural enemies in a Mediterranean vineyard agroecosystem. Agric. Ecosyst. Environ. 2018, 267, 109–118. [Google Scholar] [CrossRef]
- Gaigher, R.; Pryke, J.S.; Samways, M.J. High parasitoid diversity in remnant natural vegetation, but limited spillover into the agricultural matrix in South African vineyard agroecosystems. Biol. Conserv. 2015, 186, 69–74. [Google Scholar] [CrossRef]
- Opatovsky, I.; Lubin, Y. Coping with abrupt decline in habitat quality: Effects of harvest on spider abundance and movement. Acta. Oecol. 2012, 41, 14–19. [Google Scholar] [CrossRef]
- Adler, V.H.; Lubin, Y.; Coll, M. Spillover of crop herbivores into adjacent desert habitats. Agric. Ecosyst. Environ. 2014, 193, 117–124. [Google Scholar] [CrossRef]
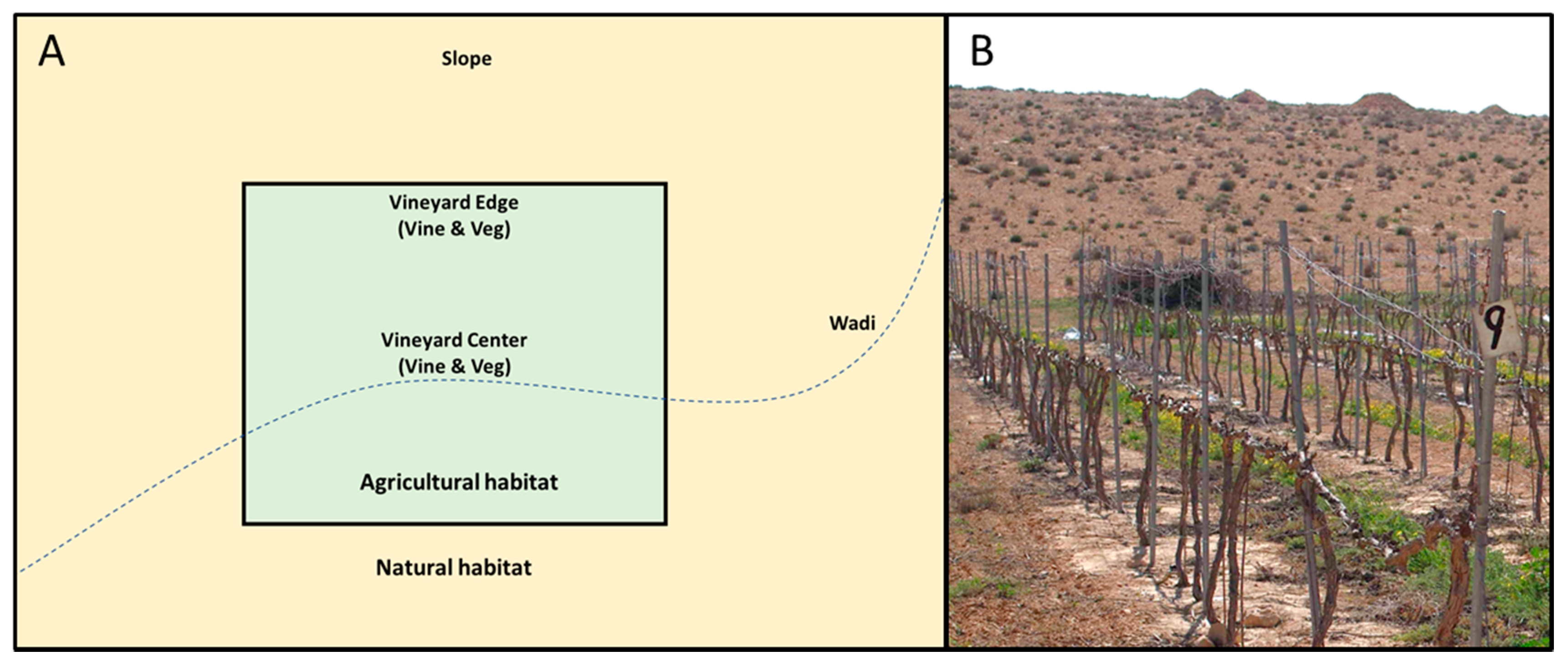
| Location | Sampling Points | Timings | Vineyards | Total |
|---|---|---|---|---|
| Vineyard center, ground vegetation (Veg-center) | 2 | 3 (March, June, August) | 6 | 36 |
| Vineyard center, grape foliage (Vine-center) | 2 | 2 (June, August) | 6 | 24 |
| Vineyard edge, ground vegetation (Veg-edge) | 2 | 3 (March, June, August) | 6 | 36 |
| Vineyard edge, grape foliage (Vine-edge) | 2 | 2 (June, August) | 6 | 24 |
| Slope, natural vegetation (Slope) | 2 | 3 (March, June, August) | 6 | 36 |
| Wadi, natural vegetation (Wadi) | 2 | 3 (March, June, August) | 6 | 36 |
| Total | 192 |
| Tested Variable | χ2 | df | p-Value |
|---|---|---|---|
| Month | 10.41 | 2 | <0.01 |
| Habitat (Center/Edge/Slope/Wadi) | 11.54 | 3 | <0.001 |
| Ground vegetation/Vine | 30.36 | 1 | <0.0001 |
| Plant species richness | 1.69 | 2 | NS |
| Month × habitat | 25.69 | 6 | <0.0001 |
| Species (Family) | Potential Host Group (Primary Hosts) | N (% in Vineyards) | Results of GLMM | ||
|---|---|---|---|---|---|
| Month | Habitat | Plant Richness | |||
| Aphelinoidea sp. (Trichogrammatidae) | Leafhoppers [43] | 399 (84%) | χ22 = 24.29, p < 0.0001 | χ24 = 14.11, p < 0.01 | χ21 =1.62, NS |
| Lymaenon litoralis (Mymaridae) | Leafhoppers [44] | 158 (73%) | χ22 = 9.69, p < 0.01 | χ24 = 6.70, NS | χ21 = 7.01, p < 0.01 |
| Mesopolobus sp. (Pteromalidae) | Coleoptera, Diptera, Hymenoptera and Lepidoptera [33] | 65 (66%) | χ22 = 30.56, p < 0.0001 | χ24 = 13.44, p < 0.01 | χ21 =0.07, NS |
