Automatic Field Detection of Western Corn Rootworm (Diabrotica virgifera virgifera; Coleoptera: Chrysomelidae) with a New Probe
Abstract
1. Introduction
2. Materials and Methods
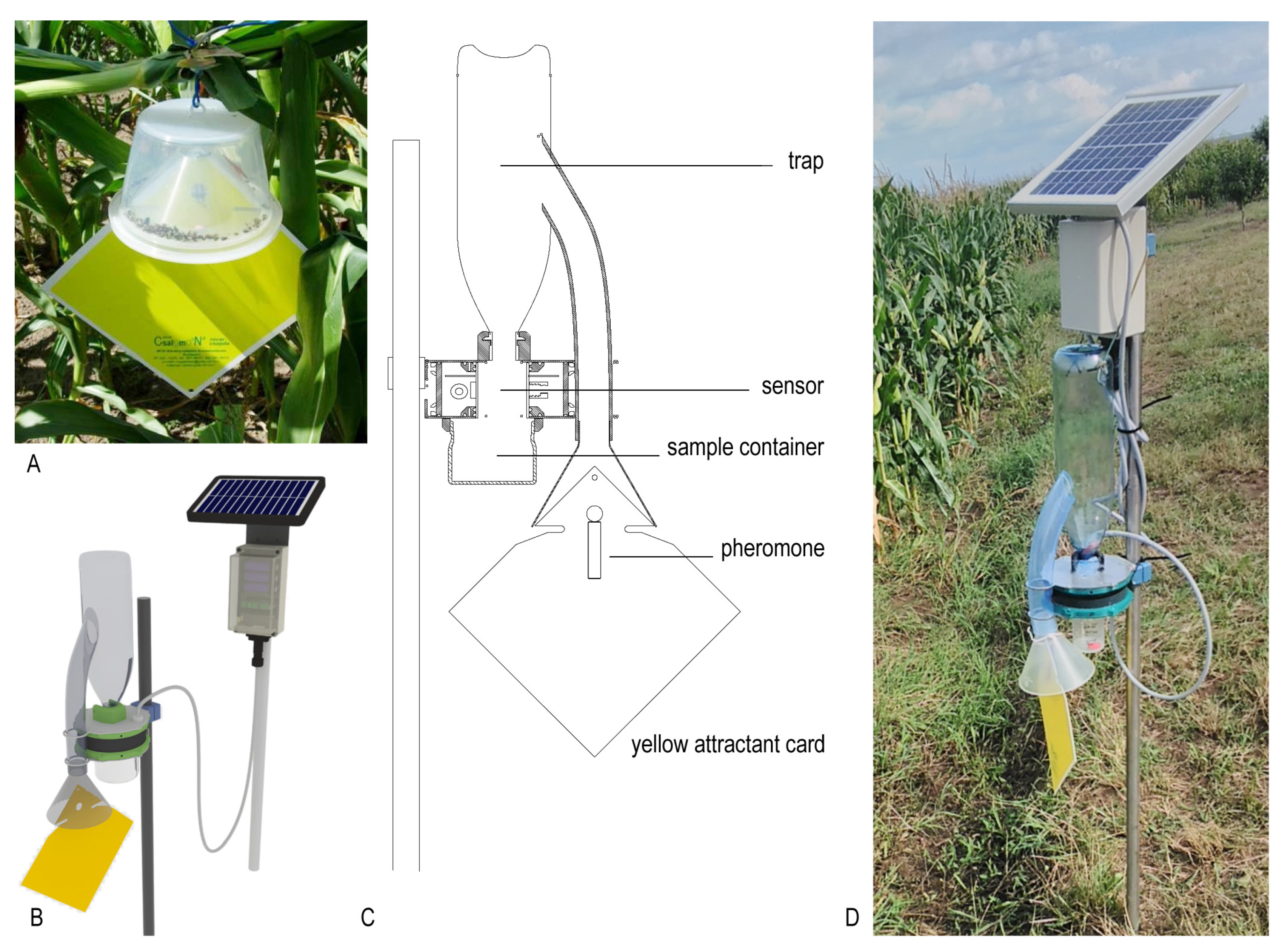
2.1. Description of the New ZooLog KLP Probe
2.1.1. Description of the Trapping Element
2.1.2. IR Sensor-Ring
2.1.3. Data Collection
2.2. Field Observations
