The Biology and Immature Stages of the Moss-Eating Flea Beetle Cangshanaltica fuanensis sp. nov. (Coleoptera, Chrysomelidae, Galerucinae, Alticini), with Description of a Fan-Driven High-Power Berlese Funnel
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological Methods
2.2. Biological Methods
2.3. DNA Barcoding
2.4. The Modified Fan-Driven Berlese Funnel
3. Results
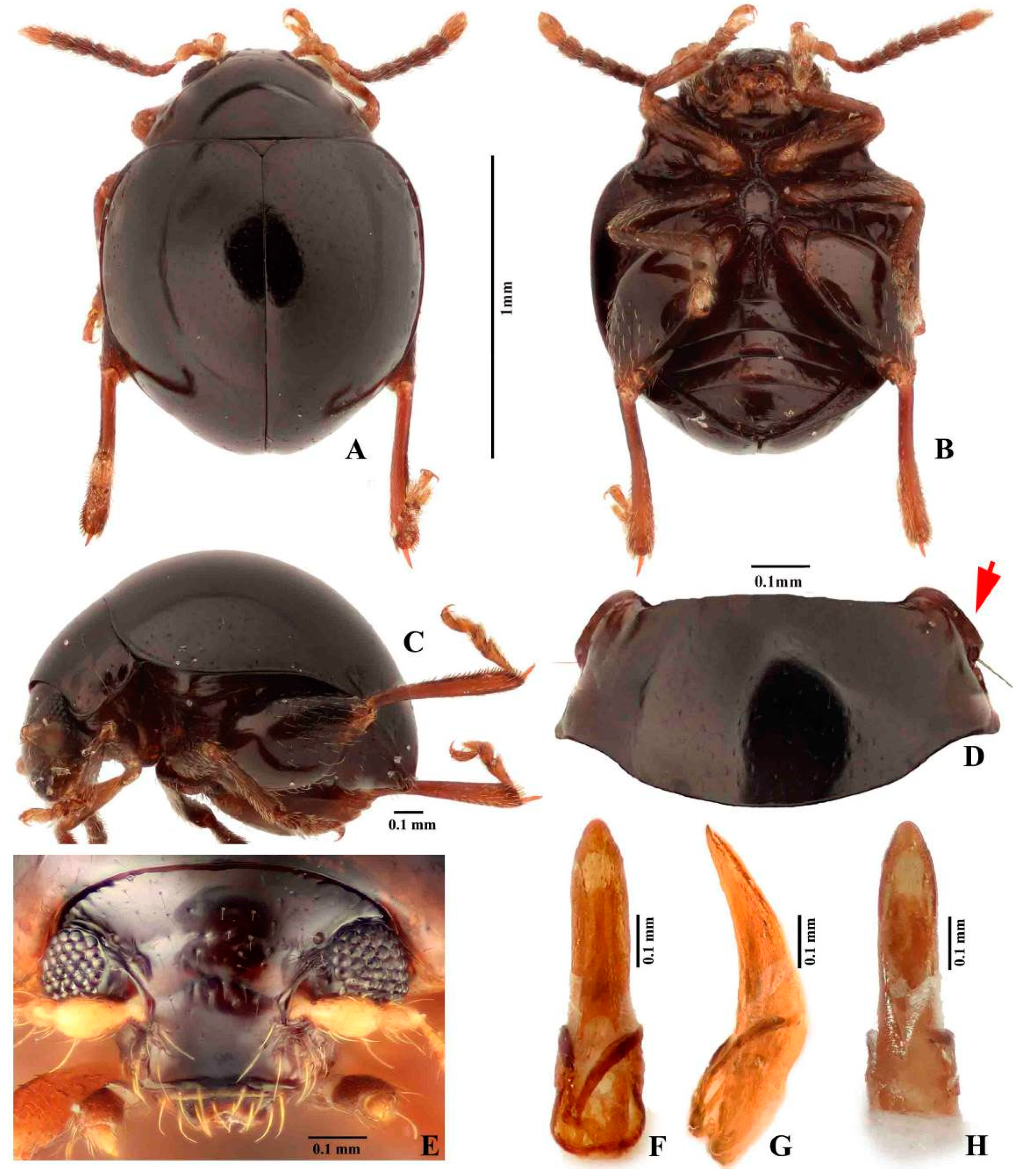
3.1. Taxonomy
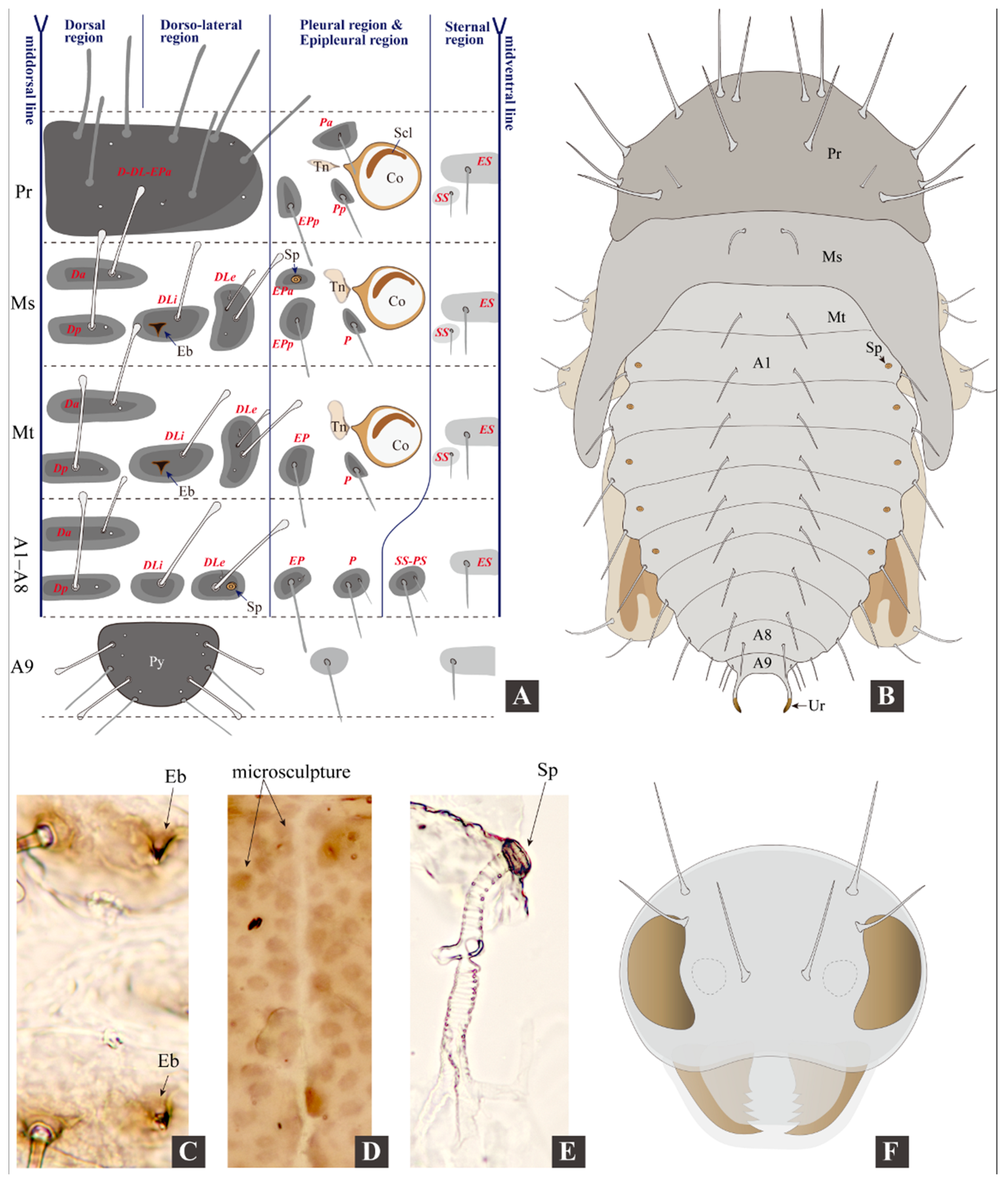
3.2. Morphology of Immature Stages
3.2.1. Larval Morphology
- First instar: head width 0.27–0.30 mm; body length 0.90–1.50 mm, body width 0.30–0.40 mm.
