Wing Design in Flies: Properties and Aerodynamic Function
Abstract
1. Introduction
2. Aerodynamic Properties of Root-Flapping Rectangular Wings
3. The Aerodynamic Benefits of an Ideal Planform
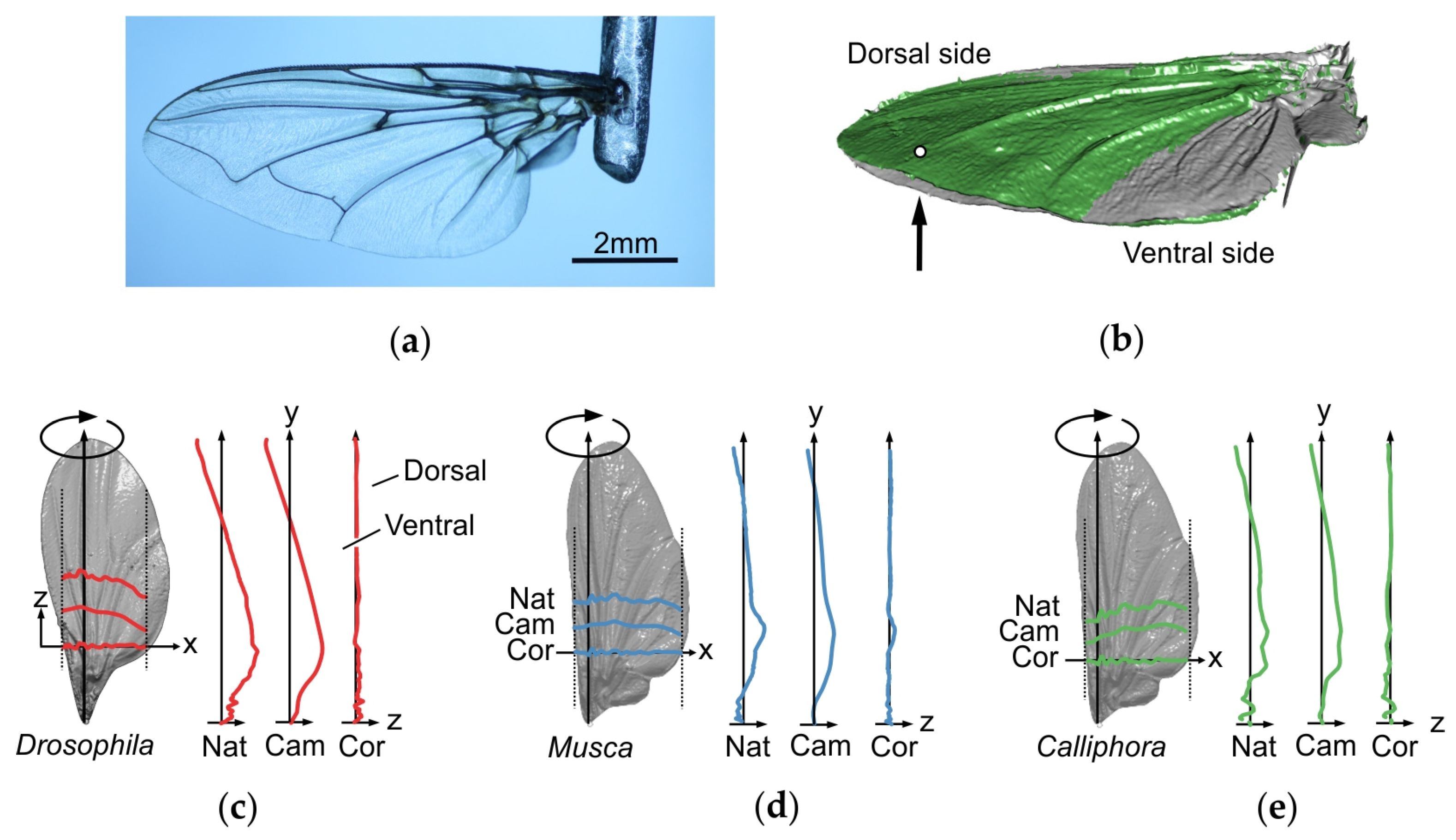
4. Functional Relevance of Three-Dimensional Wing Shape
5. Wing Stiffness and Benefits of Elastic Wing Deformation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sotavalta, O. The flight-tone (wing stroke frequency) of insects. Acta Entomologica Fenn. 1947, 4, 1–117. [Google Scholar]
- Chin, D.D.; Lentink, D. Flapping wing aerodynamics: From insects to vertebrates. J. Exp. Biol. 2016, 219, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ravi, S.; Kolomenskiy, D.; Tanaka, H. Biomechanics and biomimetics in insect-inspired flight systems. Phil. Trans. R. Soc. Lond. B 2016, 371, 20150390. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J. Insect flight: From Newton’s law to neurons. Ann. Rev. Condens. Matter Phys. 2016, 7, 281–300. [Google Scholar] [CrossRef]
- Shyy, W.; Kang, C.-k.; Chirarattananon, P.; Ravi, S.; Liu, H. Aerodynamics, sensing and control of insect-scale flapping-wing flight. Proc. Roy. Soc. Lond. A 2016, 472, 20150712. [Google Scholar] [CrossRef]
- Shyy, W.; Lian, Y.; Tang, J.; Viieru, D.; Liu, H. Aerodynamics of Low Reynolds Number Flyers; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Lehmann, F.-O. The mechanisms of lift enhancement in insect flight. Naturwissenschaften 2004, 91, 101–122. [Google Scholar] [CrossRef]
- Lehmann, F.-O. When wings touch wakes: Understanding locomotor force control by wake–wing interference in insect wings. J. Exp. Biol. 2008, 211, 224–233. [Google Scholar] [CrossRef]
- Sane, S. The aerodynamics of insect flight. J. Exp. Biol. 2003, 206, 4191–4208. [Google Scholar] [CrossRef]
- Wang, Z.J. Dissecting insect flight. Annu. Rev. Fluid Mech. 2005, 37, 183–210. [Google Scholar] [CrossRef]
- Salami, E.; Ward, T.A.; Montazer, E.; Ghazali, N.N.N. A review of aerodynamic studies on dragonfly flight. J. Mech. Eng. Sci. 2019, 233, 6519–6537. [Google Scholar] [CrossRef]
- Vishnudas, V.; Vigoreaux, J.O. Sustained high power performance: Possible strategies for integrating energy supply and demands in flight muscle. In Nature’s Versatile Engine: Insect Flight Muscle Inside and Out; Vigoreaux, J.O., Ed.; Springer: New York, NY, USA, 2006; p. 288. [Google Scholar] [CrossRef]
- Lehmann, F.-O. Muscle Systems design and integration. In Nature’s Versatile Engine: Insect Flight Muscle Inside and Out; Vigoreaux, J.O., Ed.; Landes Bioscience: Georgetown, DC, USA, 2006; pp. 230–239. [Google Scholar]
- Ellington, C.P. Limitations on animal flight performance. J. Exp. Biol. 1991, 160, 71–91. [Google Scholar]
- Lehmann, F.-O.; Bartussek, J. Neural control and precision of flight muscle activation in Drosophila. J. Comp. Physiol. A 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sane, S.P. Neurobiology and biomechanics of flight in miniature insects. Curr. Opin. Neurobiol. 2016, 41, 158–166. [Google Scholar] [CrossRef]
- Lehmann, F.-O. Neural control and precision of spike phasing in flight muscles. J. Neurol. Neuromedicine 2017, 2, 15–19. [Google Scholar] [CrossRef]
- Bomphrey, R.J.; Godoy-Diana, R. Insect and insect-inspired aerodynamics: Unsteadiness, structural mechanics and flight control. Curr. Opin. Insect. Sci. 2018, 30, 26–32. [Google Scholar] [CrossRef]
- Sun, X.; Gong, X.; Huang, D. A review on studies of the aerodynamics of different types of maneuvers in dragonflies. Arch. Appl. Mech. 2017, 87, 521–554. [Google Scholar] [CrossRef]
- Taylor, G.K. Mechanics and aerodynamics of insect flight control. Biol. Rev. 2001, 76, 449–471. [Google Scholar] [CrossRef]
- Dickinson, M.H.; Muijres, F.T. The aerodynamics and control of free flight manoeuvres in Drosophila. Phil. Trans. R. Soc. Lond. B 2016, 371, 20150388. [Google Scholar] [CrossRef]
