EPNs Exhibit Repulsion to Prenol in Pluronic Gel Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
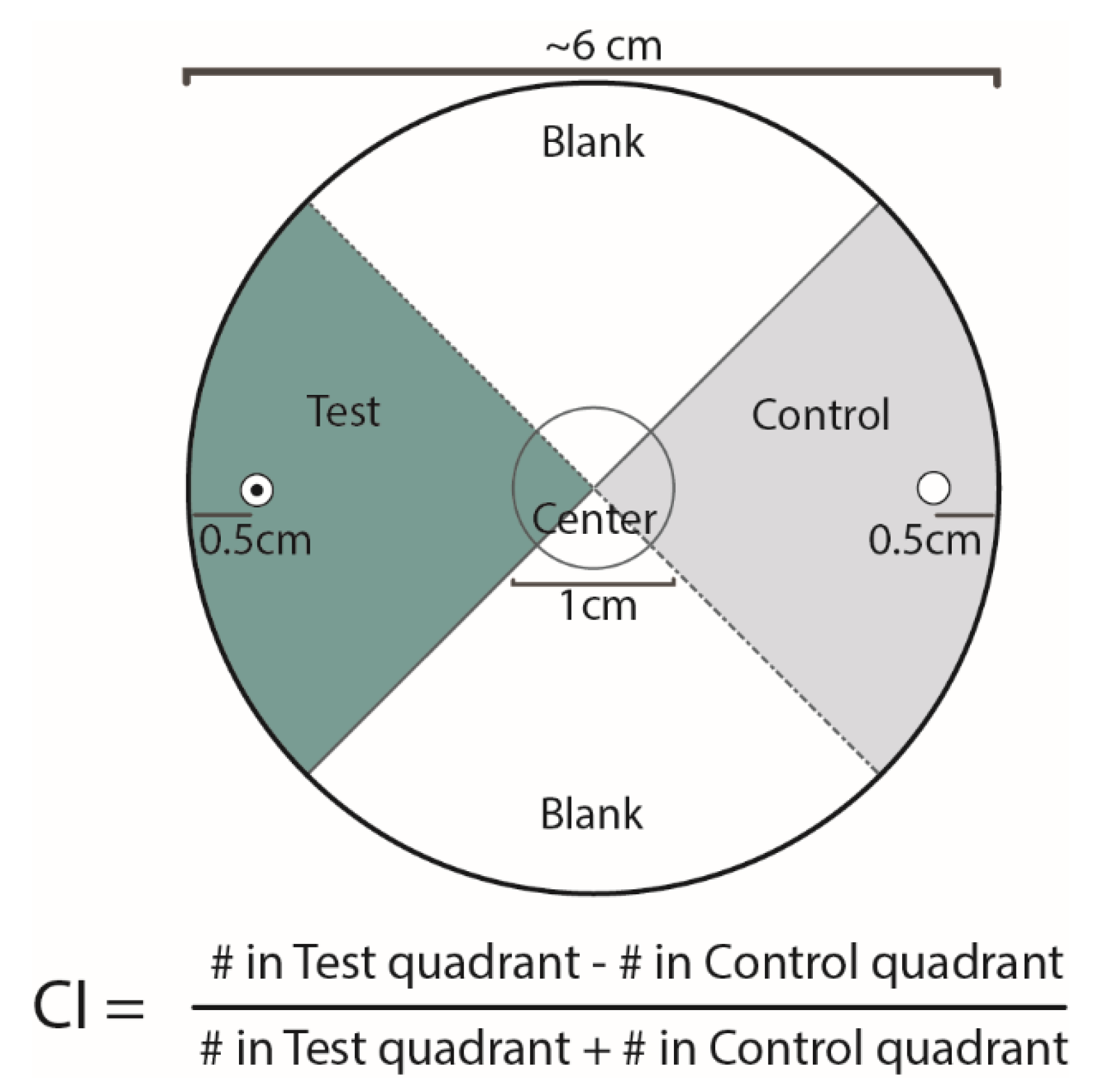
2.2. Pluronic Gel Behavioral Assay
2.3. Experimental Replicates and Normalization
2.4. Statistical Analysis
3. Results
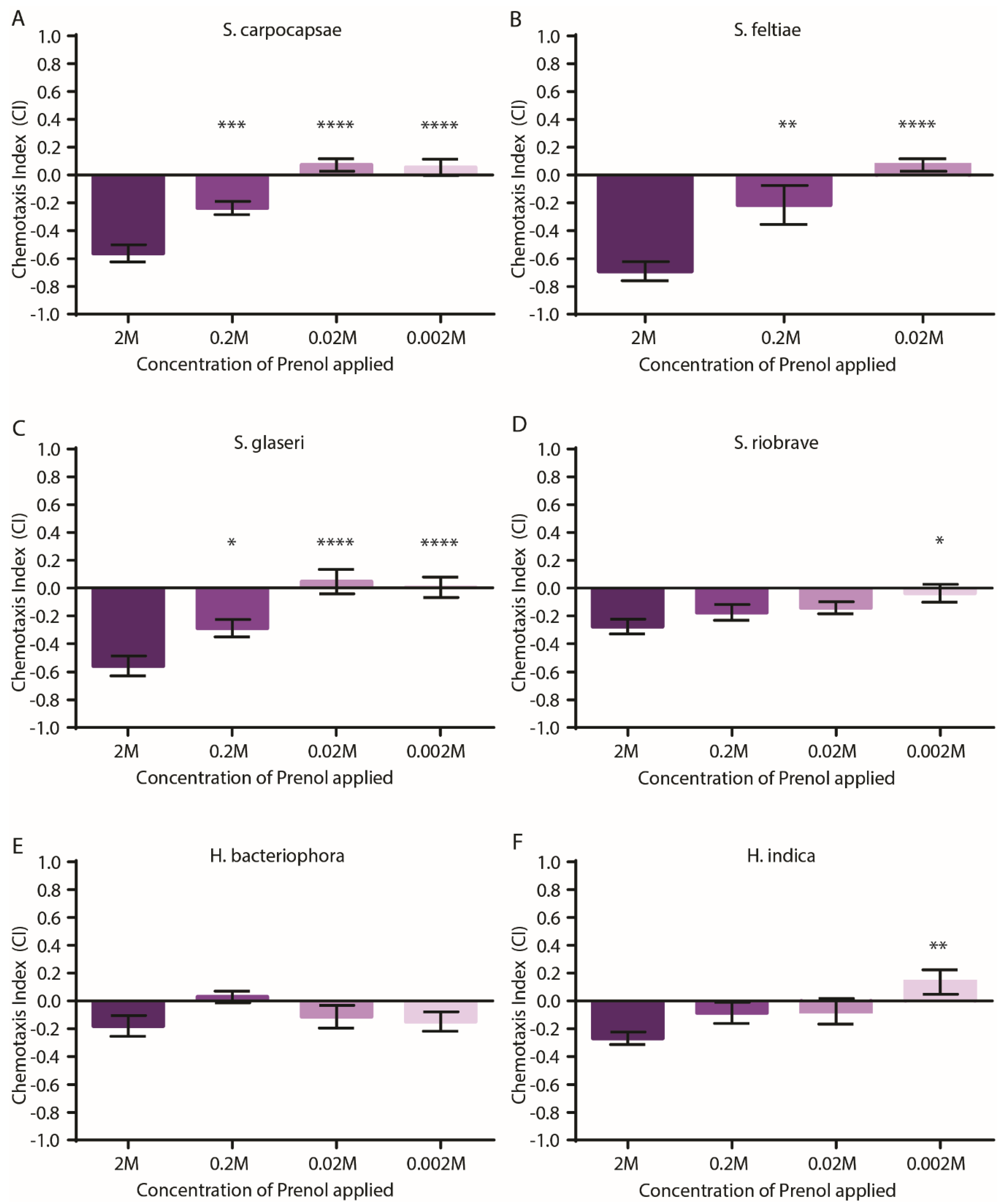
3.1. EPNs Are Repelled by Prenol in Pluronic Gel Assays
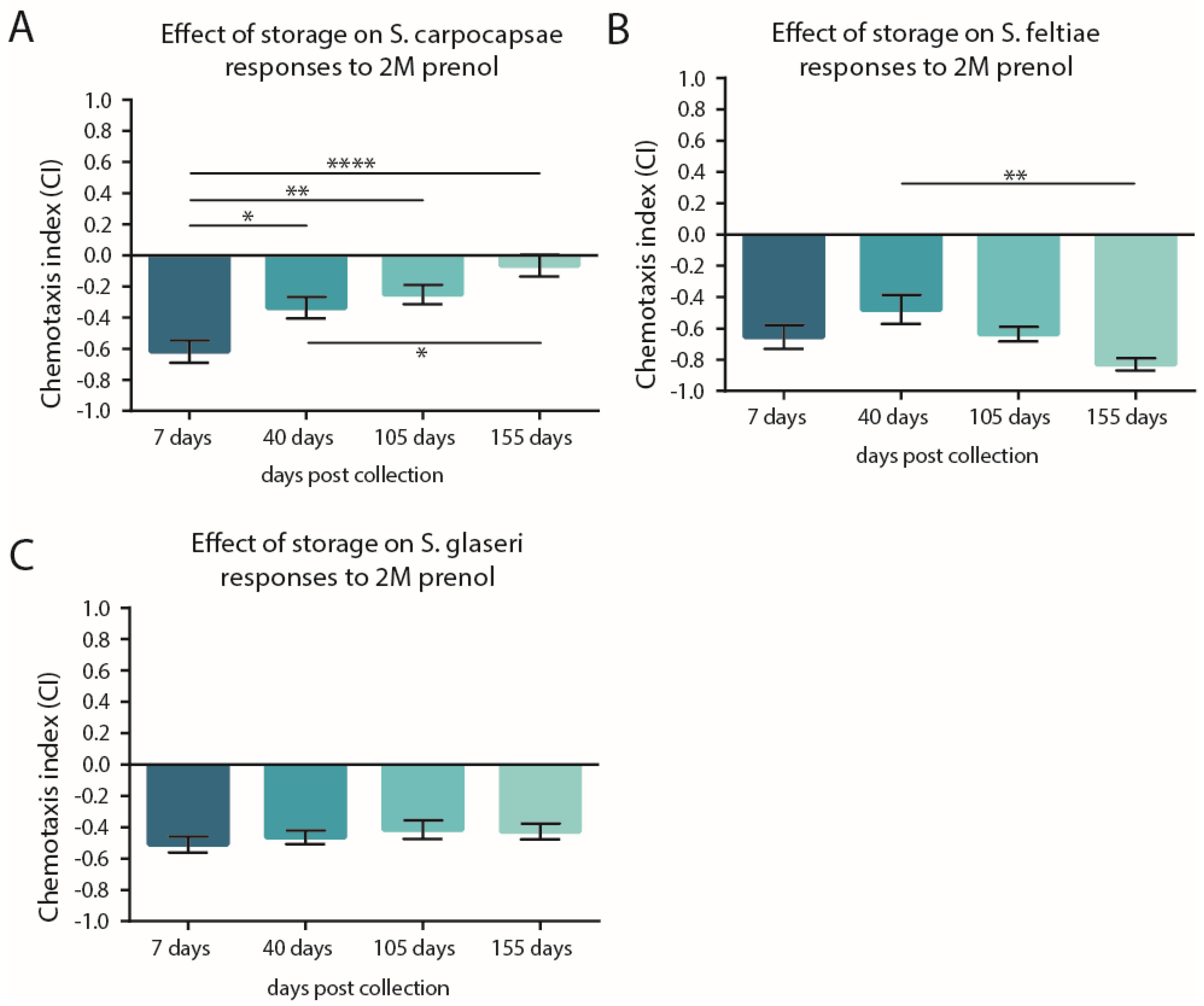
3.2. Storage Time May Not Shift the CI but Can Impact Participation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Georgis, R.; Koppenhöfer, A.; Lacey, L.; Bélair, G.; Duncan, L.; Grewal, P.; Samish, M.; Tan, L.; Torr, P.; Van Tol, R. Successes and failures in the use of parasitic nematodes for pest control. Biol. Control 2006, 38, 103–123. [Google Scholar] [CrossRef]
- Grewal, P.; Georgis, R. Entomopathogenic nematodes. In Biopesticides: Use and Delivery; Springer: Berlin/Heidelberg, Germany, 1999; pp. 271–299. [Google Scholar]
- Lee, J.H.; Dillman, A.R.; Hallem, E.A. Temperature-dependent changes in the host-seeking behaviors of parasitic nematodes. BMC Biol. 2016, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.K.; Gaugler, R. Entomopathogenic nematodes. Annu. Rev. Entomol. 1993, 38, 181–206. [Google Scholar] [CrossRef]
- Baiocchi, T.; Lee, G.; Choe, D.H.; Dillman, A.R. Host seeking parasitic nematodes use specific odors to assess host resources. Sci. Rep. 2017, 7, 6270. [Google Scholar] [CrossRef] [PubMed]
