Impact of Protoporphyrin Lysine Derivatives on the Ability of Nosema ceranae Spores to Infect Honeybees
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Pre-Treatment of Microsporidia with Porphyrins (In Vitro Study)
2.3. Infection of Honeybees with Porphyrin-Pre-Treated Spores (In Vivo Study)
2.4. Visualization and Determination of the Abundance of Nosema Spores in the Intestines of Honeybees
2.5. Statistical Analysis
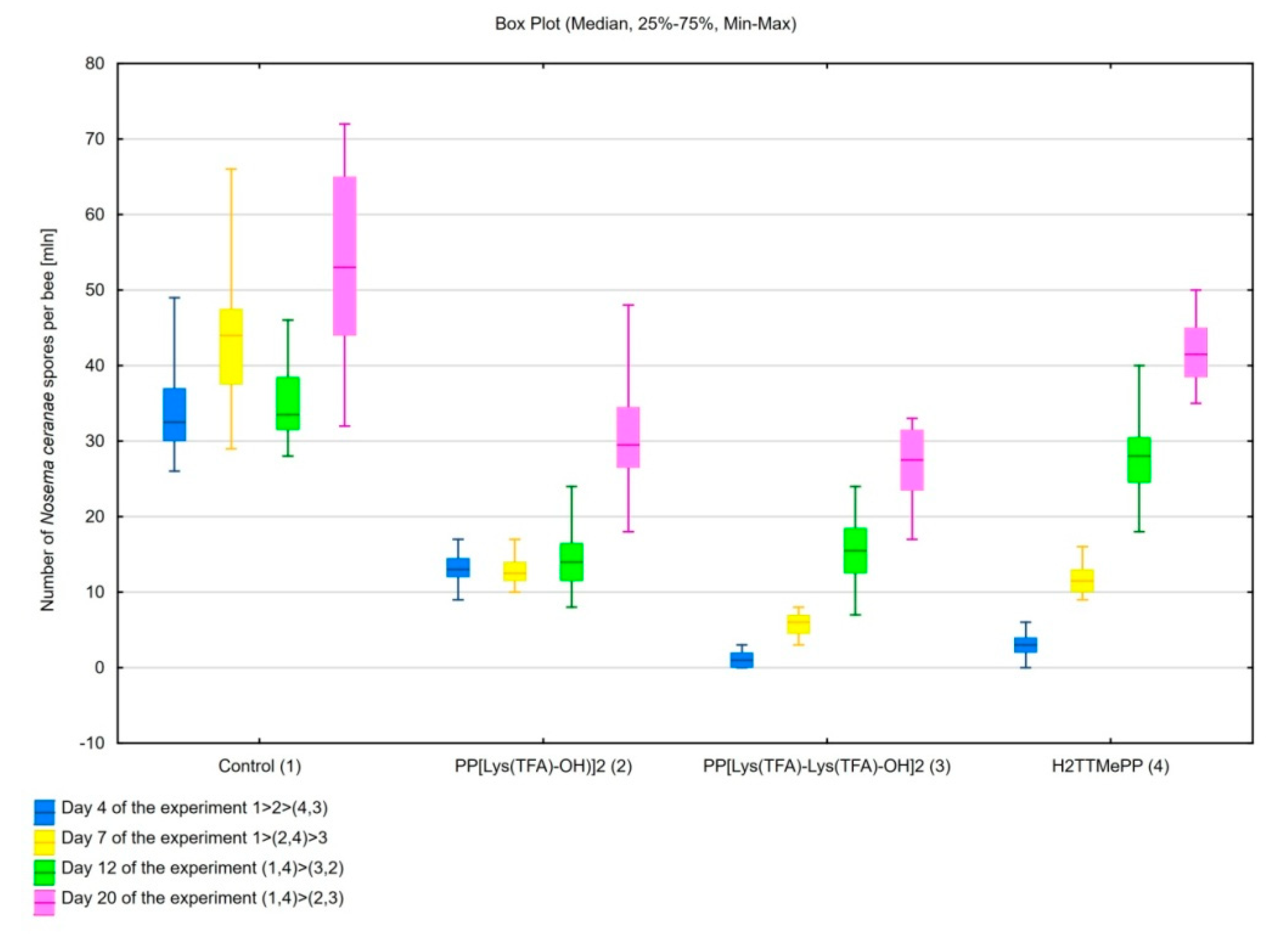
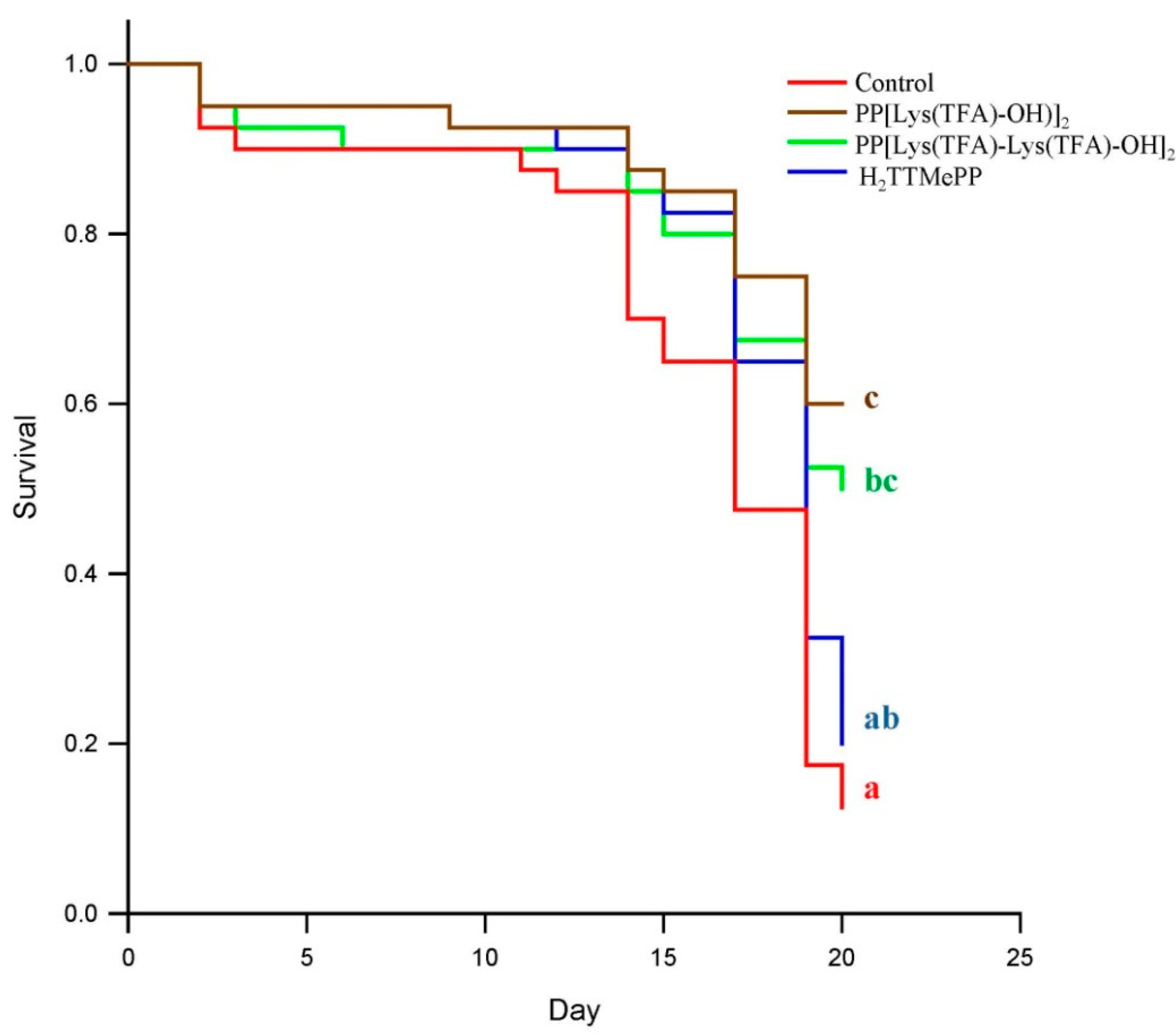
3. Results
3.1. Influence of Porphyrins on Reducing the Infective Capacity of Nosema ceranae Spores
3.2. Feed Intake by Honeybees
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Becnel, J.J.; Andreadis, T.G. Microsporidia in Insect. In The Microsporidia and Microsporidiosis; Wittner, M., Weiss, L.M., Eds.; ASM Press: Washington, DC, USA, 1999; pp. 447–501. [Google Scholar]
- Keeling, P.J.; Fast, N.M. Microsporidia: Biology and evolution of highly reduced intracellular parasites. Annu. Rev. Microbiol. 2002, 56, 93–116. [Google Scholar] [CrossRef] [PubMed]
- Keeling, P.J.; Luker, M.A.; Palmer, J.D. Evidence from beta-tubulin phylogeny that microsporidia evolved from within the fungi. Mol. Boil. Evol. 2000, 17, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Corradi, N.; Byrnes, E.J.; Torres-Martínez, S.; Dietrich, F.S.; Keeling, P.J.; Heitman, J. Microsporidia evolved from ancestral sexual fungi. Curr. Boil. 2008, 18, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Corradi, N.; Keeling, P.J. Microsporidia: A journey through radical taxonomical revisions. Fungal Boil. Rev. 2009, 23, 1–8. [Google Scholar] [CrossRef]
- Dong, S.; Shen, Z.; Xu, L.; Zhu, F. Sequence and phylogenetic analysis of SSU rRNA gene of five microsporidia. Curr. Microbiol. 2009, 60, 30–37. [Google Scholar] [CrossRef]
- Yang, D.; Pan, L.; Chen, Z.; Du, H.; Luo, B.; Luo, J.; Pan, G. The roles of microsporidia spore wall proteins in the spore wall formation and polar tube anchorage to spore wall during development and infection processes. Exp. Parasitol. 2018, 187, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, E.; Sacchi, L. Cell biology and invasion of the microsporidia. Microbes Infect. 2001, 3, 373–379. [Google Scholar] [CrossRef]
- Zander, E. Tierische parasitenals krankheitserregerbei der biene. Münchener Bienenztg. 1909, 31, 196–204. [Google Scholar]
