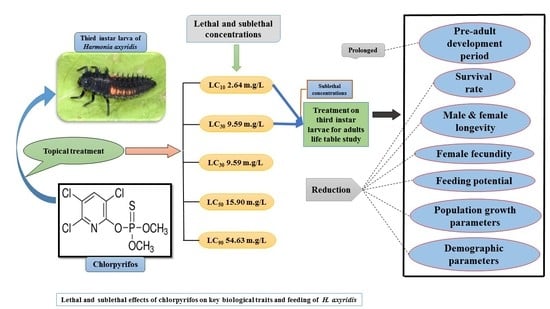
Lethal and Sublethal Effects of Chlorpyrifos on Biological Traits and Feeding of the Aphidophagous Predator Harmonia axyridis
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Culture
2.2. Acute Toxicity Determination
2.3. Evaluation of Sublethal Effects on Life Table of H. axyridis
2.4. Assessment of Feeding Potential of H. axyridis
2.5. Effects of Sublethal Chlorpyrifos Concentrations on Population Growth Parameters
2.6. Effect of Chlorpyrifos Sublethal Concentrations on Demographic Parameters
2.7. Statistical Analysis
3. Results
3.1. Chlorpyrifos Toxicity on Third Instar H. axyridis Larvae
3.2. Sublethal Effects of Chlorpyrifos on H. axyridis
3.2.1. Effects on Pre-Adult Development
3.2.2. Effects on Life Table Parameters of H. axyridis Adults
3.3. Effects on H. axyridis Population Growth Parameters
3.4. Effects on H. axyridis Feeding Potential
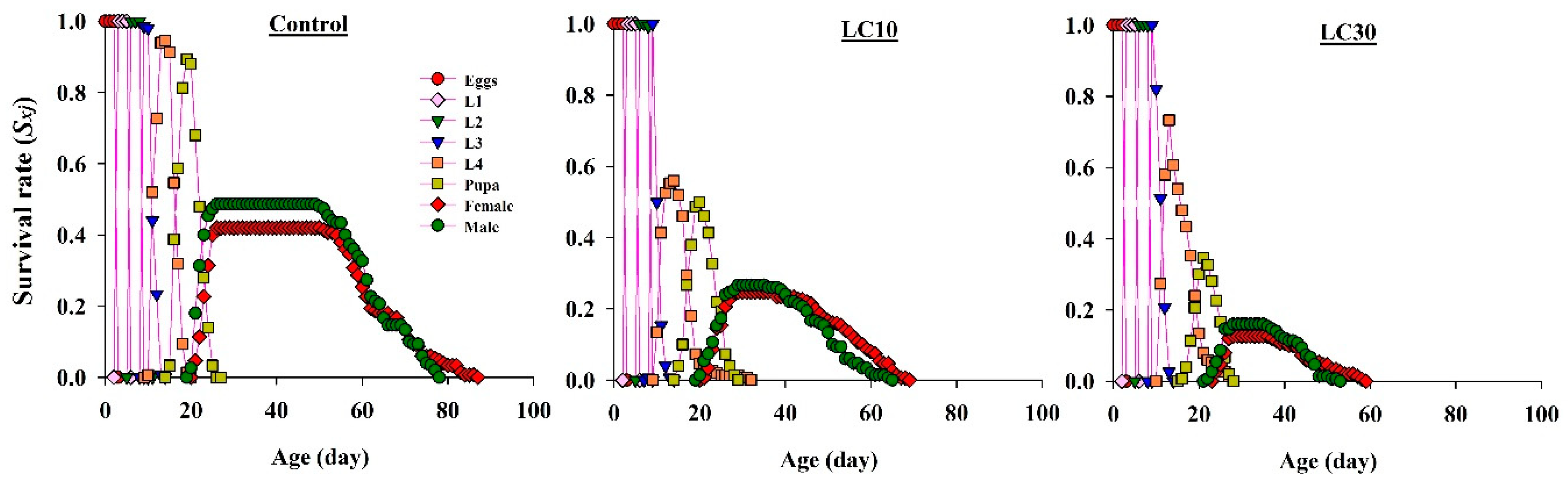
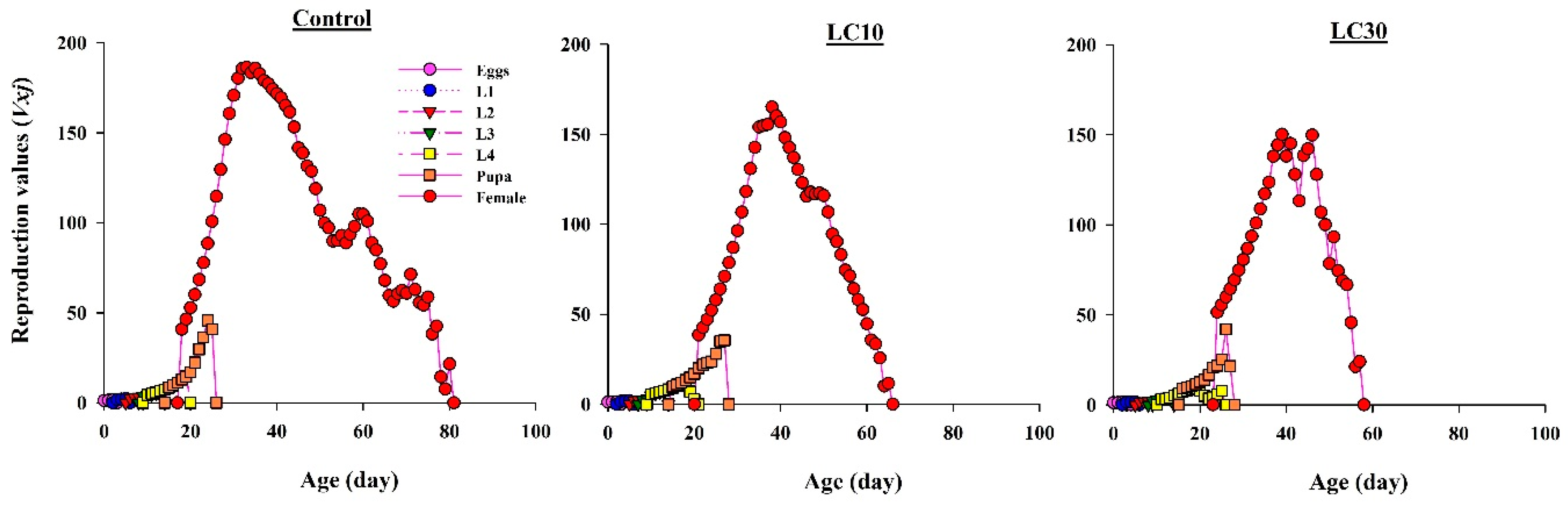
3.5. Effects on H. axyridis Demographic Parameters
3.5.1. Effect on Survival Rate
3.5.2. Effect on Life Expectancy
3.5.3. Effect on Reproduction
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Juen, A.; Hogendoorn, K.; Ma, G.; Schmidt, O.; Keller, M.A. Analysing the diets of invertebrate predators using terminal restriction fragments. J. Pest Sci. 2012, 85, 89–100. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, K.; Jiang, Y.; Guo, Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362–365. [Google Scholar] [CrossRef]
- Ali, S.; Li, S.; Jaleel, W.; Khan, M.M.; Wang, J.; Zhou, X. Using a Two-Sex Life Table Tool to Calculate the Fitness of Orius strigicollis as a Predator of Pectinophora gossypiella. Insects 2020, 11, 275. [Google Scholar] [CrossRef]
- Hagen, K.S. The significance of predaceous Coccinellidae in biological and integrated control of insects. Entomophaga Mem. Hors Ser. 1974, 7, 25–44. [Google Scholar]
- Minks, A.K.; Harrewijn, P. Aphids Their Biology, Natural Enemies and Control; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Gerling, D. Natural enemies of whiteflies: Predators and parasitoids. In Whiteflies Their Bionomics, Pest Status Manag; Intercept Press: Hants, UK, 1990; pp. 147–185. [Google Scholar]
- Hodek, I.; Honek, A.; Van Emden, H.F. Ecology and Behaviour of the Ladybird Beetles (Coccinellidae); John Wiley & Sons: Oxford, UK, 2012. [Google Scholar]