- Ellington, C.P. Power and efficiency of insect flight muscle. J. Exp. Biol. 1985, 115, 293–304. [Google Scholar]
- Lehmann, F.-O. The efficiency of aerodynamic force production in Drosophila. Comp. Biochem. Physiol. A 2001, 131, 77–88. [Google Scholar] [CrossRef]
- Vincent, J.F.V.; Wegst, U.G.K. Design and mechanical properties of insect cuticle. Arthropod. Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Xiao, K.W.; Bai, K.; Bai, Y.L. Microstructure and nanomechanical properties of the wing membrane of dragonfly. Mat. Sci. Eng. A 2007, 457, 254–260. [Google Scholar] [CrossRef]
- Wang, X.-S.; Li, Y.; Shi, Y.-F. Effects of sandwich microstructures on mechanical behaviors of dragonfly wing vein. Comp. Sci. Tech. 2008, 68, 186–192. [Google Scholar] [CrossRef]
- Ma, Y.; Ren, H.; Ning, J.; Zhang, P. Functional morphology and bending characteristics of the honeybee forewing. J. Bionic. Eng. 2017, 14, 111–118. [Google Scholar] [CrossRef]
- Rees, C.J.C. Form and function in corrugated insect wings. Nature 1975, 256, 200–203. [Google Scholar] [CrossRef]
- Combes, S.A.; Daniel, T.L. Flexural stiffness in insect wings I. Scaling and the influence of wing venation. J. Exp. Biol. 2003, 206, 2979–2987. [Google Scholar] [CrossRef]
- Ennos, A.R. Comparative functional morpholoy of the wings of Diptera. Zool. J. Linn. Soc. 1989, 96, 27–47. [Google Scholar] [CrossRef]
- Rajabi, H.; Rezasefat, M.; Darvizeh, A.; Dirks, J.H.; Eshghi, S.; Shafiei, A.; Mostofi, T.M.; Gorb, S.N. A comparative study of the effects of constructional elements on the mechanical behaviour of dragonfly wings. Appl. Physics A 2016, 122, 19. [Google Scholar] [CrossRef]
- Rajabi, H.; Shafiei, A.; Darvizeh, A.; Dirks, J.-H.; Appel, E.; Gorb, S.N. Effect of microstructure on the mechanical and damping behaviour of dragonfly wing veins. R. Soc. Open Sci. 2016, 3, 160006. [Google Scholar] [CrossRef]
- Sunada, S.; Zeng, L.; Kawachi, K. The relationship between dragonfly wing structure and torsional deformation. J. Theor. Biol. 1998, 193, 39–45. [Google Scholar] [CrossRef]
- Meyers, M.A.; Chen, P.-Y.; Lin, A.Y.-M.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mat. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef]
- Wootton, R.J. Support and deformability in insect wings. J. Zoo. Lond. 1981, 193, 447–468. [Google Scholar] [CrossRef]
- Wootton, R.J. The mechanical design of insect wings. Sci. Am. 1990, 263, 114–121. [Google Scholar] [CrossRef]
- Combes, S.A.; Daniel, T.L. Flexural stiffness in insect wings II. Spatial distribution and dynamic wing bending. J. Exp. Biol. 2003, 206, 2989–2997. [Google Scholar] [CrossRef]
- Combes, S.A.; Daniel, T.L. Into thin air: Contributions of aerodynamic and intertial-elastic forces to wing bending in the hawkmoth Manduca sexta. J. Exp. Biol. 2003, 206, 2999–3006. [Google Scholar] [CrossRef]
- Appel, E.; Heepe, L.; Lin, C.-P.; Gorb, S.N. Ultrastructure of dragonfly wing veins: Composite structure of fibrous material supplemented by resilin. J. Anat. 2015, 227, 561–582. [Google Scholar] [CrossRef]
- Dirks, J.-H.; Taylor, D. Veins improve fracture toughness of insect wings. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Rajabi, H.; Darvizeh, A.; Shafiei, A.; Taylor, D.; Dirks, J.-H. Numerical investigation of insect wing fracture behaviour. J. Biomech. 2015, 48, 89–94. [Google Scholar] [CrossRef]
- Agrawal, S.; Grimaldi, D.; Fox, J.L. Haltere morphology and campaniform sensilla arrangement across Diptera. Arthropod. Struct. Dev. 2017, 46, 215–229. [Google Scholar] [CrossRef]
- Pratt, B.; Deora, T.; Mohren, T.; Daniel, T. Neural evidence supports a dual sensory-motor role for insect wings. Proc. Roy. Soc. Lond. B 2017, 284, 20170969. [Google Scholar] [CrossRef]
- Dickinson, M.H. Comparison of encoding properties of campaniform sensilla on the fly wing. J. Exp. Biol. 1990, 151, 245–261. [Google Scholar]
