Ecological Drivers and Sex-Based Variation in Body Size and Shape in the Queensland Fruit Fly, Bactrocera tryoni (Diptera: Tephritidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Sampling
2.2. Climate Data
2.3. Data Analysis
2.3.1. Trait Variation in Males Versus Females
2.3.2. Investigate the Effect of Environmental Variables on Trait Variation
3. Results
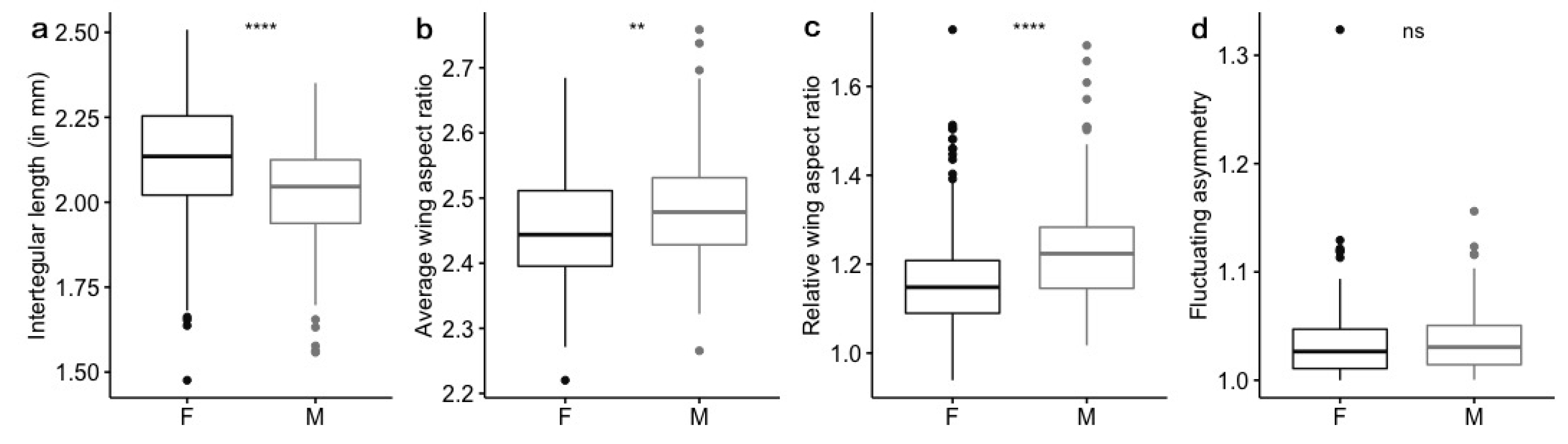
3.1. Body Size Variation
3.2. Wing Shape Variation
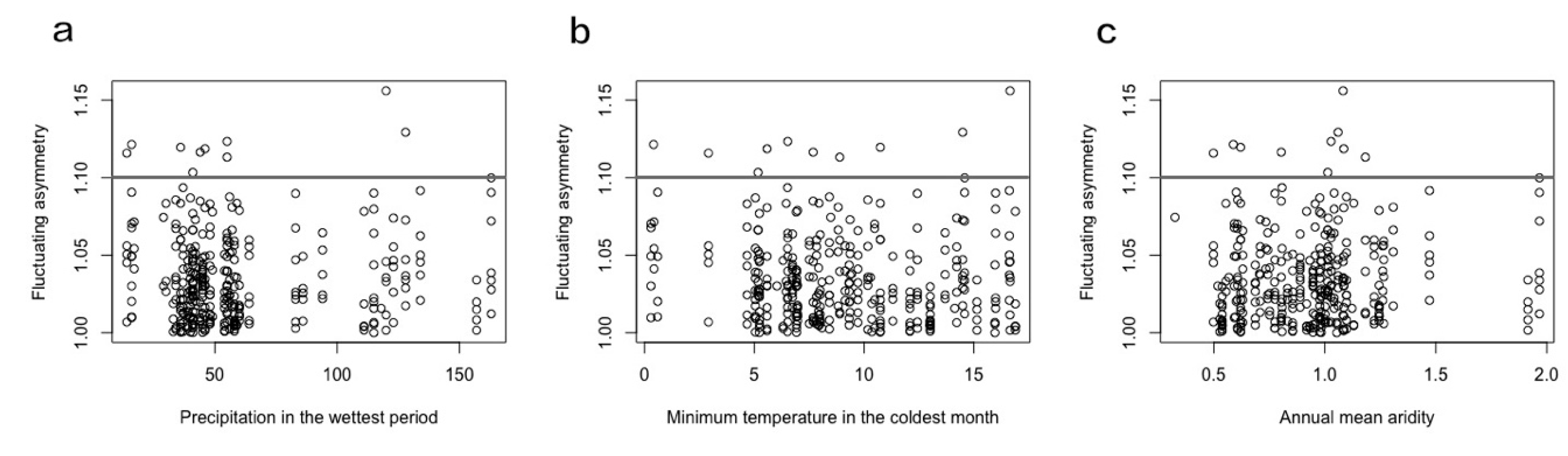
3.3. Fluctuating Asymmetry
4. Discussion
4.1. Body Size Variation
4.2. Wing Shape Variation
4.3. Fluctuating Asymmetry Variation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blacket, M.J.; Malipatil, M.B.; Semeraro, L.; Gillespie, P.S.; Dominiak, B.C. Screening mitochondrial DNA sequence variation as an alternative method for tracking established and outbreak populations of Queensland fruit fly at the species southern range limit. Ecol. Evol. 2017, 7, 2604–2616. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Baumgartner, J.B.; Dominiak, B.C.; Royer, J.E.; Beaumont, L.J. Potential impacts of climate change on habitat suitability for the Queensland fruit fly. Sci. Rep. 2017, 7, 13025. [Google Scholar] [CrossRef] [PubMed]
- Sutherst, R.W.; Collyer, B.S.; Yonow, T. The vulnerability of Australian horticulture to the Queensland fruit fly, Bactrocera (Dacus) tryoni, under climate change. Aust. J. Agric. Res. 2000, 51, 467. [Google Scholar] [CrossRef]
- Dominiak, B.C.; Mavi, H.S.; Nicol, H.I. Effect of town microclimate on the Queensland fruit fly Bactrocera tryoni. Aust. J. Exp. Agric. 2006, 46, 1239. [Google Scholar] [CrossRef]
- Dominiak, B.C.; Daniels, D. Review of the past and present distribution of Mediterranean fruit fly (Ceratitis capitata Wiedemann) and Queensland fruit fly (Bactrocera tryoni Froggatt) in Australia: Fruit fly distribution in Australia. Aust. J. Entomol. 2012, 51, 104–115. [Google Scholar] [CrossRef]
- Popa-Baez, A.D.; Lee, S.F.; Yeap, H.L.; Prasad, S.S.; Schiffer, M.; Mourant, R.; Castro-Vergas, C.; Edwards, O.R.; Taylor, P.W.; Oakeshott, J.G. Genome-wide patterns of differentiation over space and time in the Queensland fruit fly. Sci. Rep. 2020, in press. [Google Scholar]
- Clarke, A.R.; Powell, K.S.; Weldon, C.W.; Taylor, P.W. The ecology of Bactrocera tryoni (Diptera: Tephritidae): What do we know to assist pest management? Ann. Appl. Biol. 2011, 158, 26–54. [Google Scholar] [CrossRef]
- Yonow, T.; Sutherst, R.W. The geographical distribution of the Queensland fruit fly, Bactrocera (Dacus) tryoni, in relation to climate. Aust. J. Agric. Res. 1998, 49, 20. [Google Scholar]