- Fries, I.; Feng, F.; Da Silva, A.; Slemenda, S.B.; Pieniazek, N.J. Nosema ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honey bee Apis cerana (Hymenoptera, Apidae). Eur. J. Protistol. 1996, 32, 356–365. [Google Scholar] [CrossRef]
- Fries, I.; Martín, R.; Meana, A.; García-Palencia, P.; Higes, M. Natural infections of Nosema ceranae in European honey bees. J. Apic. Res. 2006, 45, 230–233. [Google Scholar] [CrossRef]
- Huang, W.-F.; Jiang, J.-H.; Chen, Y.-W.; Wang, C.-H. A Nosema ceranae isolate from the honeybee Apis mellifera. Apidologie 2007, 38, 30–37. [Google Scholar] [CrossRef]
- Higes, M.; García-Palencia, P.; Urbieta, A.; Nanetti, A.; Martín-Hernández, R. Nosema apis and Nosema ceranae tissue tropism in worker honey bees (Apis mellifera). Vet. Pathol. 2019, 57, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Paxton, R.; Klee, J.; Korpela, S.; Fries, I. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 2007, 38, 558–565. [Google Scholar] [CrossRef]
- Chen, Y.; Evans, J.D.; Smith, I.B.; Pettis, J.S. Nosema ceranae is a long-present and wide-spread microsporidian infection of the European honey bee (Apis mellifera) in the United States. J. Invertebr. Pathol. 2008, 97, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Fenoy, S.; Rueda, C.; Higes, M.; Hernández, R.M.; Del Aguila, C. High-level resistance of Nosema ceranae, a parasite of the honeybee, to temperature and desiccation. Appl. Environ. Microbiol. 2009, 75, 6886–6889. [Google Scholar] [CrossRef]
- Cilia, G.; Sagona, S.; Giusti, M.; Dos Santos, P.E.J.; Nanetti, A.; Felicioli, A. Nosema ceranae infection in honeybee samples from Tuscanian archipelago (Central Italy) investigated by two qPCR methods. Saudi J. Boil. Sci. 2019, 26, 1553–1556. [Google Scholar] [CrossRef]
- Broadrup, R.L.; Mayack, C.; Schick, S.J.; Eppley, E.J.; White, H.K.; Macherone, A. Honey bee (Apis mellifera) exposomes and dysregulated metabolic pathways associated with Nosema ceranae infection. PLoS ONE 2019, 14, e0213249. [Google Scholar] [CrossRef]
- Glavinic, U.; Tesovnik, T.; Stevanovic, J.; Zorc, M.; Cizelj, I.; Stanimirovic, Z.; Narat, M. Response of adult honey bees treated in larval stage with prochloraz to infection with Nosema ceranae. PeerJ 2019, 7, e6325. [Google Scholar] [CrossRef]
- Tesovnik, T.; Zorc, M.; Ristanić, M.; Glavinić, U.; Stevanović, J.; Narat, M.; Stanimirović, Z. Exposure of honey bee larvae to thiamethoxam and its interaction with Nosema ceranae infection in adult honey bees. Environ. Pollut. 2020, 256, 113443. [Google Scholar] [CrossRef]
