The Inability of Spotted Lanternfly (Lycorma delicatula) to Vector a Plant Pathogen between its Preferred Host, Ailanthus altissima, in a Laboratory Setting
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Verticillium nonalfalfae Inoculum
2.3. Ailanthus altissima Seedlng Source
2.4. Ailanthus altissima Mature Tree Source
2.5. Collecting L. delicatula
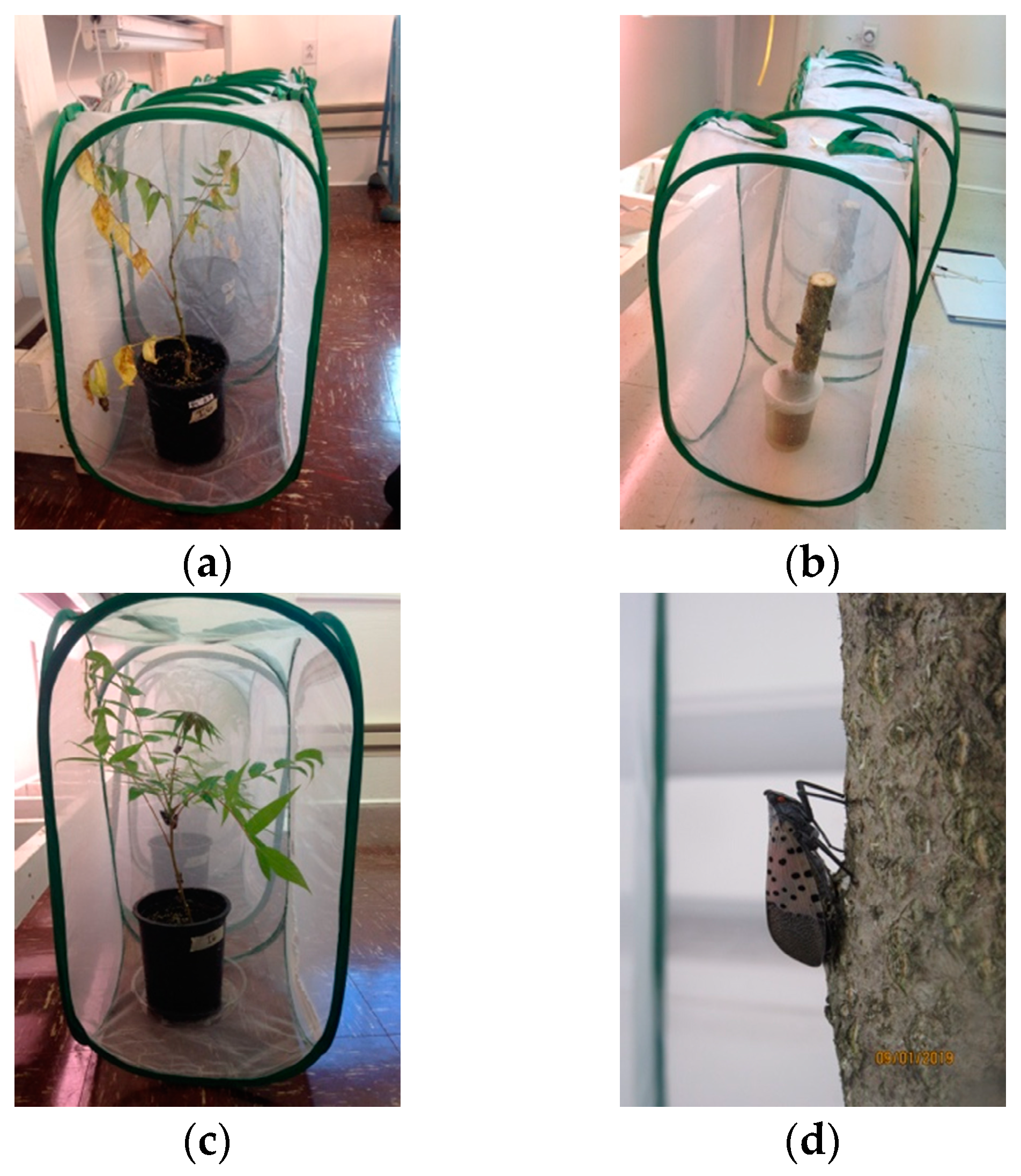
2.6. Exposing L. delicatula to Symptomatic Trees
2.7. Culturing V. nonalfalfae from L. delicatula
2.8. Monitoring and Destructively Sampling A. altissima Seedlings
2.9. Analysis
3. Results
3.1. Culturing V. nonalfalfae from L. delicatula
3.2. Culturing V. nonalfalfae from A. altissima
3.3. Active Feeding and Mortality
4. Discussion
4.1. Lycorma delicatula was Unable to Vector V. nonalfalfae between A. altissima in Laboratory Conditions
4.2. Active Feeding Numbers Indicate Method Realistic
4.3. Mortality Insignificant between Feeding Treatments
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barringer, L.E.; Donovall, L.R.; Spichiger, S.E.; Lynch, D.; Henry, D. The First new world record of Lycorma delicatula (Insecta: Hemiptera: Fulgoridae). Entomol. News 2015, 25, 20–23. [Google Scholar] [CrossRef]
- Lee, D.-H.; Park, Y.-L.; Leskey, T.C. A Review of biology and management of Lycorma delicatula (Hemiptera: Fulgoridae), an emerging global invasive species. J. Asia-Pac. Entomol. 2019, 22, 589–596. [Google Scholar] [CrossRef]
- Urban, J.M. Perspective: Shedding light on spotted lanternfly impacts in the USA. Pest Mgmt. Sci. 2020, 76, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Moon, S.-R.; Ahn, H.-G.; Cho, S.-R.; Yang, J.-O.; Yoon, C.-M.; Kim, G.-H. Feeding behavior of Lycorma delicatula (Hemiptera: Fulgoridae) and response on feeding stimulants of some plants. Korean J. Appl. Entomol. 2009, 48, 467–477. [Google Scholar] [CrossRef]
- Kim, J.G.; Lee, E.H.; Seo, Y.M.; Kim, N.Y. Cyclic behavior of Lycorma delicatula (Insecta: Hemiptera: Fulgoridae) on host plants. J. Ins. Behav. 2011, 24, 423–435. [Google Scholar] [CrossRef]
- Dara, S.K.; Barringer, L.; Arthurs, S.P. Lycorma delicatula (Hemiptera: Fulgoridae): A new invasive pest in the United States. J. Integr. Pest. Mgmt. 2015, 6, 20. [Google Scholar] [CrossRef]
- Song, S.; Kim, S.; Kwon, S.W.; Lee, S.-I.; Jablonski, P.G. Defense sequestration associated with narrowing of diet and ontogenetic change to aposematic colours in the spotted lanternfly. Scient. Repts. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Kowarik, I.; Säumel, I. Biological flora of Central Europe: Ailanthus altissima (Mill.) Swingle. Perspect. Plant Ecol. Evol. Syst. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Asaro, C.; Becker, C.; Creighton, J. Control and Utilization of Tree-of-Heaven: A Guide for Virginia Landowners; No. P00144; Department of Forestry Publication: Charlottesville, VA, USA, 2009; p. 15. [Google Scholar]