- O’Loughlin, G.T.; East, R.A.; Meats, A. Survival, Development Rates and Generation Times of the Queensland Fruit Fly, Dacus tuyoni, in a Marginally Favourable Climate: Experiments in Victoria. Aust. J. Zool. 1984, 32, 353–361. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Woods, R.E.; Collins, E.; Wallin, K.; White, A.; McKenzie, J.A. Wing shape versus asymmetry as an indicator of changing environmental conditions in insects. Aust. J. Entomol. 2005, 44, 233–243. [Google Scholar] [CrossRef]
- Markow, T.A. Evolutionary Ecology and Developmental Instability. Ann. Rev. Entomol. 1995, 40, 105–120. [Google Scholar] [CrossRef]
- Bateman, M.A. The Ecology of Fruit Flies. Ann. Rev. Entomol. 1972, 17, 493–518. [Google Scholar] [CrossRef]
- Bergmann, C. Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Größe; Göttinger Studien: Göttingen, German, 1848; Volume 3, pp. 595–701. (in German) [Google Scholar]
- Bidau, C.J.; Martí, D.A. Geographic and Climatic Factors Related to a Body-Size Cline in Dichroplus pratensis Bruner, 1900 (Acrididae, Melanoplinae). J. Orthoptera Res. 2008, 17, 149–156. [Google Scholar] [CrossRef]
- Kennington, W.J.; Killeen, J.R.; Goldstein, D.B.; Partridge, L. Rapid laboratory evolution of adult wing area in drosophila melanogaster in response to humidity. Evolution 2003, 57, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Morales Vargas, R.E.; Ya-umphan, P.; Phumala-Morales, N.; Komalamisra, N.; Dujardin, J.-P. Climate associated size and shape changes in Aedes aegypti (Diptera: Culicidae) populations from Thailand. Infect. Genet. Evol. 2010, 10, 580–585. [Google Scholar] [CrossRef]
- Przybylska, M.S.; Roque, F.; Tidon, R. Drosophilid Species (Diptera) in the Brazilian Savanna Are Larger in the Dry Season. Ann. Entomol. Soc. Am. 2014, 107, 994–999. [Google Scholar] [CrossRef]
- Allen, J.A. The influence of physical conditions in the genesis of species. Radic. Rev. 1877, 1, 108–140. [Google Scholar]
- Chown, S.L.; Klok, C.J. Altitudinal body size clines: Latitudinal effects associated with changing seasonality. Ecography 2003, 26, 445–455. [Google Scholar] [CrossRef]
- Davidowitz, G.; D’Amico, L.J.; Nijhout, H.F. The effects of environmental variation on a mechanism that controls insect body size. Evol. Ecol. 2004, 6, 49–62. [Google Scholar]
- Breuker, C.J.; Brakefield, P.M.; Gibbs, M. The association between wing morphology and dispersal is sex-specific in the glanville fritillary butterfly Melitaea cinxia (Lepidoptera: Nymphalidae). Eur. J. Entomol. 2007, 104, 445–452. [Google Scholar] [CrossRef]