- Dillman, A.R.; Guillermin, M.L.; Lee, J.H.; Kim, B.; Sternberg, P.W.; Hallem, E.A. Olfaction shapes host-parasite interactions in parasitic nematodes. Proc. Natl. Acad. Sci. USA 2012, 109, E2324–E2333. [Google Scholar] [CrossRef] [PubMed]
- Hallem, E.A.; Dillman, A.R.; Hong, A.V.; Zhang, Y.; Yano, J.M.; DeMarco, S.F.; Sternberg, P.W. A sensory code for host seeking in parasitic nematodes. Curr. Biol. 2011, 21, 377–383. [Google Scholar] [CrossRef]
- Spence, K.O.; Lewis, E.E.; Perry, R.N. Host-finding and invasion by entomopathogenic and plant-parasitic nematodes: Evaluating the ability of laboratory bioassays to predict field results. J. Nematol. 2008, 40, 93–98. [Google Scholar]
- Chatterjee, S.; Hui, P.C.; Kan, C.W.; Wang, W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 2019, 9, 11658. [Google Scholar] [CrossRef]
- Castelletto, M.L.; Gang, S.S.; Okubo, R.P.; Tselikova, A.A.; Nolan, T.J.; Platzer, E.G.; Lok, J.B.; Hallem, E.A. Diverse host-seeking behaviors of skin-penetrating nematodes. PLoS Pathog. 2014, 10, e1004305. [Google Scholar] [CrossRef]
- Rasmann, S.; Kollner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef]
- Swierczek, N.A.; Giles, A.C.; Rankin, C.H.; Kerr, R.A. High-throughput behavioral analysis in C. elegans. Nat. Methods 2011, 8, 592. [Google Scholar] [CrossRef] [PubMed]
- Čepulytė, R.; Danquah, W.B.; Bruening, G.; Williamson, V.M. Potent attractant for root-knot nematodes in exudates from seedling root tips of two host species. Sci. Rep. 2018, 8, 10847. [Google Scholar] [CrossRef] [PubMed]