- Cole, E.S.; Palka, J. The pattern of campaniform sensilla on the wing and haltere of Drosophila melanogaster and several of its homeotic mutants. Development 1982, 71, 41–61. [Google Scholar]
- Gnatzy, W.; Grünert, U.; Bender, M. Campaniform sensilla of Calliphora vicina (Insecta, Diptera): I. Topography. Zoomorphology 1987, 106, 312–319. [Google Scholar] [CrossRef]
- Ray, R.P.; Nakata, T.; Henningsson, P.; Bomphrey, R.J. Enhanced flight performance by genetic manipulation of wing shape in Drosophila. Nat. Comm. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Gorb, S.; Kesel, A.; Berger, J. Microsculpture of the wing surface in Odonata: Evidence for cuticular wax covering. Arthropod. Struct. Dev. 2000, 29, 129–135. [Google Scholar] [CrossRef]
- Wootton, R.J. Functional morphology of insect wings. Annu. Rev. Entomol. 1992, 37, 113–140. [Google Scholar] [CrossRef]
- Luo, G.; Sun, M. The effects of corrugation and wing planform on the aerodynamic force production of sweeping model insect wings. Acta Mech. Sin. 2005, 21, 531–541. [Google Scholar] [CrossRef]
- Koehler, C.; Liang, Z.; Gaston, Z.; Wan, H.; Dong, H. 3D reconstruction and analysis of wing deformation in free-flying dragonflies. J. Exp. Biol. 2012, 215, 3018–3027. [Google Scholar] [CrossRef]
- Jongerius, S.R.; Lentink, D. Structural analysis of a dragonfly wing. Exp. Mech. 2010, 50, 1323–1334. [Google Scholar] [CrossRef]
- Barnes, C.J.; Visbal, M.R. Numerical exploration of the origin of aerodynamic enhancements in [low-Reynolds number] corrugated airfoils. Phys. Fluids 2013, 25, 115106. [Google Scholar] [CrossRef]
- Feaster, J.; Battaglia, F.; Bayandor, J. A computational study on the influence of insect wing geometry on bee flight mechanics. Biol. Open 2017, 6, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Le, T.Q.; Truong, T.V.; Tran, H.T.; Park, S.H.; Ko, J.H.; Park, H.C.; Yoon, K.J.; Byun, D. Two- and three-dimensional simulations of beetle hind wing flapping during free forward flight. J. Bionic. Eng. 2013, 10, 316–328. [Google Scholar] [CrossRef]
- Okamoto, M.; Yasuda, K.; Azuma, A. Aerodynamic characteristics of the wings and body of a dragonfly. J. Exp. Biol. 1996, 199, 281–294. [Google Scholar] [PubMed]
- Harbig, R.R.; Sheridan, J.; Thompson, M.C. Reynolds number and aspect ratio effects on the leading-edge vortex for rotating insect wing planforms. J. Fluid Mech. 2013, 717, 166–192. [Google Scholar] [CrossRef]
- Jain, S.; Bhatt, V.D.; Mittal, S. Shape optimization of corrugated airfoils. Comp. Mech. 2015, 56, 917–930. [Google Scholar] [CrossRef]
- Meng, X.G.; Mao, S. Aerodynamic effects of wing corrugation at gliding flight at low Reynolds numbers. Phys. Fluids 2013, 25, 071905. [Google Scholar] [CrossRef]
- Brandt, J.; Doig, G.; Tsafnat, N. Computational aerodynamic analysis of a micro-CT based bio-realistic fruit fly wing. PLoS ONE 2015, 10, e0124824. [Google Scholar] [CrossRef]
- Rees, C.J.C. Aerodynamic properties of an insect wing section and a smooth aerofoil compared. Nature 1975, 258, 141–142. [Google Scholar] [CrossRef]
- Ortega Ancel, A.; Eastwood, R.; Vogt, D.; Ithier, C.; Smith, M.; Wood, R.; Kovač, M. Aerodynamic evaluation of wing shape and wing orientation in four butterfly species using numerical simulations and a low-speed wind tunnel, and its implications for the design of flying micro-robots. Interface focus 2017, 7, 20160087. [Google Scholar] [CrossRef]
- New, T.H.; Chan, Y.X.; Koh, G.C.; Hoang, M.C.; Shi, S. Effects of corrugated aerofoil surface features on flow-separation control. AIAA J. 2014, 52, 206–211. [Google Scholar] [CrossRef]
- Murphy, J.T.; Hu, H. An experimental study of a bio-inspired corrugated airfoil for micro air vehicle applications. Exp. Fluids 2010, 49, 531–546. [Google Scholar] [CrossRef]
- Chen, Y.; Skote, M. Gliding performance of 3-D corrugated dragonfly wing with spanwise variation. J. Fluid Struct. 2016, 62, 1–13. [Google Scholar] [CrossRef]
