Seed Predation on Oil-Polluted and Unpolluted Vachellia (Acacia) Trees in a Hyper-Arid Desert Ecosystem
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Statistical Analysis
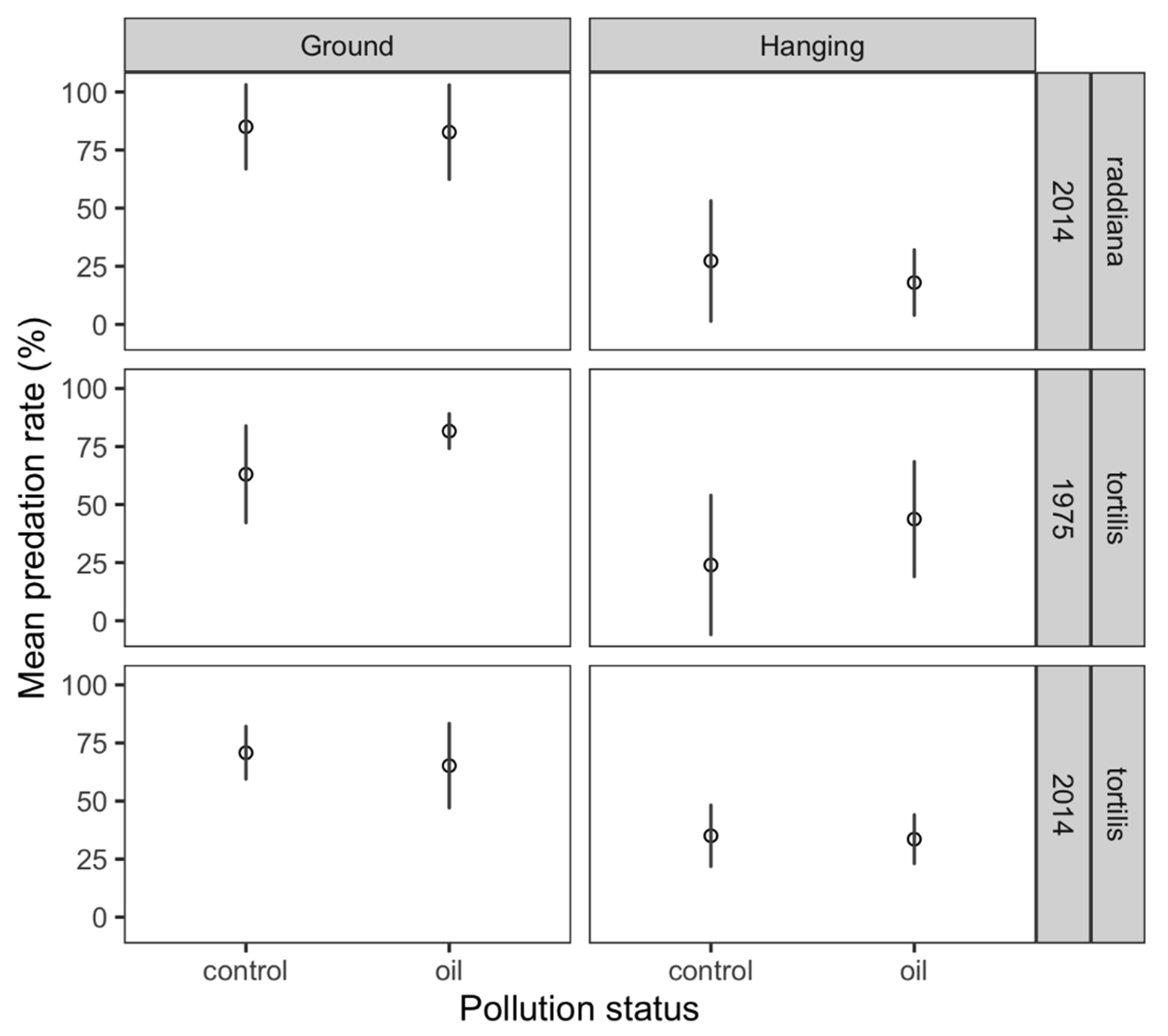
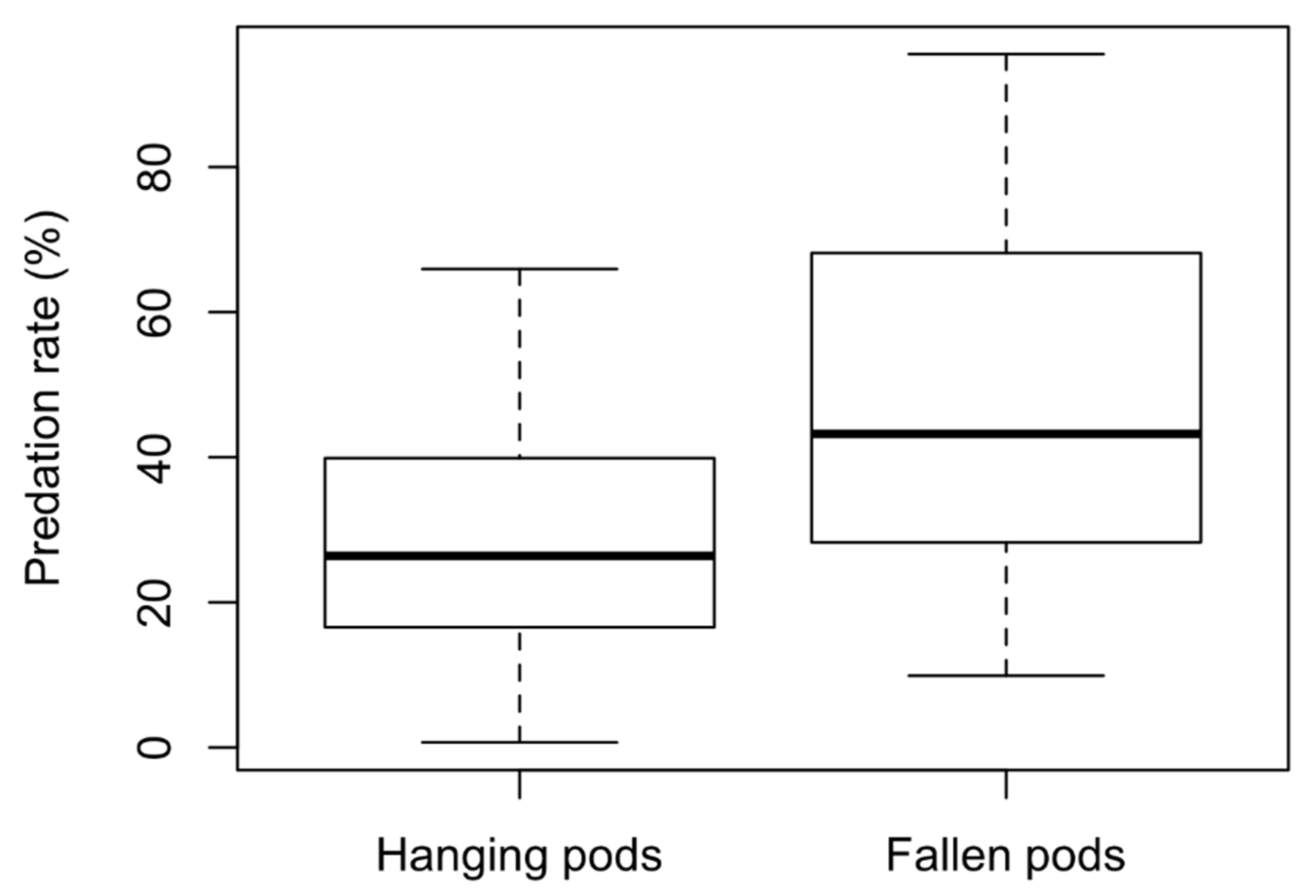
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paine, R. A note on trophic complexity and community stability. Am. Nat. 1969, 103, 91–93. [Google Scholar]
- Munzbergova, Z.; Ward, D. Acacia trees as keystone species in Negev desert ecosystems. J. Veg. Sci. 2002, 13, 227–236. [Google Scholar] [CrossRef]
- Kyalangalilwa, B.; Boatwright, J.S.; Daru, B.H.; Maurin, O.; van der Bank, M. Phylogenetic position and revised classification of Acacia s.l. (Fabaceae: Mimosoideae) in Africa, including new combinations in Vachellia and Senegalia. Bot. J. Linn. Soc. 2013, 172, 500–523. [Google Scholar] [CrossRef]
- Ward, D. The Biology of Deserts; Oxford University Press: Oxford, UK, 2016; ISBN 0-19-104730-9. [Google Scholar]
- Martins, D.J. Foraging patterns of managed honeybees and wild bee species in an arid African environment: Ecology, biodiversity and competition. Int. J. Trop. Insect Sci. 2004, 24, 105–115. [Google Scholar] [CrossRef]
- Young, T.P.; Stubblefield, C.H.; Isbell, L.A. Ants on swollen-thorn acacias: Species coexistence in a simple system. Oecologia 1997, 109, 98–107. [Google Scholar] [CrossRef]
- Hackett, T.D.; Korine, C.; Holderied, M.W. The importance of Acacia trees for insectivorous bats and arthropods in the Arava Desert. PLoS ONE 2013, 8, e52999. [Google Scholar] [CrossRef]
- Fagg, C.W.; Stewart, J.L. The value of Acacia and Prosopis in arid and semi-arid environments. J. Arid Environ. 1994, 27, 3–25. [Google Scholar] [CrossRef]