- :Hu, Y.; You, J.; Li, C.; Williamson, V.M.; Wang, C. Ethylene response pathway modulates attractiveness of plant roots to soybean cyst nematode Heterodera glycines. Sci. Rep. 2017, 7, 41282. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Hu, Y.; Hua, C.; Wang, C. Three dimensional study of wounded plant roots recruiting entomopathogenic nematodes with pluronic gel as a medium. Biol. Control 2015, 89, 68–74. [Google Scholar] [CrossRef]
- Li, C.; Zhou, X.; Lewis, E.E.; Yu, Y.; Wang, C. Study on host-seeking behavior and chemotaxis of entomopathogenic nematodes using Pluronic F-127 gel. J. Invertebr. Pathol. 2019, 161, 54–60. [Google Scholar] [CrossRef]
- Baiocchi, T.; Braun, L.; Dillman, A.R. Touch-stimulation increases host-seeking behavior in Steinernema Carpocapsae. J. Nematol. 2019, 51, 1–5. [Google Scholar] [CrossRef]
- Kin, K.; Baiocchi, T.; Dillman, A.R. Dispersal and repulsion of entomopathogenic nematodes to prenol. Biology 2019, 8, 58. [Google Scholar] [CrossRef]
- Kaya, H.K.; Stock, S.P. Techniques in insect nematology. In Manual of Techniques in Insect Pathology; Lacey, L., Ed.; Academic Press Limited: San Diego, CA, USA, 1997. [Google Scholar]
- Williamson, V.M.; Cepulyte, R. Assessing attraction of nematodes to host roots using pluronic Gel Medium. Methods Mol. Biol. 2017, 1573, 261–268. [Google Scholar] [CrossRef]
- Lewis, E.E.; Gaugler, R.; Harrison, R. Response of cruiser and ambusher entomopathogenic nematodes (Steinernematidae) to host volatile cues. Can. J. Zool. 1993, 71, 765–769. [Google Scholar] [CrossRef]