- Ansari, M.I.; Anwer, S.F. Numerical analysis of an insect wing in gliding flight: Effect of corrugation on suction side. FDMP 2018, 14, 259–279. [Google Scholar] [CrossRef]
- Reiser, M.B.; Dickinson, M.H. A test bed for insect-inspired robotic control. Phil. Trans. R. Soc. Lond. A 2003, 361, 2267–2285. [Google Scholar] [CrossRef]
- Wang, Z.J.; Birch, J.M.; Dickinson, M.H. Unsteady forces and flows in low Reynolds number hovering flight: Two-dimensional computations vs robotic wing experiments. J. Exp. Biol. 2004, 207, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.N.; Sayaman, R.; Dickinson, M.H. The aerodynamics of hovering flight in Drosophila. J. Exp. Biol. 2005, 208, 2303–2318. [Google Scholar] [CrossRef]
- Sane, S.; Dickinson, M.H. The control of flight force by a flapping wing: Lift and drag production. J. Exp. Biol. 2001, 204, 2607–2626. [Google Scholar]
- Birch, J.M.; Dickinson, M.H. The influence of wing-wake interactions on the production of aerodynamic forces in flapping flight. J. Exp. Biol. 2003, 206, 2257–2272. [Google Scholar] [CrossRef]
- Dickinson, M.H.; Lehmann, F.-O.; Sane, S. Wing rotation and the aerodynamic basis of insect flight. Science 1999, 284, 1954–1960. [Google Scholar] [CrossRef]
- Lehmann, F.-O. Wing–wake interaction reduces power consumption in insect tandem wings. Exp. Fluids 2008, 46, 765–775. [Google Scholar] [CrossRef]
- Maybury, W.J.; Lehmann, F.-O. The fluid dynamics of flight control by kinematic phase lag variation between two robotic insect wings. J. Exp. Biol. 2004, 207, 4707–4726. [Google Scholar] [CrossRef] [PubMed]
- Wehmann, H.-N.; Heepe, L.; Gorb, S.N.; Engels, T.; Lehmann, F.-O. Local deformation and stiffness distribution in fly wings. Biol. Open 2019, 8, bio038299. [Google Scholar] [CrossRef] [PubMed]
- Shyy, W.; Aono, H.; Chimakurthi, S.K.; Trizila, P.; Kang, C.-K.; Cesnik, C.E.S.; Liu, H. Recent progress in flapping wing aerodynamics and aeroelasticity. Prof. Aero. Sci. 2010, 46, 284–327. [Google Scholar] [CrossRef]
- Bluman, J.E.; Sridhar, M.K.; Kang, C.-k. Chordwise wing flexibility may passively stabilize hovering insects. J. R. Soc. Interface 2018, 15, 20180409. [Google Scholar] [CrossRef]
- Moses, K.C.; Michaels, S.C.; Willis, M.; Quinn, R.D. Artificial Manduca sexta forewings for flapping-wing micro aerial vehicles: How wing structure affects performance. Bioinsp. Biomim. 2017, 12, 055003. [Google Scholar] [CrossRef]
- Mountcastle, A.M.; Daniel, T.L. Aerodynamic and functional consequences of wing compliance. Exp. Fluids 2009, 46, 873–882. [Google Scholar] [CrossRef]
- Nakata, T.; Liu, H. Aerodynamic performance of a hovering hawkmoth with flexible wings: A computational approach. Proc. Roy. Soc. Lond. B 2012, 279, 722–731. [Google Scholar] [CrossRef]
- Tanaka, H.; Whitney, J.P.; Wood, R.J. Effect of flexural and torsional wing flexibility on lift generation in hoverfly flight. Integ. Comp. Biol. 2011, 51, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Tobing, S.; Young, J.; Lai, J.C.S. Effects of wing flexibility on bumblebee propulsion. J. Fluid Struct. 2017, 68, 141–157. [Google Scholar] [CrossRef]
- Engels, T.; Wehmann, H.-N.; Lehmann, F.-O. Three-dimensional wing structure attenuates aerodynamic efficiency in flapping fly wings. J. R. Soc. Interface 2020, 17, 20190804. [Google Scholar] [CrossRef]
- Stevens, R.J.; Babinsky, H.; Manar, F.; Mancini, P.; Jones, A.R.; Granlund, K.O.; Ol, M.V.; Nakata, T.; Phillips, N.; Bomphrey, R. Low Reynolds number acceleration of flat plate wings at high incidence. In Proceedings of the 54th AIAA Aerospace Sciences Meeting, San Diego, CA, USA, 4–8 January 2016; p. 0286. [Google Scholar] [CrossRef]
- Usherwood, J.R.; Ellington, C.P. The aerodynamics of revolving wings II. propeller force coefficients from mayfly to quail. J. Exp. Biol. 2002, 205, 1565–1576. [Google Scholar] [PubMed]