- Second instar: head width 0.34–0.37 mm; body length 1.50–2.30 mm, body width 0.45–0.61 mm.
- Third instar: head width 0.42–0.46 mm; body length 2.00–2.80 mm, body width 0.50–0.80 mm.
3.2.2. Pupal Morphology
3.2.3. Egg Morphology
3.3. Biology
3.3.1. Life History
3.3.2. Reduction of Ovarioles and Large Egg Size
3.3.3. Egg Hiding Behavior of Females
3.3.4. Cannibalism
3.3.5. Host Plant and Feeding Habit
3.3.6. Habitat Environment
3.3.7. Natural Enemies
3.3.8. Death Feigning (Thanatosis)
3.3.9. Jumping Ability
3.4. The Modified Fan-Driven Berlese Funnel
4. Discussion
4.1. Biology of Cangshanaltica fuanensis sp. nov.
4.2. The Modified Fan-Driven Berlese Funnel
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Konstantinov, A.S.; Konstantinova, A.A. New genus and species of flea beetles (Coleoptera, Chrysomelidae, Galerucinae, Alticini) from Puerto Rico, with comments on flea beetle diversity in the West Indies and a key to the West Indian Monoplatini genera. ZooKeys 2011, 155, 61–87. [Google Scholar] [CrossRef] [Green Version]
- Konstantinov, A.; Chamorro, M.L.; Prathapan, K.D.; Ge, S.; Yang, X. Moss-inhabiting flea beetles (Coleoptera: Chrysomelidae: Galerucinae: Alticini) with description of a new genus from Cangshan, China. J. Nat. Hist. 2013, 47, 2459–2477. [Google Scholar] [CrossRef]
- Damaška, A.F.; Konstantinov, A.S. A new species of Cangshanaltica Konstantinov et al., a moss-inhabiting flea beetle from Thailand (Coleoptera: Chrysomelidae: Galerucinae: Alticini). Zootaxa 2016, 4107, 93–97. [Google Scholar] [CrossRef]
- Ruan, Y.; Konstantinov, A.S.; Prathapan, K.D.; Yang, X. Contributions to the knowledge of Chinese flea beetle fauna (II): Baoshanaltica new genus and Sinosphaera new genus (Coleoptera, Chrysomelidae, Galerucinae, Alticini). ZooKeys 2017, 720, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Linzmeier, A.M.; Konstantinov, A.S. Andersonoplatus, a new, remarkable leaf litter inhabiting genus of Monoplatina (Coleoptera, Chrysomelidae, Galerucinae, Alticini). ZooKeys 2018, 744, 79–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linzmeier, A.M.; Konstantinov, A.S. Moss inhabiting flea beetles (Coleoptera: Chrysomelidae: Galerucinae: Alticini) of the West Indies II: Menudos, a new genus from Puerto Rico and description of methods to collect moss inhabiting flea beetles. Zootaxa 2020, 4786, 1–22. [Google Scholar] [CrossRef]
- Damaška, A.F.; Aston, P. Leaf litter and moss-inhabiting flea beetles of Hong Kong (Coleoptera: Chrysomelidae: Alticini). Acta Entomol. Musei Nationalis Pragae 2019, 59, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Biondi, M.; Iannella, M.; D’Alessandro, P. Adamastoraltica humicola, new genus and new species: The first example of possible moss-inhabiting flea beetle genus from sub-Saharan Africa (Coleoptera, Chrysomelidae, Galerucinae). Zootaxa 2020, 4763, 99–108. [Google Scholar] [CrossRef]
- Damaška, A.F.; Mohagan, D.J.; Fikáček, M. Moss-inhabiting flea beetles in the Philippines (Coleoptera, Chrysomelidae, Alticinae). ZooKeys 2020, 960, 125–142. [Google Scholar] [CrossRef]