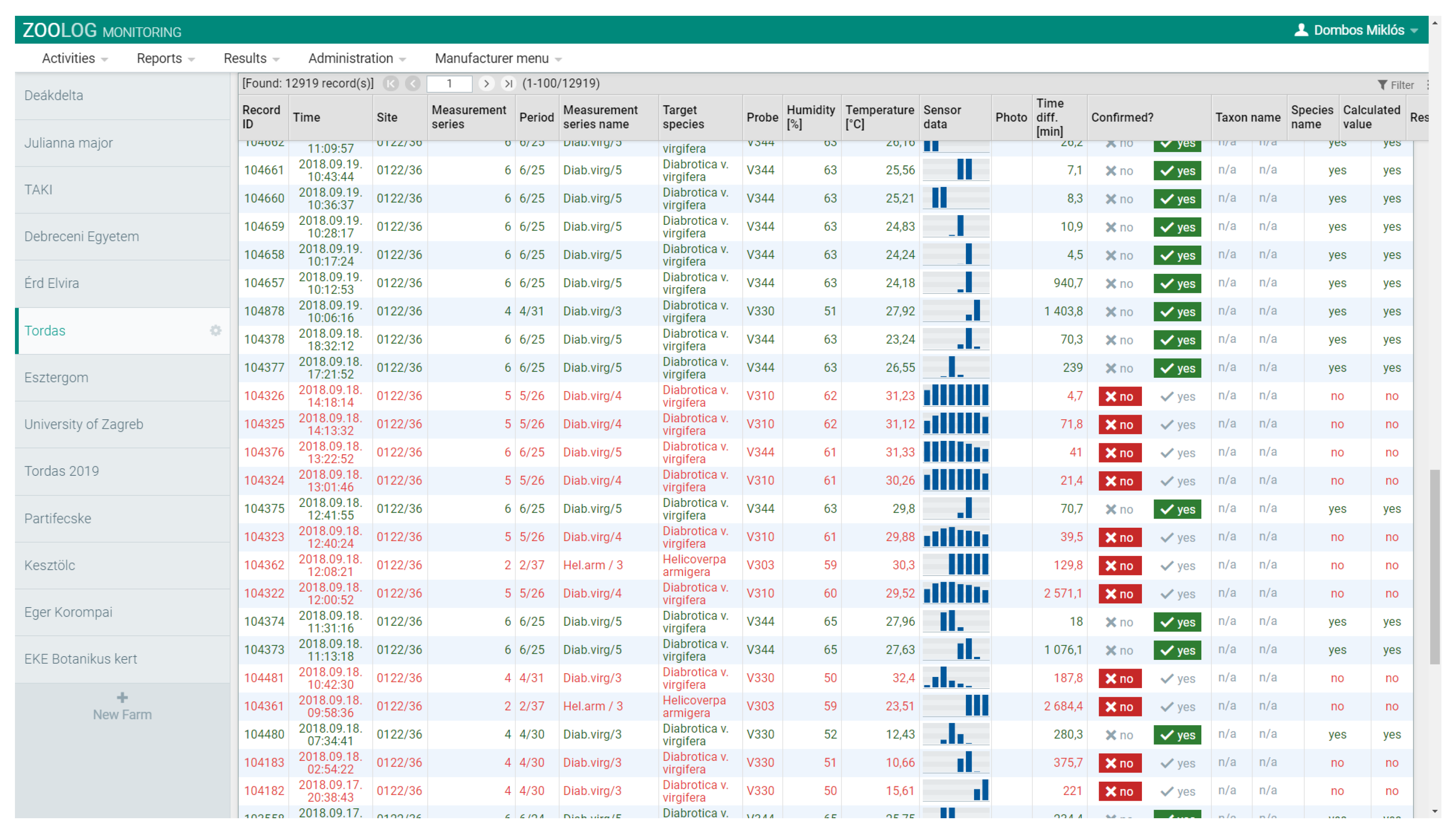
2.3. Filtering Detection Data and Data Analysis
2.3.1. Data Filtering
2.3.2. Statistical Analysis
3. Results
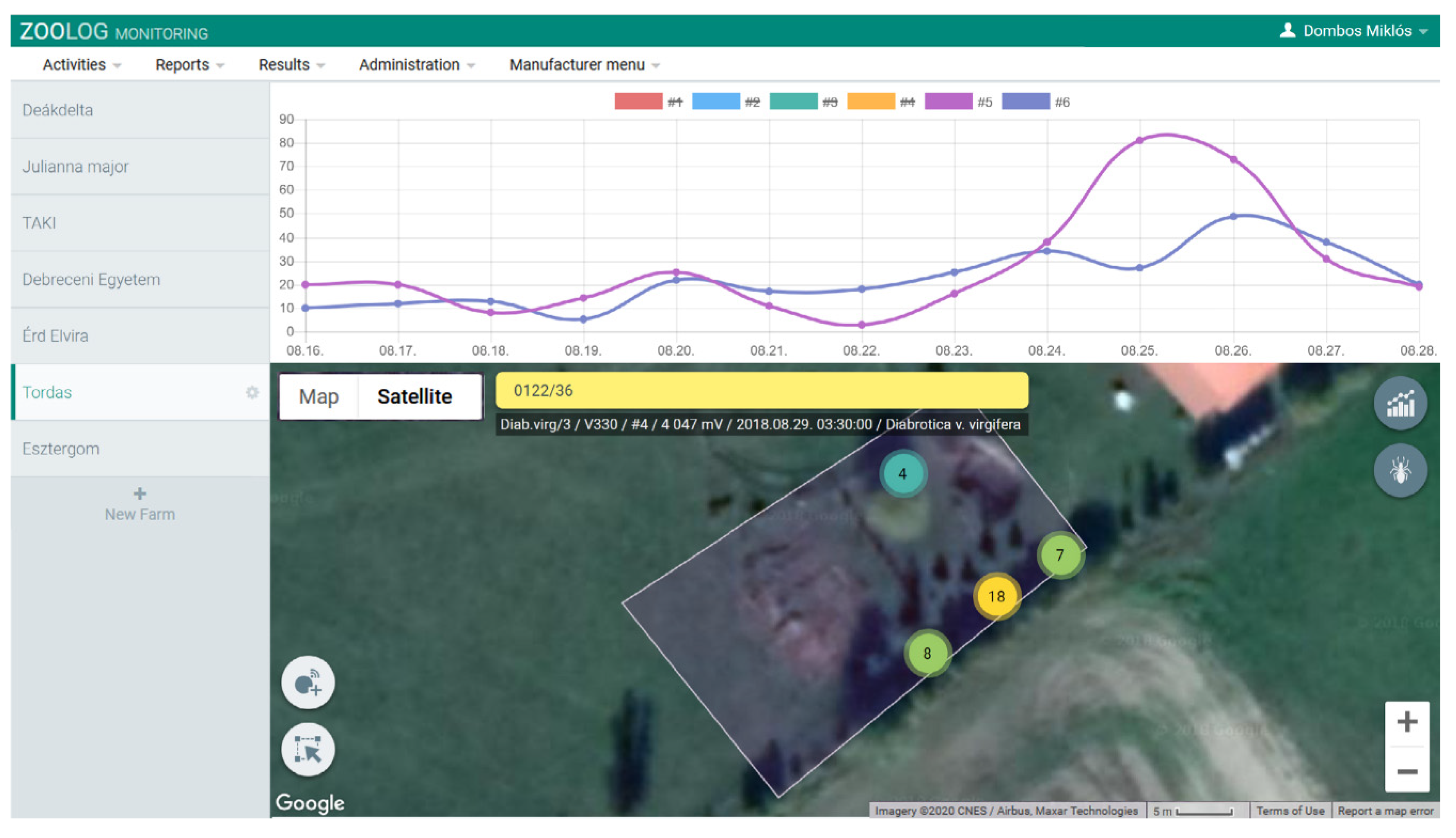
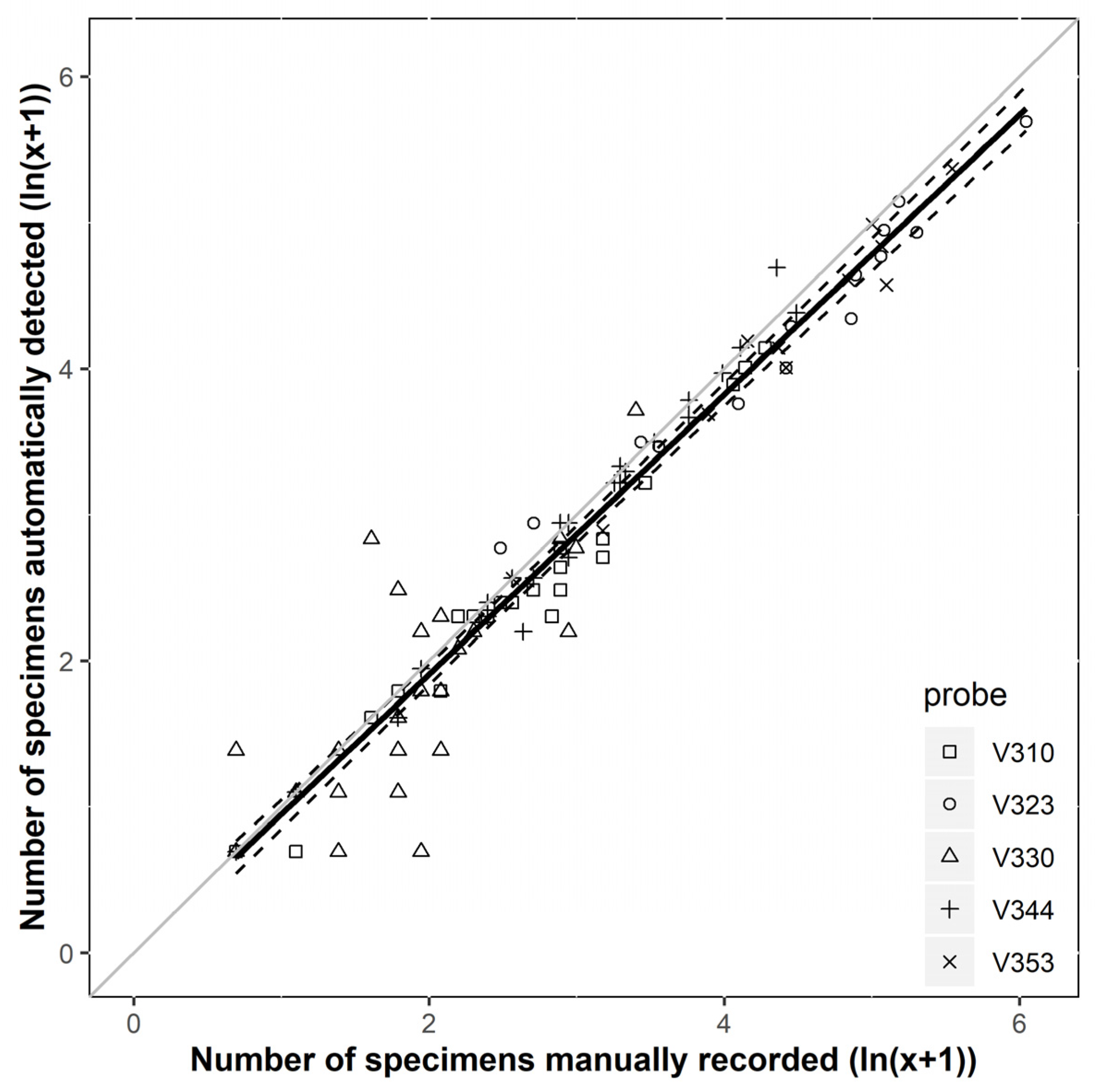
3.1. Accuracy and Performance of the Sensor System
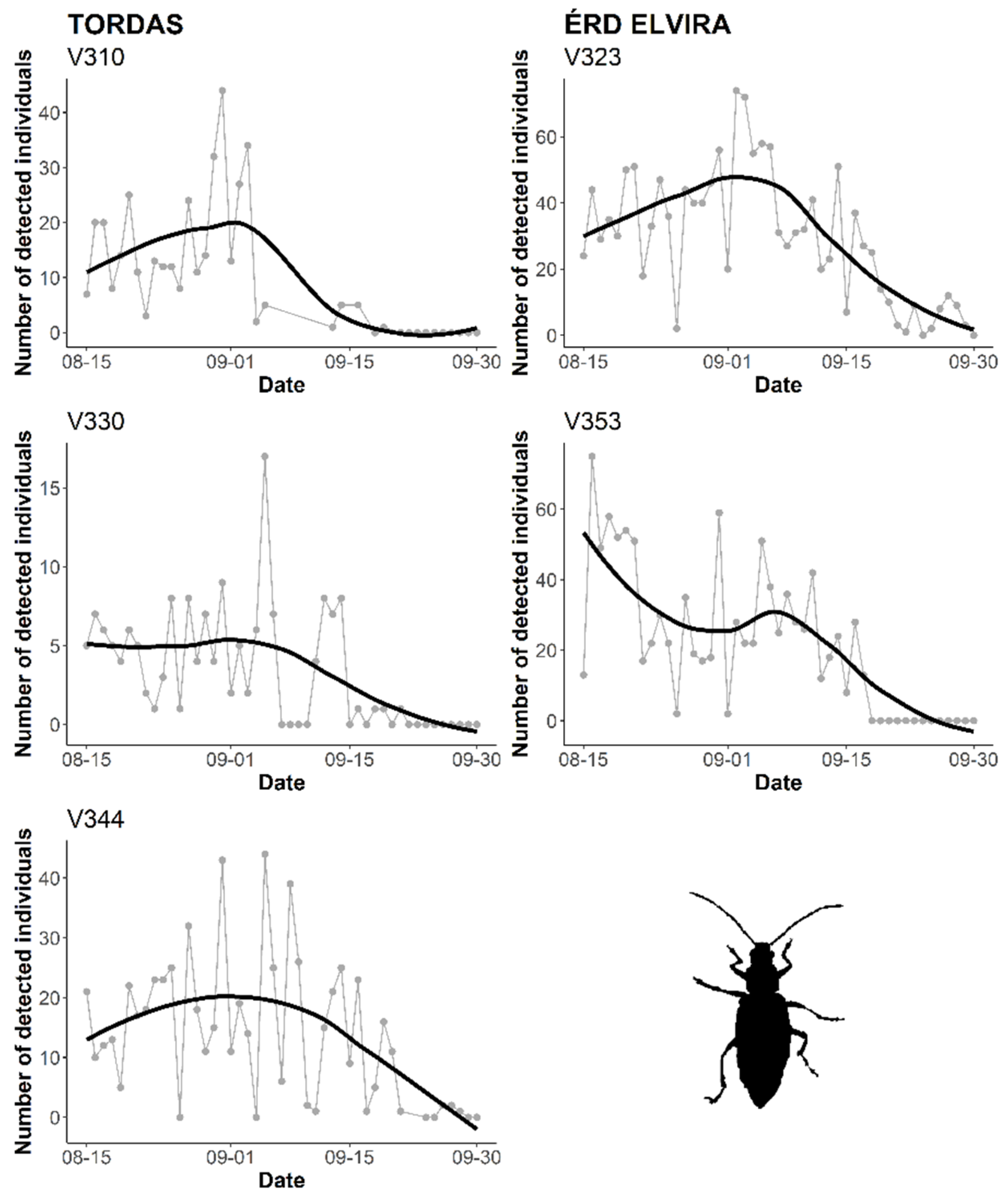
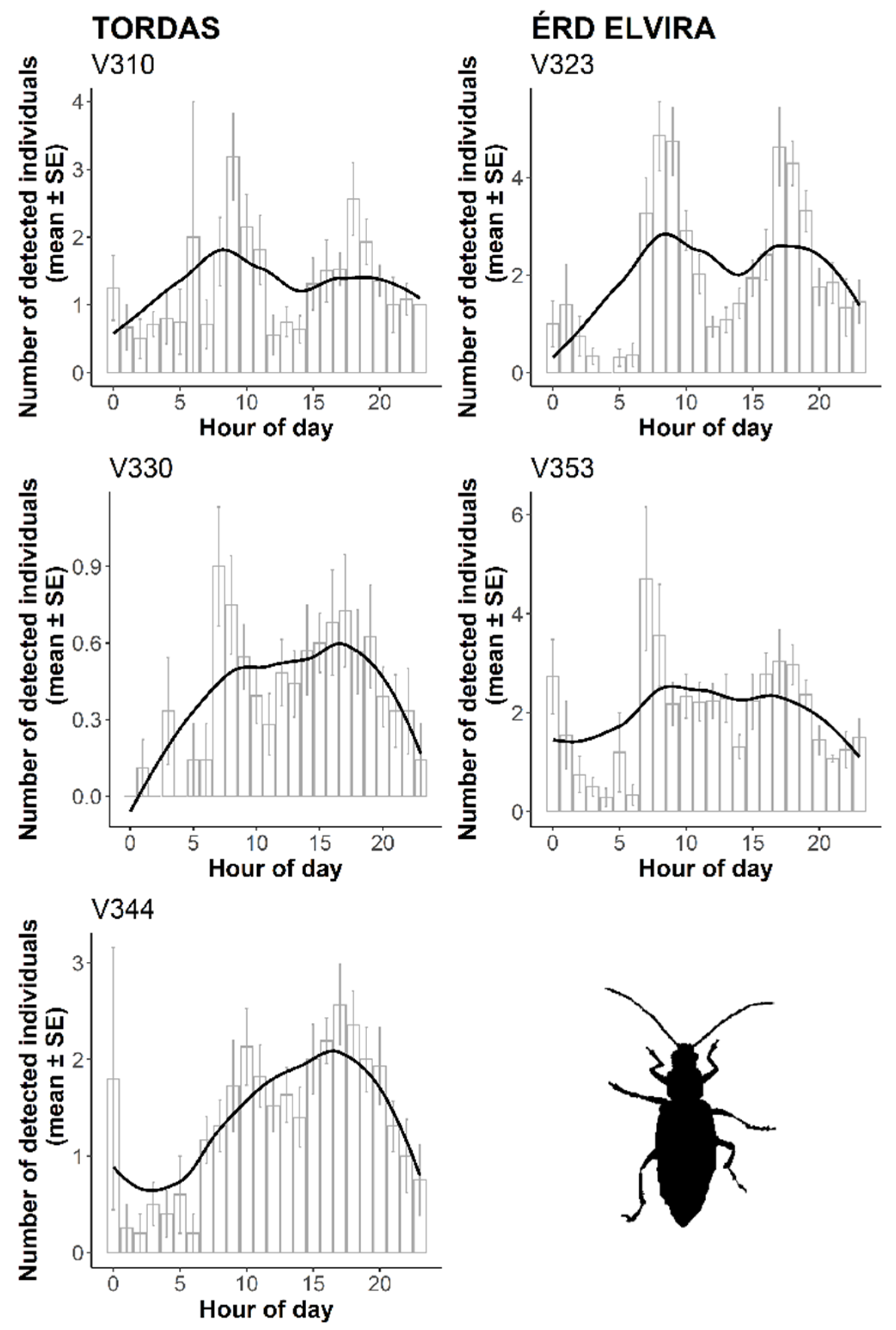