- Riddick, E.W.; Wu, Z.; Rojas, M.G. Is Tetranychus urticae suitable prey for development and reproduction of naïve Coleomegilla maculata? Insect Sci. 2014, 21, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Eliopoulos, P.A.; Kontodimas, D.C.; Stathas, G.J. Temperature-Dependent Development of Chilocorus bipustulatus (Coleoptera: Coccinellidae). Environ. Entomol. 2010, 39, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.M.J.; Thomas, C.E.; Lombaert, E.; Jeffries, D.L.; Estoup, A.; Handley, L.J.L. The global spread of Harmonia axyridis (Coleoptera: Coccinellidae): Distribution, dispersal and routes of invasion. BioControl 2011, 56, 623–641. [Google Scholar] [CrossRef]
- Castro, C.F.; Almeida, L.M.; Penteado, S.R.C. The Impact of Temperature on Biological Aspects and Life Table of Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). Fla. Entomol. 2011, 94, 923–932. [Google Scholar] [CrossRef]
- Luo, S.; Naranjo, S.E.; Wu, K. Biological control of cotton pests in China. Biol. Control 2014, 68, 6–14. [Google Scholar] [CrossRef]
- Lundgren, J.G. Nutritional aspects of non-prey foods in the life histories of predaceous Coccinellidae. Biol. Control 2009, 51, 294–305. [Google Scholar] [CrossRef]
- Pell, J.K.; Baverstock, J.; Roy, H.E.; Ware, R.L.; Majerus, M.E.N. Intraguild predation involving Harmonia axyridis: A review of current knowledge and future perspectives. BioControl 2008, 53, 147–168. [Google Scholar] [CrossRef]
- Yang, N.W.; Zang, L.S.; Wang, S.; Guo, J.Y.; Xu, H.X.; Zhang, F.; Wan, F.H. Biological pest management by predators and parasitoids in the greenhouse vegetables in China. Biol. Control 2014, 68, 92–102. [Google Scholar] [CrossRef]
- Brown, M.W.; Miller, S.S. Coccinellidae (Coleoptera) in apple orchards of eastern West Virginia and the impact of invasion by Harmonia axyridis. Entomol. News 1998, 109, 143–151. [Google Scholar]
- Tedders, W.L.; Schaefer, P.W. Release and establishment of Harmonia axyridis (Coleoptera: Coccinellidae) in the southeastern United States. Entomol. News 1994, 105, 228–243. [Google Scholar]
- Rice, N.R.; Smith, M.W.; Eikenbary, R.D.; Arnold, D.; Tedders, W.L.; Wood, B.; Landgraf, B.S.; Taylor, G.G.; Barlow, G.E. Assessment of legume and nonlegume ground covers on Coleoptera: Coccinellidae density for low-input pecan management. Am. J. Altern. Agric. 1998, 13, 111–123. [Google Scholar] [CrossRef]
- Michaud, J.P. The Asian citrus psyllid, Diaphorini citri, and its natural enemies. Citrus Ind. 2000, 81, 42–44. [Google Scholar]
- Musser, F.R.; Shelton, A.M. Bt sweet corn and selective insecticides: Impacts on pests and predators. J. Econ. Entomol. 2003, 96, 71–80. [Google Scholar] [CrossRef]
- Stuart, R.J.; Michaud, J.P.; Olsen, L.; McCoy, C.W. Lady beetles as potential predators of the root weevil diaprepesabbreviatus (coleoptera: Curculionidae) in florida citrus. Fla. Entomol. 2002, 85, 409–416. [Google Scholar] [CrossRef]