- Konstantinov, A.S.; Linzmeier, A.M.; Morais, A.C.C.; Palmer, M.W.; Scheffer, S.J.; Lewis, M.L. Discovery of the first nearctic moss-eating flea beetle, Distigmoptera borealis Blake, 1943 (Coleoptera: Chrysomelidae: Galerucinae: Alticini). Coleopts. Bull. 2019, 73, 599–610. [Google Scholar] [CrossRef]
- Konstantinov, A.S.; Linzmeier, A.M. Moss inhabiting flea beetles of the West Indies III: Erinaceialtica, a new genus from Hispaniola (Coleoptera, Chrysomelidae, Galerucinae, Alticini). Zookeys 2020, 955, 113–145. [Google Scholar] [CrossRef]
- Lee, C.F.; Beenen, R. Taiwanoshaira Lee & Beenen, a new genus and first record of moss-inhabiting Galerucinae sensu stricto (Coleoptera, Chrysomelidae) from Taiwan. ZooKeys 2020, 944, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Leschen, R.A.B.; Reid, C.A.M.; Nadein, K.S. Generic review of New Zealand Chrysomelinae (Coleoptera: Chrysomelidae). Zootaxa 2020, 4740, 1–66. [Google Scholar] [CrossRef] [PubMed]
- Duckett, C.N.; Prathapan, K.D.; Konstantinov, A.S. Notes on identity, new synonymy and larva of Ivalia Jacoby (Coleoptera: Chrysomelidae) with description of a new species. Zootaxa 2006, 1363, 49–68. [Google Scholar] [CrossRef] [Green Version]
- Cox, M.L. The larva of the flea beetle, Mniophila muscorum (Koch, 1803) (Coleoptera: Chrysomelidae, Alticinae), not a leaf-miner. Entomol. Gazette 1997, 48, 275–284. [Google Scholar]
- Kimoto, S. A phylogenetic consideration of Chrysomelinae based on immature stages of Japanese species (Coleoptera). J. Fac. Agric. Kyushu Univ. 1962, 12, 67–88. [Google Scholar]
- Takizawa, H. Supra-generic subdivisions of the subfamily Alticinae based on larval characters, with descriptions of larvae of Hispaniolan species (Coleoptera: Chrysomelidae). Insecta Matsumurana 2005, 62, 187–206. [Google Scholar]
- Wu, P.C.; Jia, Y. Flora Bryophytorum Sinicorum; Science Press: Beijing, China, 2004; Volume 8, p. 482. (In Chinese) [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial Cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- LeSage, L.; Zmudzinska-Krzesinska, A. Immature stages of the grape flea beetles Altica chalybea Illiger and A. woodsi Isley (Coleoptera: Chrysomelidae). In New Developments in the Biology of Chrysomelidae; Jolivet, P., Santiago-Blay, J.A., Schmitt, M., Eds.; SPB Academic Publishers: The Hague, The Netherlands, 2004; pp. 503–528. [Google Scholar]
- Hua, Y.; Beutel, R.G.; Ge, S.Q.; Yang, X.K. The larval head structures of Podagricomela shirahatai (Chûjô) (Chrysomelidae, Galerucinae, Alticini) and morphological effects of leaf mining. J. Morphol. 2015, 276, 446–457. [Google Scholar] [CrossRef]
- Konstantinov, A.S.; Volkovitsh, M.G.; Cristofaro, M. New data on Palearctic Aphthona (Coleoptera: Chrysomelidae) with description of a new species: Taxonomic and faunistic results of biological control exploration. Entomol. News 2001, 112, 31–41. [Google Scholar]
- Watts, J.R. Eggs, larvae and biological notes on Disonycha leptolineata Blatchley (Coleoptera: Chrysomelidae). Insecta Mundi 1990, 4, 93–97. [Google Scholar]