3.2. Automatic Detection of WCR Over Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kogan, M. Integrated pest management: Historical perspectives and contemporary developments. Annu. Rev. Entomol. 1998, 43, 243–270. [Google Scholar] [CrossRef]
- Nishimatsu, T.; Jackson, J.J. Interaction of insecticides, entomopathogenic nematodes, and larvae of the western corn root worm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1998, 91, 410–418. [Google Scholar] [CrossRef]
- Toepfer, S.; Kuhlmann, U. Survey for natural enemies of the invasive alien chrysomelid, Diabrotica virgifera virgifera, in Central Europe. BioControl 2004, 49, 385–395. [Google Scholar] [CrossRef]
- Liu, H.; Lee, S.-H.; Chahl, J.S. A review of recent sensing technologies to detect invertebrates on crops. Precis. Agric. 2017, 18, 635–666. [Google Scholar] [CrossRef]
- Čamprag, D.; Bača, F. Diabrotica virgifera (Coleoptera, Chrysomelidae); a new pest of maize in Yugoslavia. Pestic. Sci. 1995, 45, 291–292. [Google Scholar] [CrossRef]
- CABI. Diabrotica Virgifera Virgifera (Western Corn Rootworm). Invasive Species Compendium Datasheet. Available online: https://www.cabi.org/isc/datasheet/ (accessed on 5 June 2020).