- Michaud, J.P. Sources of mortality in colonies of brown citrus aphid, Toxoptera citricida. BioControl 1999, 44, 347–367. [Google Scholar] [CrossRef]
- Wang, Y.P.; Lv, F.; Wang, Z.P. Progress of Harmonia axyridis (Pallas) utilization. Entomol. J. East China 2007, 16, 310–314. [Google Scholar]
- ELZEN, G.W. Lethal and Sublethal Effects of Insecticide Residues on Orius insidiosus (Hemiptera: Anthocoridae) and Geocoris punctipes (Hemiptera: Lygaeidae). J. Econ. Entomol. 2009, 94, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.N.; Seo, M.J.; Shin, J.G.; Jang, C.; Yu, Y.M. Toxicity of greenhouse pesticides to multicolored Asian lady beetles, Harmonia axyridis (Coleoptera: Coccinellidae). Biol. Control 2003, 28, 164–170. [Google Scholar] [CrossRef]
- Garratt, J.; Kennedy, A. Use of models to assess the reduction in contamination of water bodies by agricultural pesticides through the implementation of policy instruments: A case study of the Voluntary Initiative in the UK. Pest Manag. Sci. 2006, 62, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Nawaz, M.; Hua, H.; Cai, W.; Zhao, J. Lethal and sublethal effects of emamectin benzoate on the rove beetle, Paederus fuscipes, a non-target predator of rice brown planthopper, Nilaparvata lugens. Ecotoxicol. Environ. Saf. 2018, 165, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Puinean, A.M.; Denholm, I.; Millar, N.S.; Nauen, R.; Williamson, M.S. Characterisation of imidacloprid resistance mechanisms in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Pestic. Biochem. Physiol. 2010, 97, 129–132. [Google Scholar] [CrossRef]
- Kavi, L.A.K.; Kaufman, P.E.; Scott, J.G. Genetics and mechanisms of imidacloprid resistance in house flies. Pestic. Biochem. Physiol. 2014, 109, 64–69. [Google Scholar] [CrossRef]
- Bass, C.; Denholm, I.; Williamson, M.S.; Nauen, R. The global status of insect resistance to neonicotinoid insecticides. Pestic. Biochem. Physiol. 2015, 121, 78–87. [Google Scholar] [CrossRef]
- Saddiq, B.; Shad, S.A.; Aslam, M.; Ijaz, M.; Abbas, N. Monitoring resistance of Phenacoccus solenopsis (Homoptera: Pseudococcidae) to new chemical insecticides in Punjab, Pakistan. Crop Prot. 2015, 74, 24–29. [Google Scholar] [CrossRef]
- Galvan, T.L.; Koch, R.L.; Hutchison, W.D. Effects of spinosad and indoxacarb on survival, development, and reproduction of the multicolored Asian lady beetle (Coleoptera: Coccinellidae). Biol. Control 2005, 34, 108–114. [Google Scholar] [CrossRef]