- Rohner, C.; Ward, D. Large mammalian herbivores and the conservation of arid Acacia stands in the Middle East. Conserv. Biol. 1999, 13, 1162–1171. [Google Scholar] [CrossRef]
- Rodger, Y.S.; Greenbaum, G.; Silver, M.; Bar-David, S.; Winters, G. Detecting hierarchical levels of connectivity in a population of Acacia tortilis at the northern edge of the species’ global distribution: Combining classical population genetics and network analyses. PLoS ONE 2018, 13, e0194901. [Google Scholar] [CrossRef]
- Zohary, M. Flora Palestina Volume 2; Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1972. [Google Scholar]
- Sher, A.A.; Wiegand, K.; Ward, D. Do Acacia and Tamarix trees compete for water in the Negev desert? J. Arid Environ. 2010, 74, 338–343. [Google Scholar] [CrossRef]
- Stavi, I.; Silver, M.; Avni, Y. Latitude, basin size, and microhabitat effects on the viability of Acacia trees in the Negev and Arava, Israel. CATENA 2014, 114, 149–156. [Google Scholar] [CrossRef]
- Ward, D.; Rohner, C. Anthropogenic causes of high mortality and low recruitment in three Acacia tree taxa in the Negev desert, Israel. Biodivers. Conserv. 1997, 6, 877–893. [Google Scholar] [CrossRef]
- Kennenni, L.; van der Maarel, E. Population ecology of Acacia tortilis in the semi-arid region of the Sudan. J. Veg. Sci. 1990, 1, 419–424. [Google Scholar]
- Todt, H.; Breckle, S.-W.; Veste, M. The mistletoe Loranthus acaciae (Loranthaceae) on halophytic and non-halophytic hosts in the southern Arava-Valley (Israel). In I Schimpter Symposium—Ergebnisse Weltweiter Forschungen; Verlag Günter Heimbach: Stuttgart, Germany, 2000; pp. 475–480. [Google Scholar]