- Dun, Z.; Mitchell, P.; Agosti, M. Estimating Diabrotica virgifera virgifera damage functions with field trial data: Applying an unbalanced nested error component model. J. Appl. Entomol. 2010, 134, 409–419. [Google Scholar] [CrossRef]
- Wesseler, J.; Fall, E.H. Potential damage costs of Diabrotica virgifera virgifera infestation in Europe—The ‘no control’ scenario. J. Appl. Entomol. 2010, 134, 385–394. [Google Scholar] [CrossRef]
- Musick, G.; Chiang, H.; Luckmann, W.; Mayo, Z.; Turpin, F. Impact of planting dates of field corn on beetle emergence and damage by the western and the northern corn rootworms in the Corn Belt. Ann. Entomol. Soc. Am. 1980, 73, 207–215. [Google Scholar] [CrossRef]
- Szalai, M.; Kiss, J.; Kövér, S.; Toepfer, S. Simulating crop rotation strategies with a spatiotemporal lattice model to improve legislation for the management of the maize pest Diabrotica virgifera virgifera. Agric. Syst. 2014, 124, 39–50. [Google Scholar] [CrossRef]
- Kiss, J. Monitoring of Western Corn Rootworm (Diabrotica virgifera virgifera LeConte) in Europe 1992–2003. In Western Corn Rootworm: Ecology and Management; Vidal, S., Kuhlmann, U., Edwards, C.R., Eds.; CABI Publishing: Wallingford, UK, 2005. [Google Scholar]
- Gray, M.E.; Sappington, T.W.; Miller, N.J.; Moeser, J.; Bohn, M.O. Adaptation and invasiveness of western corn rootworm: Intensifying research on a worsening pest. Annu. Rev. Entomol. 2009, 54, 303–321. [Google Scholar] [CrossRef]
- Boriani, M.; Agosti, M.; Kiss, J.; Edwards, C. Sustainable management of the western corn rootworm, Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae), in infested areas: Experiences in Italy, Hungary and the USA. Eppo. Bull. 2006, 36, 531–537. [Google Scholar] [CrossRef]
- Lemic, D.; Mikac, K.M.; Kozina, A.; Benitez, H.A.; McLean, C.M.; Bažok, R. Monitoring techniques of the western corn rootworm are the precursor to effective IPM strategies. Pest. Manag. Sci. 2016, 72, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Levine, E.; Spencer, J.L.; Isard, S.A.; Onstad, D.W.; Gray, M.E. Adaptation of the western corn rootworm to crop rotation: Evolution of a new strain in response to a management practice. Am. Entomol. 2002, 48, 94–107. [Google Scholar] [CrossRef]
- Meinke, L.J.; Sappington, T.W.; Onstad, D.W.; Guillemaud, T.; Miller, N.J.; Komáromi, J.; Levay, N.; Furlan, L.; Kiss, J.; Toth, F. Western corn rootworm (Diabrotica virgifera virgifera LeConte) population dynamics. Agric. For. Entomol. 2009, 11, 29–46. [Google Scholar] [CrossRef]
- Toshova, T.B.; Velchev, D.I.; Abaev, V.D.; Subchev, M.A.; Atanasova, D.Y.; Tóth, M. Detection and monitoring of Diabrotica virgifera virgifera LeConte, 1868 (Coleoptera: Chrysomelidae) by KLP+ traps with dual (pheromone and floral) lures in Bulgaria. Acta Zool. Bulg. 2017, 9, 247–254. [Google Scholar]
- Witkowski, J.; Owens, J.; Tollefson, J. Diel activity and vertical flight distribution of adult western corn rootworms in Iowa cornfields. J. Econ. Entomol. 1975, 68, 351–352. [Google Scholar] [CrossRef]
- Naranjo, S.E. Comparative flight behavior of Diabrotica virgifera virgifera and Diabrotica barberi in the laboratory. Entomol. Exp. et Appl. 1990, 55, 79–90. [Google Scholar] [CrossRef]
- Tóth, M.; Törőcsik, G.; Imrei, Z.; Vörörs, G. Diel rhythmicity of field responses to synthetic pheromonal or floral lures in the western corn rootworm Diabrotica v. virgifera. Acta Phytopathol. Entomol. Hung. 2010, 45, 323–328. [Google Scholar] [CrossRef]
- Tóth, M. In Detection and monitoring devices for the invading western corn rootworm Diabrotica v. virgifera: A summary. In Proceedings of the Interntioanl Conference Alien Arthropods in South East Europe–Crossroad of Three Continents, Sofia, Bulgaria, 19–21 September 2007; p. 95. [Google Scholar]
- Komáromi, J.; Kiss, J.; Tuska, T.; Pai, B. Comparison of western corn rootworm (Diabrotica virgifera virgifera LeConte) adult captures on pheromone-baited and visual traps during population build up. Acta Phytopathol. et Entomol. Hung. 2006, 41, 305–315. [Google Scholar] [CrossRef]