| Telenomus sp.1 (Platygastridae) | Lepidoptera and Heteroptera [25] | 55 (85%) | χ22 = 4.31, NS | χ24 = 13.89, p < 0.01 | χ21 = 2.08, NS |
| Anagyrus sp. (Encyrtidae) | Pseudococcidae [33] | 54 (9%) | χ22 = 22.57, p < 0.0001 | χ24 = 18.75, p < 0.001 | NA |
| Paracentrobia sp. (Trichogrammatidae) | Auchenorrhyncha [34] | 36 (100%) | NA | ||
| Eurytoma (Eurytomidae) | Coleoptera, Diptera, Hymenoptera and Lepidoptera [33] | 35 (54%) | NA | ||
| Diglyphus isaea (Eulophidae) | Agromyzidae [45] | 34 (35%) | NA | ||
| Pseudoligosita sp. (Trichogrammatidae) | Auchenorrhyncha [34] | 33 (94%) | NA | ||
| Telenomus sp.2 (Platygastridae) | Lepidoptera and Heteroptera [25] | 33 (94%) | NA | ||
| Telenomus sp.3 (Platygastridae) | Lepidoptera and Heteroptera [25] | 33 (97%) | NA | ||
| Tumidiclava tamariska (Trichogrammatidae) | Cicadellidae [46] | 30 (83%) | NA | ||
| Tested Variable | df | F | R2 | p-Value |
|---|---|---|---|---|
| Month | 2, 69 | 3.083 | 0.069 | 0.0001 |
| Habitat (Center/Edge/Slope/Wadi) | 3, 69 | 1.238 | 0.042 | 0.024 |
| Vine/Veg | 1, 69 | 1.303 | 0.001 | NS |
| Plant species richness | 1, 72 | 2.015 | 0.023 | <0.001 |
| Month × Habitat | 6, 69 | 1.049 | 0.007 | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segoli, M.; Kishinevsky, M.; Rozenberg, T.; Hoffmann, I. Parasitoid Abundance and Community Composition in Desert Vineyards and Their Adjacent Natural Habitats. Insects 2020, 11, 580. https://doi.org/10.3390/insects11090580
Segoli M, Kishinevsky M, Rozenberg T, Hoffmann I. Parasitoid Abundance and Community Composition in Desert Vineyards and Their Adjacent Natural Habitats. Insects. 2020; 11(9):580. https://doi.org/10.3390/insects11090580
Chicago/Turabian StyleSegoli, Michal, Miriam Kishinevsky, Tamir Rozenberg, and Ishai Hoffmann. 2020. "Parasitoid Abundance and Community Composition in Desert Vineyards and Their Adjacent Natural Habitats" Insects 11, no. 9: 580. https://doi.org/10.3390/insects11090580
APA StyleSegoli, M., Kishinevsky, M., Rozenberg, T., & Hoffmann, I. (2020). Parasitoid Abundance and Community Composition in Desert Vineyards and Their Adjacent Natural Habitats. Insects, 11(9), 580. https://doi.org/10.3390/insects11090580