- Higes, M.; García-Palencia, P.; Hernández, R.M.; Meana, A. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 2007, 94, 211–217. [Google Scholar] [CrossRef]
- Dussaubat, C.; Brunet, J.-L.; Higes, M.; Colbourne, J.K.; Lopez, J.; Choi, J.-H.; Hernández, R.M.; Botías, C.; Cousin, M.; McDonnell, C.; et al. Gut pathology and responses to the microsporidium Nosema ceranae in the honey bee Apis mellifera. PLoS ONE 2012, 7, e37017. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L. The honey bee parasite Nosema ceranae: Transmissible via food exchange? PLoS ONE 2012, 7, e43319. [Google Scholar] [CrossRef]
- Lecocq, A.; Jensen, A.B.; Kryger, P.; Nieh, J.C. Parasite infection accelerates age polyethism in young honey bees. Sci. Rep. 2016, 6, 22042. [Google Scholar] [CrossRef] [PubMed]
- Naug, D.; Gibbs, A. Behavioral changes mediated by hunger in honeybees infected with Nosema ceranae. Apidologie 2009, 40, 595–599. [Google Scholar] [CrossRef]
- Sulborska, A.; Horecka, B.; Cebrat, M.; Kowalczyk, M.; Skrzypek, T.H.; Kazimierczak, W.; Trytek, M.; Borsuk, G. Microsporidia Nosema spp.—obligate bee parasites are transmitted by air. Sci. Rep. 2019, 9, 14376. [Google Scholar] [CrossRef] [PubMed]
- Diaz, T.; Del-Val, E.; Ayala, R.; Larsen, J. Alterations in honey bee gut microorganisms caused by Nosema spp. and pest control methods. Pest. Manag. Sci. 2018, 75, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Antúnez, K.; Hernández, R.M.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef]
- Hernández, R.M.; Botías, C.; Barrios, L.; Martínez-Salvador, A.; Meana, A.; Mayack, C.; Higes, M. Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol. Res. 2011, 109, 605–612. [Google Scholar] [CrossRef]
- Hernández, R.M.; Meana, A.; Prieto, L.; Salvador, A.M.; Garrido-Bailón, E.; Higes, M. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 2007, 73, 6331–6338. [Google Scholar] [CrossRef]
- Higes, M.; Hernández, R.M.; Botías, C.; Bailón, E.G.; González-Porto, A.V.; Barrios, L.; Del Nozal, M.; Bernal, J.L.; Jiménez, J.; Palencia, P.G.; et al. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 2008, 10, 2659–2669. [Google Scholar] [CrossRef] [PubMed]
- Vanengelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef] [PubMed]
- Currie, R.W.; Pernal, S.F.; Guzman-Novoa, E. Honey bee colony losses in Canada. J. Apic. Res. 2010, 49, 104–106. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Paleolog, J.; Borsuk, G. Nosema ceranae infection promotes proliferation of yeasts in honey bee intestines. PLoS ONE 2016, 11, e0164477. [Google Scholar] [CrossRef]