- Delettre, Y.R. Chironomid wing length, dispersal ability and habitat predictability. Ecography 1988, 11, 166–170. [Google Scholar] [CrossRef]
- Haas, H.L.; Tolley, K.A. Geographic variation of wing morphology in three Eurasian populations of the fruit fly, Drosophila lummei. J. Zool. 1998, 245, 197–203. [Google Scholar] [CrossRef]
- Kennedy, J.D.; Borregaard, M.K.; Jønsson, K.A.; Marki, P.Z.; Fjeldså, J.; Rahbek, C. The influence of wing morphology upon the dispersal, geographical distributions and diversification of the Corvides (Aves; Passeriformes). Proc. R. Soc. B 2016, 283, 20161922. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.; Knowles, K.; Bomphrey, R.J. The effect of aspect ratio on the leading-edge vortex over an insect-like flapping wing. Bioinspir. Biomim. 2015, 10, 056020. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.B.R.; James, A.C.; McCabe, J.; Partridge, L. Latitudinal variation of wing: Thorax size ratio and wing aspect ratio in Drosophila Melanogaster. Evolution 1998, 52, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Collins, E.; Woods, R. Wing Shape and Wing Size Changes as Indicators of Environmental Stress in Helicoverpa punctigera (Lepidoptera: Noctuidae) Moths: Comparing Shifts in Means, Variances, and Asymmetries. Environ. Entomol. 2002, 31, 965–971. [Google Scholar] [CrossRef]
- Mönkkönen, M. Do migrant birds have more pointed wings? A comparative study. Evol. Ecol. 1995, 9, 520–528. [Google Scholar] [CrossRef]
- Johansson, F.; Söderquist, M.; Bokma, F. Insect wing shape evolution: Independent effects of migratory and mate guarding flight on dragonfly wings: Dragonfly wing shape evolution. Biol. J. Linn. Soc. 2009, 97, 362–372. [Google Scholar] [CrossRef]
- Hill, J.K.; Thomas, C.D.; Blackeley, D.S. Evolution of flight morphology in a butterfly that has recently expanded its geographic range. Oecologia 1999, 121, 165–170. [Google Scholar] [CrossRef]
- McLachlan, A.J. Sexual Dimorphism in Midges: Strategies for Flight in the Rain-Pool Dweller Chironomus imicola (Diptera: Chironomidae). J. Anim. Ecol. 1986, 55, 261–267. [Google Scholar] [CrossRef]
- Hassall, C. Strong geographical variation in wing aspect ratio of a damselfly, Calopteryx maculata (Odonata: Zygoptera). Peer J. 2015, 3, e1219. [Google Scholar] [CrossRef] [PubMed]
- Valen, L.V. A Study of Fluctuating Asymmetry. Evolution 1962, 16, 125–142. [Google Scholar] [CrossRef]
- Parsons, P.A. Fluctuating asymmetry: A biological monitor of environmental and genomic stress. Heredity 1992, 68, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.L.; Pruett-Jones, S. Fluctuating Asymmetry, Sexual Selection, and Survivorship in Male Dark-Winged Damselflies. Ethology 2002, 108, 779–791. [Google Scholar] [CrossRef]