- Suzuki, K.; Hara, A. Supplementary report on the ovariole number in the family Chrysomelidae (Insecta: Coleoptera). J. Coll. Lib. Arts Toyama Univ. Nat. Sci. 1975, 8, 87–93. [Google Scholar]
- Zhang, L.; Xue, H.; Yang, X. Comparative study on morphological characters of seven species of Altica eggs. Chin. Bull. Entomol. 2008, 45, 303–305. [Google Scholar]
- Zhao, Q. Chaetocnema ingenua Baly. In Chinese Agricultural Encyclopedia (Volume Insects); China Agriculture Press: Beijing, China, 1990; pp. 375–376. (In Chinese) [Google Scholar]
- Konstantinov, A.S.; Baselga, A.; Grebennikov, V.V.; Prena, J.; Lingalfelter, S.W. Revision of the Palearctic Chaetocnema Species (Coleoptera: Chrysomelidae: Galerucinae: Alticini); Pensoft: Sofia, Bulgaria, 2011; p. 363. [Google Scholar]
- Ruan, Y.; Yang, X.; Konstantinov, A.S.; Prathapan, K.D.; Zhang, M. Revision of the Oriental Chaetocnema species (Coleoptera, Chrysomelidae, Galerucinae, Alticini). Zootaxa 2019, 4699, 1–206. [Google Scholar] [CrossRef]
- Vig, K. Biology of Phyllotreta (Alticinae), with emphasis on Hungarian and middle European species. In New Developments in the Biology of Chrysomelidae; Jolivet, P., Santiago-Blay, J.A., Schmitt, M., Eds.; SPB Academic Publishers: The Hague, The Netherlands, 2004; pp. 565–578. [Google Scholar]
- Yu, P.; Wang, S.; Yang, X. Coleoptera: Chrysomeloidea (II). Economic Insect Fauna of China 54; Science Press: Beijing, China, 1996; p. 324. (In Chinese) [Google Scholar]
- Ruan, Y.; Konstantinov, A.S.; Ge, S.; Yang, X. Revision of the Chaetocnema picipes species group (Coleoptera, Chrysomelidae, Galerucinae, Alticini) in China, with descriptions of three new species. Zookeys 2014, 387, 11–32. [Google Scholar] [CrossRef]
- Yang, X.; Ge, S.; Nie, R.; Ruan, Y.; Li, W. Chinese Leaf Beetles; Science Press: Beijing, China, 2015; p. 507. [Google Scholar]
- Richardson, M.L.; Reagel, P.F.; Mitchel, R.F.; Lawrence, M.W. Causes and consequences of cannibalism in non carnivorous insects. Annu. Rev. Entomol. 2009, 55, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Santana, A.F.K.; Roselino, A.C.; Cappelari, F.A.; Zucoloto, F.S. Cannibalism in insects. In Insect Bioecology and Nutrition for Integrated Pest Management; Antonio, P.R., Jose, P.R.P., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 177–194. [Google Scholar]
- Suzuki, K. Ovariole number in the family Chrysomelidae (Insecta: Coleoptera). J. Coll. Lib. Arts Toyama Univ. Nat. Sci. 1974, 7, 53–70. [Google Scholar]
- Crowson, R.A. The Biology of the Coleoptera; Academic Press: London, UK, 1982; p. 802. [Google Scholar]
- Robertson, J.G. Ovariole numbers in Coleoptera. Can. J. Zool. 1961, 39, 245–263. [Google Scholar] [CrossRef]
- Polilov, A.A. Anatomy of the feather-winged beetles Acrotrichis montandoni and Ptilium myrmecophilum (Coleoptera, Ptiliidae). Entomol. Rev. 2005, 85, 467–475. [Google Scholar]
- Paulian, R. Deux familles de Coleopteres nouvelles pour la Faune Ma1gache. Mémoires l’Institut Scientifique Madagascar A 1949, 3, 371–374. [Google Scholar]