- Didier, P.J.; Phillips, J.N.; Kuebler, D.J.; Nasr, M.; Brindley, P.J.; Stovall, M.E.; Bowers, L.C.; Didier, E.S. Antimicrosporidial activities of Fumagillin, TNP-470, Ovalicin, and Ovalicin derivatives in vitro and in vivo. Antimicrob. Agents Chemother. 2006, 50, 2146–2155. [Google Scholar] [CrossRef]
- Williams, G.R.; Sampson, M.A.; Shutler, D.; Rogers, R.E. Does fumagillin control the recently detected invasive parasite Nosema ceranae in western honey bees (Apis mellifera)? J. Invertebr. Pathol. 2008, 99, 342–344. [Google Scholar] [CrossRef]
- Nanetti, A. Api Herb as an alternative product to treat Nosema infection. COLOSS Workshop: Nosema disease: Lack of knowledge and work standardization; COST Action FA0803—Prevention of honeybee COlony LOSSes: Guadalajara, Spain, 2009; pp. 19–22. [Google Scholar]
- Huang, W.-F.; Solter, L.F.; Yau, P.M.; Imai, B.S. Nosema ceranae escapes fumagillin control in honey bees. PLoS Pathog. 2013, 9, e1003185. [Google Scholar] [CrossRef]
- Burnham, A.J. Scientific advances in controlling Nosema ceranae (Microsporidia) infections in honey bees (Apis mellifera). Front. Vet. Sci. 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Suwannapong, G.; Maksong, S.; Phainchajoen, M.; Benbow, M.; Mayack, C. Survival and health improvement of Nosema infected Apis florea (Hymenoptera: Apidae) bees after treatment with propolis extract. J. Asia-Pacific Èntomol. 2018, 21, 437–444. [Google Scholar] [CrossRef]
- Mura, A.; Pusceddu, M.; Theodorou, P.; Angioni, A.; Floris, I.; Paxton, R.; Satta, A. Propolis consumption reduces Nosema ceranae infection of european honey bees (Apis mellifera). Insects 2020, 11, 124. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Trytek, M.; Borsuk, G.; Buczek, K.; Rybicka-Jasinska, K.; Gryko, D. Porphyrins inactivate Nosema spp. microsporidia. Sci. Rep. 2018, 8, 5523. [Google Scholar] [CrossRef] [PubMed]
- Trytek, M.; Makarska, M.; Polska, K.; Radzki, S.; Fiedurek, J. Biotechnology. Biotechnologia 2005, 4, 109–127. [Google Scholar]
- Almeida, A.; Cunha, Â.; Faustino, M.A.F.; Tomé, A.C.; Neves, M.G.P.M.S. Porphyrins as Antimicrobial Photosensitizing Agents. In Photodynamic Inactivation of Microbial Pathogens: Medical and Environmental Application; Hamblin, M.R., Jori, G., Eds.; RSC Publishing: Cambridge, UK, 2011; pp. 83–160. [Google Scholar] [CrossRef]