- Tóth, M. Trap types for capturing Diabrotica virgifera virgifera (Coleoptera, Chrysomelidae) developed by the Plant Protection Institute, HAS,(Budapest, Hungary): Performance characteristics. IOBC/WPRS Bull. 2005, 28, 147–154. [Google Scholar]
- Tóth, M.; Sivcev, I.; Ujváry, I.; Tomasek, I.; Imrei, Z.; Horváth, P.; Szarukán, I. Development of trapping tools for detection and monitoring of Diabrotica v. virgifera in Europe. Acta Phytopathol. Entomol. Hung. 2003, 38, 307–322. [Google Scholar] [CrossRef]
- Tóth, M.; Csonka, É.; Szarukán, I.; Vörös, G.; Furlan, L.; Imrei, Z.; Vuts, J. The KLP+ (“hat”) trap, a non-sticky, attractant baited trap of novel design for catching the western corn rootworm (Diabrotiea v. virgifera) and cabbage flea beetles (Phyllotreta spp.)(Coleoptera: Chrysomelidae). Int. J. Hortic. Sci. 2006, 12, 57–62. [Google Scholar]
- Zhong, Y.; Gao, J.; Lei, Q.; Zhou, Y. A vision-based counting and recognition system for flying insects in intelligent agriculture. Sensors 2018, 18, 1489. [Google Scholar] [CrossRef] [PubMed]
- Rustia, D.J.A.; Lin, C.E.; Chung, J.-Y.; Zhuang, Y.-J.; Hsu, J.-C.; Lin, T.-T. Application of an image and environmental sensor network for automated greenhouse insect pest monitoring. J. Asia-Pac. Entomol. 2020, 23, 17–28. [Google Scholar] [CrossRef]
- Solis-Sánchez, L.O.; Castañeda-Miranda, R.; García-Escalante, J.J.; Torres-Pacheco, I.; Guevara-González, R.G.; Castañeda-Miranda, C.L.; Alaniz-Lumbreras, P.D. Scale invariant feature approach for insect monitoring. Comput. Electron. Agric. 2011, 75, 92–99. [Google Scholar] [CrossRef]
- Batista, G.E.; Hao, Y.; Keogh, E.; Mafra-Neto, A. In Towards automatic classification on flying insects using inexpensive sensors. In Proceedings of the 10th International Conference on Machine Learning and Applications and Workshops, Honolulu, HI, USA, 18–21 December 2011; pp. 364–369. [Google Scholar]
- Potamitis, I.; Rigakis, I.; Fysarakis, K. The electronic McPhail trap. Sensors 2014, 14, 22285–22299. [Google Scholar] [CrossRef] [PubMed]
- Rigakis, I.; Potamitis, I.; Tatlas, N.-A.; Livadaras, I.; Ntalampiras, S. A Multispectral Backscattered Light Recorder of Insects’ Wingbeats. Electronics 2019, 8, 277. [Google Scholar] [CrossRef]
- Silva, D.F.; Souza, V.M.; Ellis, D.P.; Keogh, E.J.; Batista, G.E. Exploring low cost laser sensors to identify flying insect species. J. Intell. Robot. Syst. 2015, 80, 313–330. [Google Scholar] [CrossRef]
- Balla, E.; Flórián, N.; Gergócs, V.; Gránicz, L.; Tóth, F.; Németh, T.; Dombos, M. An Opto-electronic Sensor-ring to Detect Arthropods of Significantly Different Body Sizes. Sensors 2020, 20, 982. [Google Scholar] [CrossRef]
- Dombos, M.; Kosztolányi, A.; Szlávecz, K.; Gedeon, C.; Flórián, N.; Groó, Z.; Dudás, P.; Bánszegi, O. EDAPHOLOG monitoring system: Automatic, real-time detection of soil microarthropods. Methods Ecol. Evol. 2017, 8, 313–321. [Google Scholar] [CrossRef]
- Csonka, É.; Tóth, M. Comparison of KPL+(“hat”) and VARL+(funnel) trap types baited with allyl isothiocyanate for capture of cabbage flea beetles (Phyllotreta spp.) (Coleoptera, Chrysomelidae). Növényvédelem 2006, 42, 597–6041. [Google Scholar]
- Ball, H.J. Spectral response of the adult Western Corn Rootworm (Coleoptera: Chrysomelidae) to selected wavelengths. J. Econ. Entomol. 1982, 75, 932–933. [Google Scholar] [CrossRef]
- Abadi, M.; Agarwal, A.; Barham, P.; Brevdo, E.; Chen, Z.; Citro, C.; Corrado, G.S.; Davis, A.; Dean, J.; Devin, M.; et al. Tensorflow: Large-scale machine learning on heterogeneous distributed systems. arXiv 2016, arXiv:1603.04467. [Google Scholar]
- Github. The TensorBoard Repository on GitHub. Available online: http://github.com/tensorflow/tensorboard (accessed on 15 June 2017).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. Math. Methods Biosci. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: http://www.R-project.org (accessed on 30 July 2020).