- Beasley, D.A.E.; Bonisoli-Alquati, A.; Mousseau, T.A. The use of fluctuating asymmetry as a measure of environmentally induced developmental instability: A meta-analysis. Ecol. Indic. 2013, 30, 218–226. [Google Scholar] [CrossRef]
- Bonduriansky, R. The Evolution of Condition-Dependent Sexual Dimorphism. Am. Nat. 2007, 169, 9–19. [Google Scholar] [CrossRef]
- Blanckenhorn, W.U.; Dixon, A.F.G.; Fairbairn, D.J.; Foellmer, M.W.; Gibert, P.; van der Linde, K.; Meier, R.; Nylin, S.; Pitnick, S.; Schoff, C.; et al. Proximate Causes of Rensch’s Rule: Does Sexual Size Dimorphism in Arthropods Result from Sex Differences in Development Time? Am. Nat. 2007, 169, 245–257. [Google Scholar] [CrossRef]
- Sivinski, J.M.; Dodson, G. Sexual dimorphism inAnastrepha suspensa (Loew) and other tephritid fruit flies (Diptera: Tephritidae): Possible roles of developmental rate, fecundity, and dispersal. J. Insect Behav. 1992, 5, 491–506. [Google Scholar] [CrossRef]
- Testa, N.D.; Ghosh, S.M.; Shingleton, A.W. Sex-Specific Weight Loss Mediates Sexual Size Dimorphism in Drosophila melanogaster. PLoS ONE 2013, 8, e58936. [Google Scholar] [CrossRef]
- Honěk, A. Intraspecific Variation in Body Size and Fecundity in Insects: A General Relationship. Oikos 1993, 66, 483–492. [Google Scholar] [CrossRef]
- Cane, J.H. Estimation of Bee Size Using Intertegular Span (Apoidea). J. Kans. Entomol. Soc. 1987, 60, 145–147. [Google Scholar]
- Rust, R.W. Size-Weight Relationships in Osmia lignaria propinqua Cresson (Hymenoptera: Megachilidae). J. Kans. Entomol. Soc. 1991, 64, 174–178. [Google Scholar]
- Shingleton, A.W.; Frankino, W.A.; Flatt, T.; Nijhout, H.F.; Emlen, D.J. Size and shape: The developmental regulation of static allometry in insects. Bioessays 2007, 29, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Yonow, T.; Zalucki, M.P.; Sutherst, R.W.; Dominiak, B.C.; Maywald, G.F.; Maelzer, D.A.; Kriticos, D.J. Modelling the population dynamics of the Queensland fruit fly, Bactrocera (Dacus) tryoni: A cohort-based approach incorporating the effects of weather. Ecol. Model. 2004, 173, 9–30. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Kassambara, A. ggpubr: “ggplot2” Based Publication Ready Plots; Springer-Verlag: New York, NY, USA, 2019. [Google Scholar]
- Auguie, B. gridExtra: Miscellaneous Functions for “Grid” Graphics, R package version; 2017. Available online: https://rdrr.io/cran/gridExtra/ (accessed on 23 September 2019).