- Beirão, S.; Fernandes, S.; Coelho, J.; Faustino, M.A.; Tomé, J.P.; Neves, M.D.G.P.M.S.; Tomé, A.C.; Almeida, A.; Cunha, Â. Photodynamic inactivation of bacterial and yeast biofilms with a cationic porphyrin. Photochem. Photobiol. 2014, 90, 1387–1396. [Google Scholar] [CrossRef]
- Fernandez, J.M.; Bilgin, M.D.I.; Grossweiner, L. Singlet oxygen generation by photodynamic agents. J. Photochem. Photobiol. B Boil. 1997, 37, 131–140. [Google Scholar] [CrossRef]
- Trytek, M.; Janik, E.; Maksymiec, W.; Fiedurek, J.; Lipke, A.; Majdan, M. The spectral and catalytic studies of chlorophylls and pheophytins in mimetic biotransformation of α-pinene. J. Photochem. Photobiol. A Chem. 2011, 223, 14–24. [Google Scholar] [CrossRef]
- Trytek, M.; Lipke, A.; Majdan, M.; Pisarek, S.; Gryko, D. Homo and heterogeneous α-pinene photooxidation using a protoporphyrin-derived amide. Eur. J. Org. Chem. 2013, 2013, 1653–1658. [Google Scholar] [CrossRef]
- Tovmasyan, A.; Sampaio, R.S.; Boss, M.-K.; Bueno-Janice, J.C.; Bader, B.H.; Thomas, M.; Rebouças, J.S.; Orr, M.; Chandler, J.D.; Go, Y.-M.; et al. Anticancer therapeutic potential of Mn porphyrin/ascorbate system. Free Radic. Boil. Med. 2015, 89, 1231–1247. [Google Scholar] [CrossRef]
- Bui, B.; Liu, L.; Chen, W. Latex carrier for improving protoporphryin IX properties for photodynamic therapy. Photodiagnosis Photodyn. Ther. 2016, 14, 159–165. [Google Scholar] [CrossRef]
- Gryko, D.; Maximova, K.; Pisarek, S. A Practical protocol for the conjugation of various amino acids to protoporphyrin IX. Synthsis 2013, 45, 1099–1105. [Google Scholar] [CrossRef]
- Hamiduzzaman, M.M.; Guzman-Novoa, E.; Goodwin, P.H. A multiplex PCR assay to diagnose and quantify Nosema infections in honey bees (Apis mellifera). J. Invertebr. Pathol. 2010, 105, 151–155. [Google Scholar] [CrossRef]
- Forsgren, E.; Fries, I. Comparative virulence of Nosema ceranae and Nosema apis in individual European honey bees. Vet. Parasitol. 2010, 170, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Czekońska, K. The effect of different concentrations of carbon dioxide (CO2) in a mixture with air or nitrogen upon the survival of the honey bee (Apis mellifera). J. Apic. Res. 2009, 48, 67–71. [Google Scholar] [CrossRef]
- Hornitzky, M. Nosema disease—Literature Review and Three Surveys of Beekeepers Part 2; No. 08/006; Rural Industries Research and Development Corporation: Barton, Australia, 2008. [Google Scholar]