- Polak, T.; Gutterman, Y.; Hoffman, I.; Saltz, D. Redundancy in seed dispersal by three sympatric ungulates: A reintroduction perspective. Anim. Conserv. 2014, 17, 565–572. [Google Scholar] [CrossRef]
- Or, K.; Ward, D. The effects of seed quality and pipecolic and djenkolic acids on bruchid beetle infestation in water deficit-stressed Acacia trees. J. Chem. Ecol. 2004, 30, 2297–2307. [Google Scholar] [CrossRef]
- Evans, C.S.; Qureshi, M.Y.; Bell, E.A. Free amino acids in the seeds of Acacia species. Phytochemistry 1977, 16, 565–570. [Google Scholar] [CrossRef]
- Rahman, K.S.M.; Thahira-Rahman, J.; Lakshmanaperumalsamy, P.; Banat, I.M. Towards efficient crude oil degradation by a mixed bacterial consortium. Bioresour. Technol. 2002, 85, 257–261. [Google Scholar] [CrossRef]
- Nicolotti, G.; Egli, S. Soil contamination by crude oil: Impact on the mycorrhizosphere and on the revegetation potential of forest trees. Environ. Pollut. 1998, 99, 37–43. [Google Scholar] [CrossRef]
- Baker, J.M. The effects of oils on plants. Environ. Pollut. 1970, 1, 27–44. [Google Scholar] [CrossRef]
- Malallah, G.; Afzal, M.; Kurian, M.; Gulshan, S.; Dhami, M.S.I. Impact of oil pollution on some desert plants. Environ. Int. 1998, 24, 919–924. [Google Scholar] [CrossRef]
- Odukoya, J.; Lambert, R.; Sakrabani, R. Understanding the impacts of crude oil and its induced abiotic stresses on agrifood production: A review. Horticulturae 2019, 5, 47. [Google Scholar] [CrossRef]
- Golan, S.; Faraj, T.; Rahamim, E.; Zemach, H.; Lifshitz, D.; Singer, A.; Bar, D.; Carmeli, D.; Steinberger, Y.; Sherman, C.; et al. The effect of petroleum hydrocarbons on seed germination, development and survival of wild and cultivated plants in extreme desert soil. Int. J. Agric. Environ. Res. 2016, 2, 1743–1767. [Google Scholar]