- Bal, H.K.; Taylor, R.A.; Grewal, P.S. Ambush foraging entomopathogenic nematodes employ ‘sprinters’ for long-distance dispersal in the absence of hosts. J. Parasitol. 2014, 100, 422–432. [Google Scholar] [CrossRef]
- Sharmila, R.; Subramanian, S. Effect of Storage Temperature on Survival and Infectivity of Entomopathogenic Nematodes. Madras Agric. J. 2016, 103, 146–149. [Google Scholar]
- Yadav, A.K. Effects of storage temperature on survival and infectivity of three indigenous entomopathogenic nematodes strains (Steinernematidae and Heterorhabditidae) from Meghalaya, India. J. Parasit. Dis. 2016, 40, 1150–1154. [Google Scholar]
- Gaugler, R.; Campbell, J.F.; McGuire, T.R. Selection for host-finding in Steinernema feltiae. J. Invertebr. Pathol. 1989, 54, 363–372. [Google Scholar] [CrossRef]
- Grewal, P.; Lewis, E.; Gaugler, R.; Campbell, J. Host finding behaviour as a predictor of foraging strategy in entomopathogenic nematodes. Parasitology 1994, 108, 207–215. [Google Scholar] [CrossRef]
- Kunkel, B.A.; Shapiro-Ilan, D.I.; Campbell, J.F.; Lewis, E.E. Effect of Steinernema glaseri-infected host exudates on movement of conspecific infective juveniles. J. Invertebr. Pathol. 2006, 93, 42–49. [Google Scholar] [CrossRef] [PubMed]
- El-Borai, F.E.; Campos-Herrera, R.; Stuart, R.J.; Duncan, L.W. Substrate modulation, group effects and the behavioral responses of entomopathogenic nematodes to nematophagous fungi. J. Invertebr. Pathol. 2011, 106, 347–356. [Google Scholar] [CrossRef]