- Gover, A.; Johnson, J.; Lloyd, K.; Sellmer, J. Tree-of-heaven (Ailanthus altissima). In Quicksheet 5. Wildland Weed Management; Penn State, College of Agricultural Sciences: State College, PA, USA, 2013. [Google Scholar]
- Herrick, N.J.; Salom, S.M.; Kok, L.T.; McAvoy, T.J. Life History, development, and rearing of Eucryptorrhynchus brandti (Coleoptera: Curculionidae) in quarantine. Ann. Entomol. Soc. Am. 2011, 104, 718–725. [Google Scholar] [CrossRef]
- Herrick, N.J.; McAvoy, T.J.; Snyder, A.L.; Salom, S.M.; Kok, L.T. Host-range testing of Eucryptorrhynchus brandti (Coleoptera: Curculionidae), a candidate for biological control of tree-of-heaven, Ailanthus altissima. Environ. Entomol. 2012, 41, 118–124. [Google Scholar] [CrossRef]
- Schall, M.J.; Davis, D.D. Ailanthus altissima wilt and mortality: Etiology. Plant Dis. 2009, 93, 747–751. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, E.S.; Davis, D.D. Biocontrol of Ailanthus altissima: Inoculation protocol and risk assessment for Verticillium nonalfalfae (Plectosphaerellaceae: Phyllachorales). Biocont. Sci. Tech. 2015, 25, 950–969. [Google Scholar] [CrossRef]
- O’Neal, E.S.; Davis, D.D. Intraspecific root grafts and clonal growth within Ailanthus altissima stands influence Verticillium nonalfalfae transmission. Plant Dis. 2015, 99, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Kasson, M.T.; Short, D.P.; O’Neal, E.S.; Subbarao, K.V.; Davis, D.D. Comparative pathogenicity, biocontrol efficacy, and multilocus sequence typing of Verticillium nonalfalfae from the invasive Ailanthus altissima and other hosts. Phytopath 2014, 104, 282–292. [Google Scholar] [CrossRef]
- Rebbeck, J.; Malone, M.A.; Short, D.P.G.; Kasson, M.T.; O’Neal, E.S.; Davis, D.D. First report of Verticillium wilt caused by Verticillium nonalfalfae on tree-of-heaven (Ailanthus altissima) in Ohio. Plant Dis. 2013, 97, 999. [Google Scholar] [CrossRef]
- Snyder, A.L.; Kasson, M.T.; Salom, S.M.; Davis, D.D.; Griffin, G.J.; Kok, L.T. First report of Verticillium wilt of Ailanthus altissima in Virginia caused by Verticillium nonalfalfae. Plant Dis. 2013, 97, 837. [Google Scholar] [CrossRef]
- Snyder, A.L.; Salom, S.M.; Kok, L.T.; Griffin, G.J.; Davis, D.D. Assessing Eucryptorrhynchus brandti (Coleoptera: Curculionidae) as a potential carrier for Verticillium nonalfalfae (Phyllachorales) from infected Ailanthus altissima. Biocont. Sci. Tech. 2012, 22, 1005–1019. [Google Scholar] [CrossRef]
- Talboys, P.W. A culture-medium aiding the identification of Verticillium albo-atrum and V. dahliae. Plant Path. 1960, 9, 57–58. [Google Scholar] [CrossRef]
- Brooks, R.K.; Wickert, K.L.; Baudoin, A.; Kasson, M.T.; Salom, S.M. Field-inoculated Ailanthus altissima stands reveal the biological control potential of Verticillium nonalfalfae in the mid-Atlantic region of the United States. Biol. Control. 2020, 148, 104298. [Google Scholar] [CrossRef]
- Christen, A. A selective medium for isolating Verticillium albo-atrum from soil. Phytopathology 1982, 72, 47–49. [Google Scholar] [CrossRef]