- Silva, R.A.; Carvalho, G.A.; Carvalho, C.F.; Reis, P.R.; Pereira, A.M.A.R.; Cosme, L.V. Toxicidade de produtos fitossanitários utilizados na cultura do cafeeiro a larvas de Chrysoperla externa (Hagen) (Neuroptera: Chrysopidae) e efeitos Sobre as fases subseqüentes do desenvolvimento do predador. Neotrop. Entomol. 2005, 34, 951–959. [Google Scholar] [CrossRef][Green Version]
- Campiche, S.; Becker-van Slooten, K.; Ridreau, C.; Tarradellas, J. Effects of insect growth regulators on the nontarget soil arthropod Folsomia candida (Collembola). Ecotoxicol. Environ. Saf. 2006, 63, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Cai, W.; Jing, Z.; Zhou, X.; Mabubu, J.I.; Hua, H. Toxicity and sublethal effects of chlorantraniliprole on the development and fecundity of a non-specific predator, the multicolored Asian lady beetle, Harmonia axyridis (Pallas). Chemosphere 2017, 178, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Saber, M. Acute and population level toxicity of imidacloprid and fenpyroximate on an important egg parasitoid, Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). Ecotoxicology 2011, 20, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Stara, J.; Ourednickova, J.; Kocourek, F. Laboratory evaluation of the side effects of insecticides on Aphidius colemani (Hymenoptera: Aphidiidae), Aphidoletes aphidimyza (Diptera: Cecidomyiidae), and Neoseiulus cucumeris (Acari: Phytoseidae). J. Pest Sci. 2011, 84, 25–31. [Google Scholar] [CrossRef]
- Kim, M.; Shin, D.; Suh, E.; Cho, K. An assessment of the chronic toxicity of fenpyroximate and pyridaben to Tetranychus urticae using a demographic bioassay. Appl. Entomol. Zool. 2004, 39, 401–409. [Google Scholar] [CrossRef][Green Version]
- Rimoldi, F.; Schneider, M.I.; Ronco, A.E. Short and Long-Term Effects of Endosulfan, Cypermethrin, Spinosad, and Methoxyfenozide on Adults of Chrysoperla externa (Neuroptera: Chrysopidae). J. Econ. Entomol. 2013, 105, 1982–1987. [Google Scholar] [CrossRef]
- Halappa, R.; David, M. Behavioural responses of the freshwater fish, Cyprinus carpio (Linnaeus) following sublethal exposure to chlorpyrifos. Turk. J. Fish. Aquat. Sci. 2009, 9, 233–238. [Google Scholar] [CrossRef]
- Smida, A.; Ncibi, S.; Taleb, J.; Ben Saad, A.; Ncib, S.; Zourgui, L. Immunoprotective activity and antioxidant properties of cactus (Opuntia ficus indica) extract against chlorpyrifos toxicity in rats. Biomed. Pharmacother. 2017, 88, 844–851. [Google Scholar] [CrossRef]
- Santos, K.F.A.; Zanuzo Zanardi, O.; De Morais, M.R.; Jacob, C.R.O.; De Oliveira, M.B.; Yamamoto, P.T. The impact of six insecticides commonly used in control of agricultural pests on the generalist predator Hippodamia convergens (Coleoptera: Coccinellidae). Chemosphere 2017, 186, 218–226. [Google Scholar] [CrossRef]