- Usherwood, J.R.; Ellington, C.P. The aerodynamic of revolving wings I. model hawkmoth wings. J. Exp. Biol. 2002, 205, 1547–1564. [Google Scholar] [PubMed]
- Jones, A.R.; Manar, F.; Phillips, N.; Nakata, T.; Bomphrey, R.; Ringuette, M.J.; Percin, M.; van Oudheusden, B.; Palmer, J. Leading edge vortex evolution and lift production on rotating wings. In Proceedings of the 54th AIAA Aerospace Sciences Meeting, San Diego, CA, USA, 4–8 January 2016; p. 0288. [Google Scholar] [CrossRef]
- Rival, D.; Tropea, C. Characteristics of pitching and plunging airfoils under dynamic-stall conditions. J. Aircraft 2010, 47, 80–86. [Google Scholar] [CrossRef]
- Jantzen, R.T.; Taira, K.; Granlund, K.O.; Ol, M.V. Vortex dynamics around pitching plates. Phys. Fluids 2014, 26, 053606. [Google Scholar] [CrossRef]
- Manar, F.; Medina, A.; Jones, A.R. Tip vortex structure and aerodynamic loading on rotating wings in confined spaces. Exp. Fluids 2014, 55, 1815. [Google Scholar] [CrossRef]
- Lua, K.B.; Zhang, X.; Lim, T.; Yeo, K. Effects of pitching phase angle and amplitude on a two-dimensional flapping wing in hovering mode. Exp. Fluids 2015, 56, 35. [Google Scholar] [CrossRef]
- Ellington, C.P.; Berg, C.v.d.; Willmott, A.P.; Thomas, A.L.R. Leading-edge vortices in insect flight. Nature 1996, 384, 626–630. [Google Scholar] [CrossRef]
- Lehmann, F.O.; Sane, S.P.; Dickinson, M.H. The aerodynamic effects of wing-wing interaction in flapping insect wings. J. Exp. Biol. 2005, 208, 3075–3092. [Google Scholar] [CrossRef]
- Bomphrey, R.J.; Nakata, T.; Phillips, N.; Walker, S.M. Smart wing rotation and trailing-edge vortices enable high frequency mosquito flight. Nature 2017, 544, 92–95. [Google Scholar] [CrossRef]
- Cheng, B.; Sane, S.P.; Barbera, G.; Troolin, D.R.; Strand, T.; Deng, X. Three-dimensional flow visualization and vorticity dynamics in revolving wings. Exp. Fluids 2013, 54, 1423. [Google Scholar] [CrossRef]
- Garmann, D.; Visbal, M. Dynamics of revolving wings for various aspect ratios. J. Fluid Mech. 2014, 748, 932–956. [Google Scholar] [CrossRef]
- Percin, M.; Van Oudheusden, B. Three-dimensional flow structures and unsteady forces on pitching and surging revolving flat plates. Exp. Fluids 2015, 56, 47. [Google Scholar] [CrossRef][Green Version]
- Wolfinger, M.; Rockwell, D. Transformation of flow structure on a rotating wing due to variation of radius of gyration. Exp. Fluids 2015, 56, 137. [Google Scholar] [CrossRef]
- Carr, Z.R.; DeVoria, A.C.; Ringuette, M.J. Aspect-ratio effects on rotating wings: Circulation and forces. J. Fluid Mech. 2015, 767, 497–525. [Google Scholar] [CrossRef]
- Krishna, S.; Green, M.A.; Mulleners, K. Effect of pitch on the flow behavior around a hovering wing. Exp. Fluids 2019, 60, 86. [Google Scholar] [CrossRef]
- Ellington, C.P. The novel aerodynamics of insect flight: Applications to micro-air vehicles. J. Exp. Biol. 1999, 202, 3439–3448. [Google Scholar]
- Pick, S.; Lehmann, F.-O. Stereoscopic PIV on multiple color-coded light sheets and its application to axial flow in flapping robotic insect wings. Exp. Fluids 2009, 47, 1009–1023. [Google Scholar] [CrossRef]
- Birch, J.M.; Dickinson, M.H. Spanwise flow and the attachment of the leading-edge vortex on insect wings. Nature 2001, 412, 729–733. [Google Scholar] [CrossRef]
- Ellington, C.P. The aerodynamics of hovering insect flight. V. A vortex theory. Phil. Trans. R. Soc. Lond. B 1984, 305, 115–144. [Google Scholar]
- Muijres, F.T.; Spedding, G.R.; Winter, Y.; Hedenström, A. Actuator disk model and span efficiency of flapping flight in bats based on time-resolved PIV measurements. Exp. Fluids 2011, 51, 511–525. [Google Scholar] [CrossRef]
- Milne-Thomson, L.M. Theoretical Aerodynamics, 4th ed.; Macmillan: New York, NY, USA, 1966; pp. 210–211. [Google Scholar]
- Spedding, G.; McArthur, J. Span efficiencies of wings at low Reynolds numbers. J. Aircraft 2010, 47, 120–128. [Google Scholar] [CrossRef]