- Fries, I.; Chauzat, M.-P.; Chen, Y.P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M.E.; et al. Standard methods for nosema research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Gerphagnon, M.; Latour, D.; Colombet, J.; Sime-Ngando, T. A double staining method using SYTOX green and calcofluor white for studying fungal parasites of phytoplankton. Appl. Environ. Microbiol. 2013, 79, 3943–3951. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Leblanc, P.J.; Didier, E.S. Discrimination between viable and dead Encephalitozoon cuniculi (microsporidian) spores by dual staining with sytox green and calcofluor white M2R. J. Clin. Microbiol. 2000, 38, 3811–3814. [Google Scholar] [CrossRef]
- Snow, J.W. A fluorescent method for visualization of Nosema infection in whole-mount honey bee tissues. J. Invertebr. Pathol. 2016, 135, 10–14. [Google Scholar] [CrossRef]
- Hernández, R.M.; Meana, A.; García-Palencia, P.; Marin, P.; Botías, C.; Garrido-Bailón, E.; Barrios, L.; Higes, M. Effect of temperature on the biotic potential of honeybee microsporidia. Appl. Environ. Microbiol. 2009, 75, 2554–2557. [Google Scholar] [CrossRef]
- Fries, I. Nosema ceranae in European honey bees (Apis mellifera). J. Invertebr. Pathol. 2010, 103, S73–S79. [Google Scholar] [CrossRef]
- Gisder, S.; Hedtke, K.; Möckel, N.; Frielitz, M.-C.; Linde, A.; Genersch, E. Five-year cohort study of Nosema spp. in Germany: Does climate shape virulence and assertiveness of Nosema ceranae? Appl. Environ. Microbiol. 2010, 76, 3032–3038. [Google Scholar] [CrossRef]
- Trytek, M.; Buczek, K.; Borsuk, G.; Ptaszyńska, A.A.; Gromada, A.; Rybicka-Jasińska, K.; Gryko, D. Effect of porphyrinoids on the infectivity of Nosema spp. microsporidia, Abstracts Book: 37. In Proceedings of the International Biotechnology and Research Conference, Rome, Italy, 25–27 April 2018. [Google Scholar]
- Gisder, S.; Genersch, E. Identification of candidate agents active against N. ceranae infection in honey bees: Establishment of a medium throughput screening assay based on N. ceranae infected cultured cells. PLoS ONE 2015, 10, e0117200. [Google Scholar] [CrossRef]
- Buczek, K.; Trytek, M.; Deryło, K.; Borsuk, G.; Rybicka-Jasińska, K.; Gryko, D.; Cytryńska, M.; Tchórzewski, M. Bioactivity studies of porphyrinoids against microsporidia isolated from honeybees. Sci. Rep. 2020, 10, 11553. [Google Scholar] [CrossRef] [PubMed]