- Cutler, G.C.; Purdy, J.; Giesy, J.P.; Solomon, K.R. Risk to pollinators from the use of chlorpyrifos in the United States. In Ecological Risk Assessment for Chlorpyrifos in Terrestrial and Aquatic Systems in the United States; Springer: Cham, Switzerland, 2014; pp. 219–265. [Google Scholar]
- Delpuech, J.M.; Gareau, E.; Terrier, O.; Fouillet, P. Sublethal effects of the insecticide chlorpyrifos on the sex pheromonal communication of Trichogramma brassicae. Chemosphere 1998, 36, 1775–1785. [Google Scholar] [CrossRef]
- Guillade, A.C.; Folgarait, P.J. Natural enemies of Atta vollenweideri (Hymenoptera: Formicidae) leaf-cutter ants negatively affected by synthetic pesticides, chlorpyrifos and fipronil. J. Econ. Entomol. 2014, 107, 105–114. [Google Scholar] [CrossRef] [PubMed]
- McCornack, B.P.; Koch, R.L.; Ragsdale, D.W. A Simple Method for In-Field Sex Determination of the Multicolored Asian Lady Beetle Harmonia axyridis. J. Insect Sci. 2007, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, J.; Zheng, Y.; Desneux, N.; Wu, K. Lethal effect of imidacloprid on the coccinellid predator Serangium japonicum and sublethal effects on predator voracity and on functional response to the whitefly Bemisia tabaci. Ecotoxicology 2012, 5, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.I.; Sanchez, N.; Pineda, S.; Chi, H.; Ronco, A. Impact of glyphosate on the development, fertility and demography of Chrysoperla externa (Neuroptera: Chrysopidae): Ecological approach. Chemosphere 2009, 76, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Bandani, A.R. Sublethal concentrations of thiamethoxam adversely affect life table parameters of the aphid predator, Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae). Crop Prot. 2013, 54, 168–175. [Google Scholar] [CrossRef]
- Chi, H.; Yang, T.-C. Two-Sex Life Table and Predation Rate of Propylaea japonica Thunberg (Coleoptera: Coccinellidae) Fed on Myzus persicae (Sulzer) (Homoptera: Aphididae). Environ. Entomol. 2003, 32, 327–333. [Google Scholar] [CrossRef]
- Huang, Y.B.; Chi, H. Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age-specific life tables to insect populations. Insect Sci. 2012, 19, 263–273. [Google Scholar] [CrossRef]
- LeOra, S. Poloplus, A User’s Guide to Probit or Logit Analysis; LeOra Software: Berkeley, CA, USA, 2003. [Google Scholar]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-Table Analysis Incorporating Both Sexes and Variable Development Rates Among Individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. National Chung Hsing University: Taichung, Taiwan. Available online: http://140.1202015.197.193/Ecology/Download/Twosex-MSChart.rar (accessed on 25 December 2019).