- Wang, C.; Bruening, G.; Williamson, V.M. Determination of preferred pH for root-knot nematode aggregation using pluronic F-127 gel. J. Chem. Ecol. 2009, 35, 1242–1251. [Google Scholar] [CrossRef]
- Wang, C.; Lower, S.; Williamson, V.M. Application of pluronic gel to the study of root-knot nematode behaviour. Nematology 2009, 11, 453–464. [Google Scholar] [CrossRef]
- Campbell, J.F.; Guagler, R.R. Inter-specifie variation in entomopathogenic nematode foraging strategy: Dichotomy or variation along a continuum? Fundam. Appl. Nematol. 1997, 20, 393–398. [Google Scholar]
- Bal, H.K.; Michel, A.P.; Grewal, P.S. Genetic selection of the ambush foraging entomopathogenic nematode, Steinernema carpocapsae for enhanced dispersal and its associated trade-offs. Evol. Ecol. 2014, 28, 923–939. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baiocchi, T.; Li, C.; Dillman, A.R. EPNs Exhibit Repulsion to Prenol in Pluronic Gel Assays. Insects 2020, 11, 457. https://doi.org/10.3390/insects11080457
Baiocchi T, Li C, Dillman AR. EPNs Exhibit Repulsion to Prenol in Pluronic Gel Assays. Insects. 2020; 11(8):457. https://doi.org/10.3390/insects11080457
Chicago/Turabian StyleBaiocchi, Tiffany, Chunjie Li, and Adler R. Dillman. 2020. "EPNs Exhibit Repulsion to Prenol in Pluronic Gel Assays" Insects 11, no. 8: 457. https://doi.org/10.3390/insects11080457
APA StyleBaiocchi, T., Li, C., & Dillman, A. R. (2020). EPNs Exhibit Repulsion to Prenol in Pluronic Gel Assays. Insects, 11(8), 457. https://doi.org/10.3390/insects11080457