- Borges, D.; Guzman-Novoa, E.; Goodwin, P.H. Control of the microsporidian parasite Nosema ceranae in honey bees (Apis mellifera) using nutraceutical and immuno-stimulatory compounds. PLoS ONE 2020, 15, e0227484. [Google Scholar] [CrossRef] [PubMed]
- Ptaszyńska, A.A.; Małek, W.; Borsuk, G.; Grzęda, M.; Wicha, M.; Pachla, A. Bacterial strains of the Lactobacillus and Fructobacillus genera, isolated from alimentary tract of honeybees to be applied for fighting and prevention of bees' diseases and the probiotic preparations based on such bacteria strains. Polish patent application No. PL423363, 6 November 2017. [Google Scholar]
- Mortensen, A.N.; Jack, C.J.; A Bustamante, T.; Schmehl, D.R.; Ellis, J.D. Effects of Supplemental pollen feeding on honey bee (Hymenoptera: Apidae) colony strength and Nosema spp. infection. J. Econ. Èntomol. 2018, 112, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.T.; Thompson, E.E.; Di Rienzo, A. Complex signatures of natural selection at the duffy blood group locus. Am. J. Hum. Genet. 2002, 70, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Giuntini, F.; Alonso, C.M.A.; Boyle, R.W. Synthetic approaches for the conjugation of porphyrins and related macrocycles to peptides and proteins. Photochem. Photobiol. Sci. 2011. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buczek, K.; Deryło, K.; Kutyła, M.; Rybicka-Jasińska, K.; Gryko, D.; Borsuk, G.; Rodzik, B.; Trytek, M. Impact of Protoporphyrin Lysine Derivatives on the Ability of Nosema ceranae Spores to Infect Honeybees. Insects 2020, 11, 504. https://doi.org/10.3390/insects11080504
Buczek K, Deryło K, Kutyła M, Rybicka-Jasińska K, Gryko D, Borsuk G, Rodzik B, Trytek M. Impact of Protoporphyrin Lysine Derivatives on the Ability of Nosema ceranae Spores to Infect Honeybees. Insects. 2020; 11(8):504. https://doi.org/10.3390/insects11080504
Chicago/Turabian StyleBuczek, Katarzyna, Kamil Deryło, Mateusz Kutyła, Katarzyna Rybicka-Jasińska, Dorota Gryko, Grzegorz Borsuk, Beata Rodzik, and Mariusz Trytek. 2020. "Impact of Protoporphyrin Lysine Derivatives on the Ability of Nosema ceranae Spores to Infect Honeybees" Insects 11, no. 8: 504. https://doi.org/10.3390/insects11080504
APA StyleBuczek, K., Deryło, K., Kutyła, M., Rybicka-Jasińska, K., Gryko, D., Borsuk, G., Rodzik, B., & Trytek, M. (2020). Impact of Protoporphyrin Lysine Derivatives on the Ability of Nosema ceranae Spores to Infect Honeybees. Insects, 11(8), 504. https://doi.org/10.3390/insects11080504