- Fikáček, M.; Hu, F.; Aston, P.; Jia, F.; Liang, W.; Liu, H.; Minoshima, N.Y. Comparative morphology of immature stages and adults of Hydroscapha from Taiwan, with description of a new species from Hong Kong (Coleoptera: Myxophaga: Hydroscaphidae). Raffles Bull. Zool. 2020, 68, 334–349. [Google Scholar] [CrossRef]
- Clark, E.W.; Williamson, A.L.; Richmond, C.A. A collecting technique for pink bollworms and other insects using a Berlese funnel with an improved heater. J. Econ. Entomol. 1959, 52, 1010–1012. [Google Scholar] [CrossRef]
- Dondale, C.D.; Nicholls, C.F.; Redner, J.H.; Semple, R.B.; Turnbull, A.L. An improved Berlese-Tullgren funnel and a flotation separator for extracting grassland arthropods. Can. Entomol. 1971, 103, 1549–1552. [Google Scholar] [CrossRef]
- Bremner, G. A Berlese funnel for the rapid extraction of grassland surface macro-arthropods. N. Z. Entomol. 1990, 13, 76–80. [Google Scholar] [CrossRef]
- Krell, F.T.; Chung, A.Y.; DeBoise, E.; Eggleton, P.; Giusti, A.; Inward, K.; Krell-Westerwalbesloh, S. Quantitative extraction of macro-invertebrates from temperate and tropical leaf litter and soil: Efficiency and time-dependent taxonomic biases of the Winkler extraction. Pedobiologia 2005, 49, 175–186. [Google Scholar] [CrossRef]
- Besuchet, C.; Burckhardt, D.H.; Löbl, I. The ‘Winkler/Moczarski’ eclector as an efficient extractor for fungus and litter Coleoptera. Coleopts. Bull. 1987, 414, 392–394. [Google Scholar]
- Wheeler, Q.D.; McHugh, J.V. A portable and convertible “Moczarski/Tullgren” extractor for fungus and litter Coleoptera. Coleopts. Bull. 1987, 41, 9–12. [Google Scholar]
- Tie, L.; Zhang, L.; Feng, M.; Bai, W.; Wang, L.; He, P. Baking temperature and time on the effect of soil meso- and micro- fauna separation results. J. Sichuan Agric. Univ. 2015, 33, 45–50. [Google Scholar]







| Cangshanaltica fuanensis sp. nov. | Ivalia korakundah, Parathapan et al. | Distigmoptera borealis Blake | Agasicles hygrophila Selman and Vogt | Podagricomela shirahatai (Chûjô) | Aphthona russica Konstantinov et al. | |
|---|---|---|---|---|---|---|
| Feeding type | Leaf feeding (moss) | Leaf feeding (moss) | Leaf feeding (moss) | Leaf feeding (angiosperm) | Leaf mining (angiosperm) | Root feeding (angiosperm) |
| Body color (mature larva) | Lemon yellow | White | Yellow | Green-black to black | Yellow | Whitish |
| Body shape | Eruciform; short and robust | Eruciform; short and robust | Eruciform; short and robust | Eruciform; short and robust | Slightly flattened dorso-ventrally; robust | Subcylindrical; elongate and slender |
| Sclerotization of tubercles | Weak, edge poorly defined | Moderate, edge well defined | Strong, edge well defined | Absent | Absent | Absent |
| Long capitate setae on dorsum | Present | Present | Present | Absent | Unknown | Absent |
| Head orientation | Hypognathous | Hypognathous | Hypognathous | Hypognathous | Prognathous | Hypognathous |
| Head shape | Globular | Globular | Globular | Globular | Flattened dorso-ventrally | Almost parallel-sided; slightly elongated |
| Posterior emargination of head | Shallow | Shallow | Moderately deep | Shallow | Strongly developed and V-shaped | Moderately deep |