- Inderbitzin, P.; Davis, R.M.; Bostock, R.M.; Subbarao, K.V. Identification and differentiation of Verticillium species and V. longisporum lineages by simplex and multiplex PCR assays. PLoS ONE 2013, 8, e65990. [Google Scholar] [CrossRef] [PubMed]
- Pompon, J.; Quiring, D.; Goyer, C.; Giordanengo, P.; Pelletier, Y. A phloem-sap feeder mixes phloem and xylem sap to regulate osmotic potential. J. Insect Physiol. 2011, 57, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.R.; Saxena, R.C. Technique for demonstrating phloem of xylem feeding by leafhoppers (Homoptera: Cicadellidae) and planthoppers (Homoptera: Delphacidae) in rice plant. J. Econ. Entomol. 1984, 77, 550–552. [Google Scholar] [CrossRef]
- Weintraub, P.G.; Beanland, L. Insect vectors of phytoplasmas. Rev. Entomol. 2006, 51, 91–111. [Google Scholar] [CrossRef]
- Mitchell, P.L. Heteroptera as vectors of plant pathogens. Neotrop. Entomol. 2004, 33, 519–545. [Google Scholar] [CrossRef]
- Leach, J.G. Insect Transmission of Plant Diseases; McGraw-Hill Book Co., Inc.: New York, NY, USA, 1940. [Google Scholar]

| Active Feeding (%) | Survival (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Life Stage | Type | Treatment | Mean | SD | n | p | Mean | SD | n | p |
| fourth instar | seedling | control | 87.2 | 7.2 | 25 | 0.079 | 100 | 0.0 | 5 | 0.148 |
| fourth instar | seedling | V. nonalfalfae | 68.7 | 20.2 | 50 | 74 | 32.7 | 10 | ||
| adult | seedling | control | 90.4 | 7.8 | 25 | 0.194 | 100 | 0.0 | 5 | 1.000 |
| adult | seedling | V. nonalfalfae | 85.5 | 6.2 | 50 | 98 | 6.3 | 10 | ||
| adult | log | control | 87.2 | 15.1 | 25 | 1.000 | 100 | 0.0 | 5 | 0.519 |
| adult | log | V. nonalfalfae | 87.3 | 9.3 | 50 | 94 | 9.7 | 10 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brooks, R.K.; Toland, A.; Dechaine, A.C.; McAvoy, T.; Salom, S. The Inability of Spotted Lanternfly (Lycorma delicatula) to Vector a Plant Pathogen between its Preferred Host, Ailanthus altissima, in a Laboratory Setting. Insects 2020, 11, 515. https://doi.org/10.3390/insects11080515
Brooks RK, Toland A, Dechaine AC, McAvoy T, Salom S. The Inability of Spotted Lanternfly (Lycorma delicatula) to Vector a Plant Pathogen between its Preferred Host, Ailanthus altissima, in a Laboratory Setting. Insects. 2020; 11(8):515. https://doi.org/10.3390/insects11080515
Chicago/Turabian StyleBrooks, Rachel K., Ashley Toland, Andrew C. Dechaine, Thomas McAvoy, and Scott Salom. 2020. "The Inability of Spotted Lanternfly (Lycorma delicatula) to Vector a Plant Pathogen between its Preferred Host, Ailanthus altissima, in a Laboratory Setting" Insects 11, no. 8: 515. https://doi.org/10.3390/insects11080515
APA StyleBrooks, R. K., Toland, A., Dechaine, A. C., McAvoy, T., & Salom, S. (2020). The Inability of Spotted Lanternfly (Lycorma delicatula) to Vector a Plant Pathogen between its Preferred Host, Ailanthus altissima, in a Laboratory Setting. Insects, 11(8), 515. https://doi.org/10.3390/insects11080515