- Akca, I.; Ayvaz, T.; Yazici, E.; Smith, C.L.; Chi, H. Demography and population projection of Aphis fabae (Hemiptera: Aphididae): With additional comments on life table research criteria. J. Econ. Entomol. 2015, 108, 1466–1478. [Google Scholar] [CrossRef]
- Dawar, F.U.; Zuberi, A.; Azizullah, A.; Khan Khattak, M.N. Effects of cypermethrin on survival, morphological and biochemical aspects of rohu (Labeo rohita) during early development. Chemosphere 2016, 144, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Galvan, T.L.; Koch, R.L.; Hutchison, W.D. Toxicity of Commonly Used Insecticides in Sweet Corn and Soybean to Multicolored Asian Lady Beetle (Coleoptera: Coccinellidae). J. Econ. Entomol. 2009, 98, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Ono, É.K.; Zanardi, O.Z.; Aguiar Santos, K.F.; Yamamoto, P.T. Susceptibility of Ceraeochrysa cubana larvae and adults to six insect growth-regulator insecticides. Chemosphere 2017, 168, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Garzón, A.; Medina, P.; Amor, F.; Viñuela, E.; Budia, F. Toxicity and sublethal effects of six insecticides to last instar larvae and adults of the biocontrol agents Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae) and Adalia bipunctata (L.) (Coleoptera: Coccinellidae). Chemosphere 2015, 132, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Coons, L.B. Acari: Mites: Integument. Microsc. Anat. Invertebr. Chelicerate Arthropoda C 1999, 8, 681–714. [Google Scholar]
- Tuan, S.J.; Yeh, C.C.; Atlihan, R.; Chi, H. Linking life table and predation rate for biological control: A comparative study of Eocanthecona furcellata (Hemiptera: Pentatomidae) fed on Spodoptera litura (Lepidoptera: Noctuidae) and Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2016, 109, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Hannig, G.T.; Ziegler, M.; Paula, G.M. Feeding cessation effects of chlorantraniliprole, a new anthranilic diamide insecticide, in comparison with several insecticides in distinct chemical classes and mode-of-action groups. Pest. Manag. Sci. 2009, 65, 969–974. [Google Scholar] [CrossRef]
- Fernandes, M.E.S.; Alves, F.M.; Pereira, R.C.; Aquino, L.A.; Fernandes, F.L.; Zanuncio, J.C. Lethal and sublethal effects of seven insecticides on three beneficial insects in laboratory assays and field trials. Chemosphere 2016, 156, 45–55. [Google Scholar] [CrossRef]
- Mahdavi, V.; Saber, M.; Rafiee-Dastjerdi, H.; Kamita, S.G. Lethal and Demographic Impact of Chlorpyrifos and Spinosad on the Ectoparasitoid Habrobracon hebetor (Say) (Hymenoptera: Braconidae). Neotrop. Entomol. 2015, 44, 626–633. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.; Yu, X.; Ma, D.; Yu, C.; Liu, F.; Mu, W. Influence of lethal and sublethal exposure to clothianidin on the seven-spotted lady beetle, Coccinella septempunctata L. (Coleoptera: Coccinellidae). Ecotoxicol. Environ. Saf. 2018, 161, 208–213. [Google Scholar] [CrossRef]