- Flórián, N.; Gránicz, L.; Gergócs, V.; Tóth, F.; Dombos, M. Detecting Soil Microarthropods with a Camera-Supported Trap. Insects 2020, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Alhady, S.; Kai, X.Y. Butterfly Species Recognition Using Artificial Neural Network. In Intelligent Manufacturing & Mechatronics; Springer: Singapore, 2018; pp. 449–457. [Google Scholar]
- Kaya, Y.; Kayci, L.; Uyar, M. Automatic identification of butterfly species based on local binary patterns and artificial neural network. Appl. Soft Comput. 2015, 28, 132–137. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Dang, L.M.; Sadeghi-Niaraki, A.; Moon, H. Crop pest recognition in natural scenes using convolutional neural networks. Comput. Electron. Agric. 2020, 169, 105174. [Google Scholar] [CrossRef]
- Wang, J.; Lin, C.; Ji, L.; Liang, A. A new automatic identification system of insect images at the order level. Knowl. Based Syst. 2012, 33, 102–110. [Google Scholar] [CrossRef]
- Potamitis, I.; Rigakis, I.; Tatlas, N.-A. In On Fresnel-Based Single and Multi Spectral Sensors for Insects’ Wingbeat Recording. In Proceedings of the 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; pp. 1901–1904. [Google Scholar]
- Potamitis, I.; Rigakis, I.; Vidakis, N.; Petousis, M.; Weber, M. Affordable bimodal optical sensors to spread the use of automated insect monitoring. J. Sens. 2018, 2018. [Google Scholar] [CrossRef]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef]
- Dombos, M. Innovative Real-Time Monitoring and Pest Control for Insects, ZooLog Sensor System. LIFE Layman Report 2017. Available online: http://zoolog.hu/insectlife/wp-content/uploads/2019/04/layman_eng_min3.pdf (accessed on 30 July 2020).
- Metcalf, R.L. Chemical Ecology of Diabroticites. In Novel Aspects of the Biology of Chrysomelidae; Springer Science+Business Media: Dordrecht, The Netherlands, 1994; pp. 153–169. [Google Scholar]
- Gerber, C.K.; Edwards, C.R.; Bledsoe, L.W.; Obermeyer, J.L.; Barna, G.; Foster, R.E. Sampling devices and decision rule development for western corn rootworm (Diabrotica virgifera virgifera LeConte) adults in soybean to predict subsequent damage to maize in Indiana. West. Corn Rootworm Ecol. Manag. 2005, 2005, 169–187. [Google Scholar]
- Spencer, J.L.; Isard, S.A.; Levine, E. Western corn rootworms on the move: Monitoring beetles in corn and soybeans. In Proceedings of the 1998 Illinois Agricultural Pesticides Conference, Cooperative Extension Service, University of Illinois, Urbana-Champaign, IL, USA, 6–8 January 1998; pp. 10–23. [Google Scholar]
- Coats, S.A.; Tollefson, J.J.; Mutchmor, J.A. Study of migratory flight in the western corn rootworm (Coleoptera: Chrysomelidae). Environ. Entomol. 1986, 15, 620–625. [Google Scholar] [CrossRef]
- Isard, S.A.; Spencer, J.L.; Nasser, M.A.; Levine, E. Aerial movement of western corn rootworm (Coleoptera: Chrysomelidae): Diel periodicity of flight activity in soybean fields. Environ. Entomol. 2000, 29, 226–234. [Google Scholar] [CrossRef]
| Location | Description | Probe | Latitude | Longitude |
|---|---|---|---|---|
| Tordas | cornfield in a privately owned farm | V310 | 47°20′54.15″ N | 18°43′58.65″ E |
| V330 | 47°20′54.43″ N | 18°43′59.22″ E | ||
| V344 | 47°20′54.31″ N | 18°43′58.95″ E | ||
| Érd Elvira | cornfield adjacent to an orchard | V323 | 47°20′23.89″ N | 18°51′53.44″ E |
| V353 | 47°20′24.91″ N | 18°51′52.71″ E |
| Automatically Detected (ANN Predicted) | ||||
|---|---|---|---|---|
| 1 (Positive) | 0 (Negative) | Sum | ||
| Manually Counted | 1 | 3266 (TP) | 313 (FN) | 3579 |
| 0 | 184 (FP) | 3611 (TN) | 3795 | |
| Sum | 3450 | 3924 | 7374 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, Z.; Tóth, M.; Jósvai, J.K.; Tóth, F.; Flórián, N.; Gergócs, V.; Dombos, M. Automatic Field Detection of Western Corn Rootworm (Diabrotica virgifera virgifera; Coleoptera: Chrysomelidae) with a New Probe. Insects 2020, 11, 486. https://doi.org/10.3390/insects11080486
Tóth Z, Tóth M, Jósvai JK, Tóth F, Flórián N, Gergócs V, Dombos M. Automatic Field Detection of Western Corn Rootworm (Diabrotica virgifera virgifera; Coleoptera: Chrysomelidae) with a New Probe. Insects. 2020; 11(8):486. https://doi.org/10.3390/insects11080486
Chicago/Turabian StyleTóth, Zsolt, Miklós Tóth, Júlia Katalin Jósvai, Franciska Tóth, Norbert Flórián, Veronika Gergócs, and Miklós Dombos. 2020. "Automatic Field Detection of Western Corn Rootworm (Diabrotica virgifera virgifera; Coleoptera: Chrysomelidae) with a New Probe" Insects 11, no. 8: 486. https://doi.org/10.3390/insects11080486
APA StyleTóth, Z., Tóth, M., Jósvai, J. K., Tóth, F., Flórián, N., Gergócs, V., & Dombos, M. (2020). Automatic Field Detection of Western Corn Rootworm (Diabrotica virgifera virgifera; Coleoptera: Chrysomelidae) with a New Probe. Insects, 11(8), 486. https://doi.org/10.3390/insects11080486