- Henningsson, P.; Bomphrey, R.J. Span efficiency in hawkmoths. J. R. Soc. Interface 2013, 10, 20130099. [Google Scholar] [CrossRef]
- Prandtl, L. Tragflügeltheorie. I. Mitteilung. Nachricht. Gesell. Wissensch. Göttingen 1918, 1918, 451–477. [Google Scholar]
- Betz, A. Schraubenpropeller mit geringstem Energieverlust. Nachricht. Gesell. Wissensch. Göttingen 1919, 1919, 193–217. [Google Scholar]
- Goldstein, S. On the vortex theory of screw propellers. Proc. Roy. Soc. Lond. A 1929, 123, 440–465. [Google Scholar]
- Larrabee, E.E.; French, S.E. Minimum induced loss windmills and propellers. J. Wind Eng. Industr. Aero. 1983, 15, 317–327. [Google Scholar] [CrossRef]
- Nabawy, M.R.; Crowther, W.J. Is Flapping Flight Aerodynamically Efficient? In Proceedings of the 32nd AIAA Applied Aerodynamics Conference, Atlanta, GA, USA, 16–20 June 2014; p. 2277. [Google Scholar]
- Nabawy, M.R.; Crowther, W.J. Aero-optimum hovering kinematics. Bioinsp. Biomim. 2015, 10, 044002. [Google Scholar] [CrossRef]
- Nabawy, M.R.; Crowther, W.J. Optimum hovering wing planform. J. Theor. Biol. 2016, 406, 187–191. [Google Scholar] [CrossRef]
- Reid, H.E.; Schwab, R.K.; Maxcer, M.; Peterson, R.K.D.; Johnson, R.L.; Jankauski, M. Wing flexibility reduces the energetic requirements of insect flight. Bioinsp. Biomim. 2019, 14, 056007. [Google Scholar] [CrossRef]
- Bhat, S.; Zhao, J.; Sheridan, J.; Hourigan, K.; Thompson, M. Aspect ratio studies on insect wings. Phys. Fluids 2019, 31, 121301. [Google Scholar] [CrossRef]
- Levy, D.-E.; Seifert, A. Simplified dragonfly airfoil aerodynamics at Reynolds numbers below 8000. Phys. Fluids 2009, 21, 071901. [Google Scholar] [CrossRef]
- Weis-Fogh, T. Quick estimates of flight fitness in hovering animals, including novel mechanisms for lift production. J. Exp. Biol. 1973, 59, 169–230. [Google Scholar]
- Ellington, C.P. The aerodynamics of hovering insect flight. II. Morphological parameters. Phil. Trans. R. Soc. Lond. B 1984, 305, 17–40. [Google Scholar]
- Ennos, A.R. The effect of size on the optimal shapes of gliding insects and seeds. J. Zoo. Lond. 1989, 219, 61–69. [Google Scholar] [CrossRef]
- Zanker, J.M. The wing beat of Drosophila melanogaster I. Kinematics. Phil. Trans. R. Soc. Lond. B 1990, 327, 1–18. [Google Scholar]
- Han, J.-S.; Chang, J.W.; Cho, H.-K. Vortices behavior depending on the aspect ratio of an insect-like flapping wing in hover. Exp. Fluids 2015, 56, 181. [Google Scholar] [CrossRef]
- Suzuki, K.; Yoshino, M. A trapezoidal wing equivalent to a Janatella leucodesma’s wing in terms of aerodynamic performance in the flapping flight of a butterfly model. Bioinsp. Biomim. 2019, 14, 036003. [Google Scholar] [CrossRef]
- Engels, T.; Kolomenskiy, D.; Schneider, K.; Sesterhenn, J. FluSI: A novel parallel simulation tool for flapping insect flight using a Fourier method with volume penalization. SIAM J. Sci. Comp. 2016, 38, S3–S24. [Google Scholar] [CrossRef]
- Sroka, M.; Engels, T.; Krah, P.; Mutzel, S.; Schneider, K.; Reiss, J. An open and parallel multiresolution framework using block-based adaptive grids. In Active Flow and Combustion Control 2018; Springer: Berlin/Heidelberg, Germany, 2019; pp. 305–319. [Google Scholar]
- Usherwood, J.R.; Lehmann, F.-O. Phasing of dragonfly wings can improve aerodynamic efficiency by removing swirl. J. R. Soc. Interface 2008, 5, 1303–1307. [Google Scholar] [CrossRef]
- Ellington, C.P. The aerodynamics of hovering insect flight. VI. Lift and power requirements. Phil. Trans. R. Soc. Lond. B 1984, 305, 145–181. [Google Scholar]
- Phillips, N.; Knowles, K.; Lawson, N. Effect of wing planform shape on the flow structures of an insect-like flapping wing in hover. In Proceedings of the 27th International Congress of the Aeronautical Sciences ICAS, Nice, France, 19–24 September 2010. [Google Scholar]