- Fogel, M.N.; Schneider, M.I.; Desneux, N.; González, B.; Ronco, A.E. Impact of the neonicotinoid acetamiprid on immature stages of the predator Eriopis connexa (Coleoptera: Coccinellidae). Ecotoxicology 2013, 22, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Perveen, F.; Miyata, T. Effects of Sublethal Dose of Chlorfluazuron on Ovarian Development and Oogenesis in the Common Cutworm Spodoptera litura (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 2006, 93, 1131–1137. [Google Scholar] [CrossRef]
- Santos, M.S.; Zanardi, O.Z.; Pauli, K.S.; Forim, M.R.; Yamamoto, P.T.; Vendramim, J.D. Toxicity of an azadirachtin-based biopesticide on Diaphorina citri Kuwayama (Hemiptera: Liviidae) and its ectoparasitoid Tamarixia radiata (Waterston) (Hymenoptera: Eulophidae). Crop Prot. 2015, 74, 116–123. [Google Scholar] [CrossRef]
- Martinou, A.F.; Seraphides, N.; Stavrinides, M.C. Lethal and behavioral effects of pesticides on the insect predator Macrolophus pygmaeus. Chemosphere 2014, 96, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.L.; Zheng, Y.; Zhao, J.W.; Desneux, N.; He, Y.X.; Weng, Q.Y. Lethal and sublethal effects of thiamethoxam on the whitefly predator Serangium japonicum (Coleoptera: Coccinellidae) through different exposure routes. Chemosphere 2015, 128, 49–55. [Google Scholar] [CrossRef]
- Stark, J.D.; Banks, J.E. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 2003, 48, 505–519. [Google Scholar] [CrossRef]
- Papachristos, D.P.; Milonas, P.G. Adverse effects of soil applied insecticides on the predatory coccinellid Hippodamia undecimnotata (Coleoptera: Coccinellidae). Biol. Control 2008, 47, 77–81. [Google Scholar] [CrossRef]
- Muslim, M.; Ansari, M.S.; Hasan, F. Non-target toxicity of synthetic insecticides on the biological performance and population growth of Bracon hebetor Say. Ecotoxicology 2018, 27, 1019–1031. [Google Scholar] [CrossRef]
- Brown, P.M.J.; Adriaens, T.; Bathon, H.; Cuppen, J.; Goldarazena, A.; Hägg, T.; Kenis, M.; Klausnitzer, B.E.M.; Kovář, I.; Loomans, A.J.M.; et al. Harmonia axyridis in Europe: Spread and distribution of a non-native coccinellid, Biological Control to Invasion: The Ladybird Harmonia axyridis as a Model Species. BioControl 2007, 53, 5–21. [Google Scholar] [CrossRef]
- Cisneros-Heredia, D.F.; Peñaherrera-Romero, E. Invasion history of Harmonia axyridis (Pallas, 1773) (Coleoptera: Coccinellidae) in Ecuador. BioRxiv 2020. [Google Scholar] [CrossRef]
- Gordon, R.D. The Coccinellidae (Coleoptera) of America North of Mexico. J. N. Y. Entomol. Soc. 1985, 93, 912. [Google Scholar]
- Koch, R.L.; Venette, R.C.; Hutchison, W.D. Invasions by Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) in the Western Hemisphere: Implications for South America. Neotrop. Entomol. 2006, 35, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, D.L.; Chapman, J.; Roy, H.E.; Humphries, S.; Harrington, R.; Brown, P.M.J.; Handley, L.J.L. Characteristics and drivers of high-altitude ladybird flight: Insights from vertical-looking entomological radar. PLoS ONE 2013, 8, e82278. [Google Scholar] [CrossRef] [PubMed]