- Nothers, M.; Segev, N.; Kreyling, J.; Hjazin, A.; Groner, E. Desert vegetation forty years after an oil spill. J. Environ. Qual. 2017, 46, 568–575. [Google Scholar] [CrossRef]
- Louda, S.M.; Collinge, S.K. Plant resistance to insect herbivores: A field test of the environmental stress hypothesis. Ecology 1992, 73, 153–169. [Google Scholar] [CrossRef]
- Girsowicz, R.; Koryachenko, O.; Sherman, C.; Mayzlish-Gati, E.; Doniger, T.; Steinberger, Y. Impact of oil-spill contamination on a soil bacterial community: A 40-year history of rehabilitation in the Arava Valley. Soil Sediment Contam. Int. J. 2018, 27, 175–185. [Google Scholar] [CrossRef]
- Tran, T.H.; Mayzlish Gati, E.; Eshel, A.; Winters, G. Germination, physiological and biochemical responses of acacia seedlings (Acacia raddiana and Acacia tortilis) to petroleum contaminated soils. Environ. Pollut. 2018, 234, 642–655. [Google Scholar] [CrossRef]
- Gordon, G.; Stavi, I.; Rosenzweig, R. Oil spill effects on soil hydrophobicity and related properties in a hyper-arid region. Geoderma 2018, 312, 114–120. [Google Scholar] [CrossRef]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer Series in Statistics; Springer: New York, NY, USA, 1998; pp. 199–213. ISBN 978-1-4612-1694-0. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; Version 3.3.3; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Haubo Bojesen Christense, R. lme4: Linear Mixed-Effects Models Using Eigen and S4. R Package Version 1. 2015, pp. 1–7. Available online: cran.r-project.org/web/packages/lme4/index.html (accessed on 18 February 2020).