- Lu, Y.; Shen, G.X.; Lai, G.J. Dual leading-edge vortices on flapping wings. J. Exp. Biol. 2006, 209, 5005–5016. [Google Scholar] [CrossRef] [PubMed]
- Rival, D.E.; Kriegseis, J.; Schaub, P.; Widmann, A.; Tropea, C. Characteristic length scales for vortex detachment on plunging profiles with varying leading-edge geometry. Exp. Fluids 2014, 55, 1660. [Google Scholar] [CrossRef]
- Wootton, R.J. Leading edge section and asymmetric twisting in the wings of flying butterflies (insecta, papilionoidea). J. Exp. Biol. 1993, 180, 105–117. [Google Scholar]
- Newman, D.J.S.; Wooton, R.J. An approach to the mechanics of pleating in dragonfly wings. J. Exp. Biol. 1986, 125, 361–372. [Google Scholar]
- Lian, Y.; Broering, T.; Hord, K.; Prater, R. The characterization of tandem and corrugated wings. Progr. Aero. Sci. 2014, 65, 41–69. [Google Scholar] [CrossRef]
- Kesel, A.B. Aerodynamic characteristics of dragonfly wing sections compared with technical aerofoils. J. Exp. Biol. 2000, 203, 3125–3135. [Google Scholar]
- Kim, W.-K.; Ko, J.H.; Park, H.C.; Byun, D. Effects of corrugation of the dragonfly wing on gliding performance. J. Theor. Biol. 2009, 260, 523–530. [Google Scholar] [CrossRef]
- Huda, N.; Anwer, S.F. The Effects of Leading Edge Orientation on the Aerodynamic Performance of Dragon Fly Wing Section in Gliding Flight; Springer: New Delhi, India, 2016; pp. 1433–1441. [Google Scholar]
- Hord, K.; Liang, Y. Numerical investigation of the aerodynamic and structural characteristics of a corrugated airfoil. J. Aircraft 2012, 49, 749–757. [Google Scholar] [CrossRef]
- Xiang, J.; Du, J.; Li, D.; Liu, K. Aerodynamic performance of the locust wing in gliding mode at low Reynolds number. J. Bionic. Eng. 2016, 13, 249–260. [Google Scholar] [CrossRef]
- Shahzad, A.; Hamdani, H.R.; Aizaz, A. Investigation of corrugated wing in unsteady motion. J. Appl. Fluid Dyn. 2017, 10, 833–845. [Google Scholar] [CrossRef]
- Meng, X.; Sun, M. Aerodynamic effects of corrugation in flapping insect wings in forward flight. J. Bionic. Eng. 2011, 8, 140–150. [Google Scholar] [CrossRef]
- Du, G.; Sun, M. Aerodynamic effects of corrugation and deformation in flapping wings of hovering hoverflies. J. Theor. Biol. 2012, 300, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Flint, T.J.; Jermy, M.C.; New, T.H.; Ho, W.H. Computational study of a pitching bio-inspired corrugated airfoil. Int. J. Heat Fluid Flow 2017, 65, 328–341. [Google Scholar] [CrossRef]
- Vargas, A.; Mittal, R.; Dong, H. A computational study of the aerodynamic performance of a dragonfly wing section in gliding flight. Bioinsp. Biomim. 2008, 3, 026004. [Google Scholar] [CrossRef]
- Du, G.; Sun, M. Effects of wing deformation on aerodynamic forces in hovering hoverflies. J. Exp. Biol. 2010, 213, 2273–2283. [Google Scholar] [CrossRef]
- Haas, F.; Gorb, S.N.; Wootton, R.J. Elastic joints in dermapteran hind wings: Materials and wing folding. Arthropod. Struct. Dev. 2000, 29, 137–146. [Google Scholar] [CrossRef]
- Hou, D.; Zhong, Z.; Yin, Y.; Pan, Y.; Zhao, H. The role of soft vein joints in dragonfly flight. J. Bionic. Eng. 2017, 14, 738–745. [Google Scholar] [CrossRef]
- Rajabi, H.; Ghoroubi, N.; Darvizeh, A.; Dirks, J.-H.; Appel, E.; Gorb, S.N. A comparative study of the effects of vein-joints on the mechanical behaviour of insect wings: I. Single joints. Bioinsp. Biomim. 2015, 10, 056003. [Google Scholar] [CrossRef]
- Andersen, S.O.; Weis-Fogh, T. Resilin, a rubber-like protein in arthropod cuticle. Adv. Insect Physiol. 1964, 2, 1–65. [Google Scholar]
- Appel, E.; Gorb, S.N. Resilin-bearing wing vein joints in the dragonfly Epiophlebia superstes. Bioinsp. Biomim. 2011, 6. [Google Scholar] [CrossRef]
- Gorb, S.N. Serial elastic elements in the damselfly wing: Mobile vein joints contain resilin. Naturwissenschaften 1999, 86, 552–555. [Google Scholar] [CrossRef]