- Vilcinskas, A.; Mukherjee, K.; Vogel, H. Expansion of the antimicrobial peptide repertoire in the invasive ladybird Harmonia axyridis. Proc. R. Soc. B Biol. Sci. 2013, 280, 2012–2113. [Google Scholar] [CrossRef]



| Insecticide | Concentration (95% CL)−1 mg (a.i.) L−1 | ||||||
|---|---|---|---|---|---|---|---|
| N | LC10 | LC30 | LC50 | LC90 | Slope ± SE | χ2 (Df) | |
| Chlorpyrifos | 360 | 4.62 | 9.59 | 15.90 | 54.63 | 2.391 ± 0.225 | 5.822 (4) |
| (2.31–6.92) | (6.27–12.89) | (11.70–21.19) | (37.66–102.01) | ||||
| Treatments | Development Period of Immature Stages | ||
|---|---|---|---|
| Third Instar Larva (Day) | Fourth Instar Larva (Day) | Pupa (Day) | |
| Control | 2.68 ± 0.07 b | 5.31 ± 0.06 c | 5.74 ± 0.06 c |
| LC10 | 2.13 ± 0.10 c | 6.46 ± 0.12 b | 6.70 ± 0.13 a |
| LC30 | 2.95 ± 0.08 a | 7.44 ± 0.22 a | 6.14 ± 0.19 b |
| Treatments | Female Adult Longevity (d) | Male Adult Longevity (d) | APOP (d) | TPOP (d) | Fecundity (Eggs/Female) |
|---|---|---|---|---|---|
| Control | 65.43 ± 1.16 a | 63.89 ± 0.9 a | 9.76 ± 0.12 c | 33.14 ± 0.21 c | 694.84 ± 17.28 a |
| LC10 | 55.41 ± 1.36 b | 50.02 ± 1.13 b | 11.76 ± 0.14 b | 36.27 ± 0.32 b | 379.03 ± 24.21 b |
| LC30 | 47.26 ± 1.55 c | 44.46 ± 0.92 c | 12.61 ± 0.30 a | 38.50 ± 0.46 a | 229.06 ± 36.88 c |
| Treatments | Population Growth Parameters | |||
|---|---|---|---|---|
| (r) | (λ) | (R0) | (T) | |
| Control | 0.12 ± 0.002 a | 1.13 ± 0.002 a | 290.33 ± 28.60 a | 43.92 ± 0.33 a |
| LC10 | 0.10 ± 0.003 b | 1.10 ± 0.004 b | 93.36 ± 14.53 b | 44.40 ± 0.50 a |
| LC30 | 0.07 ± 0.006 c | 1.07 ± 0.006 c | 27.48 ± 7.43 c | 44.22 ± 0.75 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasheed, M.A.; Khan, M.M.; Hafeez, M.; Zhao, J.; Islam, Y.; Ali, S.; Ur-Rehman, S.; e-Hani, U.; Zhou, X. Lethal and Sublethal Effects of Chlorpyrifos on Biological Traits and Feeding of the Aphidophagous Predator Harmonia axyridis. Insects 2020, 11, 491. https://doi.org/10.3390/insects11080491
Rasheed MA, Khan MM, Hafeez M, Zhao J, Islam Y, Ali S, Ur-Rehman S, e-Hani U, Zhou X. Lethal and Sublethal Effects of Chlorpyrifos on Biological Traits and Feeding of the Aphidophagous Predator Harmonia axyridis. Insects. 2020; 11(8):491. https://doi.org/10.3390/insects11080491
Chicago/Turabian StyleRasheed, Muhammad Asim, Muhammad Musa Khan, Muhammad Hafeez, Jing Zhao, Yasir Islam, Shahzaib Ali, Shakeel Ur-Rehman, Um e-Hani, and Xingmiao Zhou. 2020. "Lethal and Sublethal Effects of Chlorpyrifos on Biological Traits and Feeding of the Aphidophagous Predator Harmonia axyridis" Insects 11, no. 8: 491. https://doi.org/10.3390/insects11080491
APA StyleRasheed, M. A., Khan, M. M., Hafeez, M., Zhao, J., Islam, Y., Ali, S., Ur-Rehman, S., e-Hani, U., & Zhou, X. (2020). Lethal and Sublethal Effects of Chlorpyrifos on Biological Traits and Feeding of the Aphidophagous Predator Harmonia axyridis. Insects, 11(8), 491. https://doi.org/10.3390/insects11080491