| Stemmata | Absent | Absent | Absent | 1 pair | Absent | Absent |
| Eyespot | Present | Present (based on image) | Unknown | Absent | Present | Unknown |
| Epicranial suture | Short | Short | Short | Short | Absent | Short |
| Shape of Endocarina | Narrow ridge | Unknown | Narrow ridge | Narrow ridge | Strongly developed Median bulge | Narrow ridge |
| Anterior edge of labrum | Deeply concave | Deeply concave | Moderately concave | Slightly concave at middle | Convex | Slightly concave |
| Number and shape of mandibular teeth | 4; sharp, with similar size | 4; largest tooth bearing minute serration | 3 sharp and 1 blunt | 3 large and 1 small | 4; all blunt | 4 sharp long and 1 small |
| Egg Length and Width | Adult Body Length and Width | Egg Length to Adult Body Length Ratio | Egg Width to Adult Body Width Ratio | Egg Numbers Laid by Female | |
|---|---|---|---|---|---|
| Cangshanaltica fuanensis sp. nov. | 0.68–0.74 mm; 0.36–0.40 mm | 1.5–1.7 mm; 1.2–1.3 mm (female) | 0.40–0.50 | 0.28–0.33 | Ca. 2–4, laid separately |
| Agasicles hygrophila Selman and Vogt | 1.26 mm; 0.54 mm | 6 mm; 3 mm | 0.21 | 0.18 | >50, laid in clusters |
| Altica caerulescens (Baly) | 0.85 mm; 0.35 mm | 4 mm; 2 mm | 0.21 | 0.18 | Unknown |
| Altica fragariae Nakane | 0.84 mm; 0.37 mm | 3.8 mm; 2 mm | 0.22 | 0.19 | >100, laid in clusters |
| Chaetocnema ingenua Baly | 0.75 mm; 0.35 mm | 1.9–3 mm; 0.9–1.57 mm | 0.25–0.39 | 0.22–0.39 | Ca. 100, laid in clusters |
| Disonycha leptolineata Blatchley | 1.77–2.23 mm; 0.66–1.09 mm | 6.2–7.5 mm; 3.4–4.5 mm | 0.24–0.36 | 0.15–0.32 | Unknown, laid in clusters |
| Phyllotreta striolata (Fabricius) | 0.37 mm; 0.21 mm | 1.8–2.4 mm; 0.9 mm | 0.15–0.21 | 0.23 | >25, laid in clusters |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, Y.; Konstantinov, A.S.; Damaška, A.F. The Biology and Immature Stages of the Moss-Eating Flea Beetle Cangshanaltica fuanensis sp. nov. (Coleoptera, Chrysomelidae, Galerucinae, Alticini), with Description of a Fan-Driven High-Power Berlese Funnel. Insects 2020, 11, 571. https://doi.org/10.3390/insects11090571
Ruan Y, Konstantinov AS, Damaška AF. The Biology and Immature Stages of the Moss-Eating Flea Beetle Cangshanaltica fuanensis sp. nov. (Coleoptera, Chrysomelidae, Galerucinae, Alticini), with Description of a Fan-Driven High-Power Berlese Funnel. Insects. 2020; 11(9):571. https://doi.org/10.3390/insects11090571
Chicago/Turabian StyleRuan, Yongying, Alexander S. Konstantinov, and Albert F. Damaška. 2020. "The Biology and Immature Stages of the Moss-Eating Flea Beetle Cangshanaltica fuanensis sp. nov. (Coleoptera, Chrysomelidae, Galerucinae, Alticini), with Description of a Fan-Driven High-Power Berlese Funnel" Insects 11, no. 9: 571. https://doi.org/10.3390/insects11090571
APA StyleRuan, Y., Konstantinov, A. S., & Damaška, A. F. (2020). The Biology and Immature Stages of the Moss-Eating Flea Beetle Cangshanaltica fuanensis sp. nov. (Coleoptera, Chrysomelidae, Galerucinae, Alticini), with Description of a Fan-Driven High-Power Berlese Funnel. Insects, 11(9), 571. https://doi.org/10.3390/insects11090571