- Or, K.; Ward, D. Three–way interactions between Acacia, large mammalian herbivores and bruchid beetles—A review. Afr. J. Ecol. 2003, 41, 257–265. [Google Scholar] [CrossRef]
- Coe, M.; Coe, C. Large herbivores, acacia trees and bruchid beetles. S. Afr. J. Sci. 1987, 83, 624. [Google Scholar]
- Salman, I.N.A.; Ferrante, M.; Möller, D.M.; Gavish-Regev, E.; Lubin, Y. Trunk Refugia: A Simple, Inexpensive Method for Sampling Tree Trunk Arthropods. J. Insect Sci. 2020, 20. [Google Scholar] [CrossRef]
- Breslau, B.; Polak, T.; Shalmon, B.; Groner, E. Evidence of browsing pressure on the critically endangered Acacia gazelle (Gazella acaciae). J. Arid Environ. 2020, 173, 104019. [Google Scholar] [CrossRef]
- Ernst, W.H.O.; Tolsma, D.J.; Decelle, J.E. Predation of seeds of Acacia tortilis by insects. Oikos 1989, 54, 294–300. [Google Scholar] [CrossRef]
- Derbel, S.; Noumi, Z.; Anton, K.W.; Chaieb, M. Life cycle of the coleopter Bruchidius raddianae and the seed predation of the Acacia tortilis Subsp. raddiana in Tunisia. Comptes Rendus Biol. 2007, 330, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Toma, L.; Ramos, R.Y.; Severini, F.; Di Luca, M.; Zampetti, M.F. First record of Bruchidius raddianae in Italy: Infested seeds of Vachellia karroo from Lampedusa island (Coleoptera: Bruchidae; Fabales: Fabaceae). Fragm. Entomol. 2017, 49, 89–91. [Google Scholar] [CrossRef]
- Anton, K.W.; Halperin, J.; Calderon, M. An annotated list of the Bruchidae (Coleoptera) of Israel and adjacent areas. Isr. J. Entomol. 1997, 31, 59–96. [Google Scholar]
| Vachellia Species | Site, Tree Status | Seeds Collected | Habitat | Collection Date | Inspection Dates |
|---|---|---|---|---|---|
| V. raddiana | 2014, oil-polluted | 892 | tree | 2 June | 30 June; 14, 27 July; 11, 27 August; 4, 24 September |
| 2014, unpolluted | 759 | tree | 2 June | 30 June; 14, 27 July; 11, 27 August; 4, 24 September | |
| 2014, oil-polluted | 306 | ground | 2 October | 23 October | |
| 2014, unpolluted | 301 | ground | 2 October | 23 October | |
| V. tortilis | 2014, oil-polluted | 489 | tree | 8 August | 28 August; 13, 25 September; 14 October |
| 2014, unpolluted | 684 | tree | 8 August | 28 August; 13, 25 September; 14 October | |
| 1975, oil-polluted | 396 | tree | 8 August | 28 August; 13, 25 September; 14 October | |
| 1975, unpolluted | 393 | tree | 8 August | 28 August; 13, 25 September; 14 October | |
| 2014, oil-polluted | 361 | ground | 2 October | 23 October | |
| 2014, unpolluted | 402 | ground | 2 October | 23 October | |
| 1975, oil-polluted | 399 | ground | 2 October | 23 October | |
| 1975, unpolluted | 335 | ground | 2 October | 23 October |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrante, M.; Möller, D.M.; Möller, G.M.; Lubin, Y.; Segoli, M. Seed Predation on Oil-Polluted and Unpolluted Vachellia (Acacia) Trees in a Hyper-Arid Desert Ecosystem. Insects 2020, 11, 665. https://doi.org/10.3390/insects11100665
Ferrante M, Möller DM, Möller GM, Lubin Y, Segoli M. Seed Predation on Oil-Polluted and Unpolluted Vachellia (Acacia) Trees in a Hyper-Arid Desert Ecosystem. Insects. 2020; 11(10):665. https://doi.org/10.3390/insects11100665
Chicago/Turabian StyleFerrante, Marco, Daniella M. Möller, Gabriella M. Möller, Yael Lubin, and Michal Segoli. 2020. "Seed Predation on Oil-Polluted and Unpolluted Vachellia (Acacia) Trees in a Hyper-Arid Desert Ecosystem" Insects 11, no. 10: 665. https://doi.org/10.3390/insects11100665
APA StyleFerrante, M., Möller, D. M., Möller, G. M., Lubin, Y., & Segoli, M. (2020). Seed Predation on Oil-Polluted and Unpolluted Vachellia (Acacia) Trees in a Hyper-Arid Desert Ecosystem. Insects, 11(10), 665. https://doi.org/10.3390/insects11100665