- Kovalev, A.; Filippov, A.; Gorb, S.N. Slow viscoelastic response of resilin. J. Comp. Physiol. A 2018, 204, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Hu, X.; Cebe, P.; Kaplan, D.L. Mechanism of resilin elasticity. Nat. Comm. 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.C.; Pearson, R.D.; Elvin, C.M.; Merritt, D.J. Expression of the rubber-like protein, resilin, in developing and functional insect cuticle determined using a Drosophila anti-rec 1 resilin antibody. Dev. Dyn. 2012, 241, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Weis-Fogh, T. A rubber-like protein in insect cuticles. J. Exp. Biol. 1960, 37, 889–907. [Google Scholar]
- Jensen, M.; Weis-Fogh, T. Biology and physics of locust flight. V. Strength and elasticity of locust cuticle. Phil. Trans. Roy. Soc. London B 1962, 245, 137–169. [Google Scholar]
- Biewener, A.A.; Roberts, T.J. Muscle and tendon contributions to force, work, and elastic energy savings: A comparative perspective. Exerc. Sport Sci. Rev. 2000, 28, 99–107. [Google Scholar]
- Lichtwark, G.A.; Barclay, C.J. The influence of tendon compliance on muscle power output and efficiency during cyclic contractions. J. Exp. Biol. 2010, 213, 707–714. [Google Scholar] [CrossRef]
- Roberts, T.J.; Marsh, R.L.; Weyand, P.G.; Taylor, C.R. Muscular force in running turkeys: The economy of minimizing work. Science 1997, 275, 1113–1115. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Q.; Deng, X.; Sane, S.P. Aerodynamic effects of flexibility in flapping wings. J. R. Soc. Interface 2010, 7, 485–497. [Google Scholar] [CrossRef]
- Lehmann, F.-O.; Gorb, S.; Nasir, N.; Schützner, P. Elastic deformation and energy loss of flapping fly wings. J. Exp. Biol. 2011, l214, 2949–2961. [Google Scholar] [CrossRef] [PubMed]
- Wootton, R.J. Invertebrate paraxial locomotory appendages: Design, deformation and control. J. Exp. Biol. 1999, 202, 3333–3345. [Google Scholar] [PubMed]
- Young, J.; Walker, S.M.; Bomphrey, R.J.; Taylor, G.K.; Thomas, A.L.R. Details of insect wing design and deformation enhance aerodynamic function and flight efficiency. Science 2009, 325, 1549–1552. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hedrick, T.L.; Mittal, R. Time-varying wing-twist improves aerodynamic efficiency of forward flight in butterflies. PLoS ONE 2013, 8, e53060. [Google Scholar] [CrossRef]
- Mistick, E.A.; Mountcastle, A.M.; Combes, S.A. Wing flexibility improves bumblebee flight stability. J. Exp. Biol. 2016, 219, 3384–3390. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Sundar, D.S.; Yeo, K.S.; Lim, T.T. Modeling and analysis of insect-like flexible wings at low Reynolds number. J. Fluid Struct. 2016, 62, 294–317. [Google Scholar] [CrossRef]




| Kinematics | Property | Rectangular Wing | Ideal Wing | Fly Wing |
|---|---|---|---|---|
| Revolving 1 | Vertical force (μN) | 471 | 215 | 431 |
| Revolving 1 | Paero (μW) | 1696 | 724 | 1434 |
| Revolving 1 | Efficiency | 0.22 | 0.16 | 0.23 |
| Flapping 2 | Vertical force (μN) | 479 | n.a. | 458 |
| Flapping 2 | Paero (μW) | 2340 | n.a. | 2361 |
| Flapping 2 | Efficiency | 0.27 | n.a. | 0.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishna, S.; Cho, M.; Wehmann, H.-N.; Engels, T.; Lehmann, F.-O. Wing Design in Flies: Properties and Aerodynamic Function. Insects 2020, 11, 466. https://doi.org/10.3390/insects11080466
Krishna S, Cho M, Wehmann H-N, Engels T, Lehmann F-O. Wing Design in Flies: Properties and Aerodynamic Function. Insects. 2020; 11(8):466. https://doi.org/10.3390/insects11080466
Chicago/Turabian StyleKrishna, Swathi, Moonsung Cho, Henja-Niniane Wehmann, Thomas Engels, and Fritz-Olaf Lehmann. 2020. "Wing Design in Flies: Properties and Aerodynamic Function" Insects 11, no. 8: 466. https://doi.org/10.3390/insects11080466
APA StyleKrishna, S., Cho, M., Wehmann, H.-N., Engels, T., & Lehmann, F.-O. (2020). Wing Design in Flies: Properties and Aerodynamic Function. Insects, 11(8), 466. https://doi.org/10.3390/insects11080466