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation, R package version; 2019. Available online: https://dplyr.tidyverse.org/reference/dplyr-package.html (accessed on 23 September 2019).
- Scheiner, S.M.; Gurevitch, J. Design and Analysis of Ecological Experiments; Oxford University Press: Oxford, UK, 2001; ISBN 978-0-19-803022-5. [Google Scholar]
- Zhang, Z. Variable selection with stepwise and best subset approaches. Ann. Transl. Med. 2016, 4, 136. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S., 4th ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Nelder, J.A.; Wedderburn, R.W.M. Generalized Linear Models. J. R. Stat. Soc. Ser. A (Gen.) 1972, 135, 370. [Google Scholar] [CrossRef]
- Sivinski, J.M.; Calkins, C.O. Sexually Dimorphic Developmental Rates in the Caribbean Fruit Fly (Diptera: Tephritidae). Environ. Entomol. 1990, 19, 1491–1495. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Gaston, K.J.; Loder, N. Geographic gradients in body size: A clarification of Bergmann’s rule. Divers. Distrib. 1999, 5, 165–174. [Google Scholar] [CrossRef]
- Lloyd-Jones, L.R.; Wang, Y.-G.; Courtney, A.J.; Prosser, A.J.; Montgomery, S.S. Latitudinal and seasonal effects on growth of the Australian eastern king prawn (Melicertus plebejus). Can. J. Fish. Aquat. Sci. 2012, 69, 1525–1538. [Google Scholar] [CrossRef]
- Mayr, E. Geographical character gradients and climatic adaptation. Evolution 1956, 10, 105–108. [Google Scholar] [CrossRef]
- Bujan, J.; Yanoviak, S.P.; Kaspari, M. Desiccation resistance in tropical insects: Causes and mechanisms underlying variability in a Panama ant community. Ecol. Evol. 2016, 6, 6282–6291. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Li, G.; Li, H.; Wang, Q.; Wan, L. The Effect of Dietary Fat Levels on the Size and Development of Chrysomya megacephala (Diptera: Calliphoridae). J. Insect Sci. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Dmitriew, C.; Rowe, L. The Effects of Larval Nutrition on Reproductive Performance in a Food-Limited Adult Environment. PLoS ONE 2011, 6, e17399. [Google Scholar] [CrossRef] [PubMed]
- Dalby-Ball, G.; Meats, A. Influence of the odour of fruit, yeast and cue-lure on the flight activity of the Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). Aust. J. Entomol. 2000, 39, 195–200. [Google Scholar] [CrossRef]
- Pritchard, G. The ecology of a natural population of Queensland fruit fly, Dacus tryoni II. The distribution of eggs and its relation to behaviour. Aust. J. Zool. 1969, 17, 293. [Google Scholar] [CrossRef]
- Senger, S.E.; Roitberg, B.D.; Thistlewood, H.M.A. Relative flight responses of Rhagoletis indifferens as influenced by crowding, sex, and resources. Entomol. Exp. Appl. 2007, 123, 91–100. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Romig, M.C.; Drew, R.A.I. Facilitation in Ovipositional Behavior of Bactrocera tryoni Flies. J. Insect Behav. 1999, 12, 815–832. [Google Scholar] [CrossRef]
- De Oliveira Christe, R.; Wilke, A.B.B.; Vidal, P.O.; Marrelli, M.T. Wing sexual dimorphism in Aedes fluviatilis (Diptera: Culicidae). Infect. Genet. Evol. 2016, 45, 434–436. [Google Scholar] [CrossRef]
- Clarke, G.M. Fluctuating asymmetry of invertebrate populations as a biological indicator of environmental quality. Environ. Pollut. 1993, 82, 207–211. [Google Scholar] [CrossRef]
- Hulthen, A.D.; Clarke, A.R. The influence of soil type and moisture on pupal survival of Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). Aust. J. Entomol. 2006, 45, 16–19. [Google Scholar] [CrossRef]
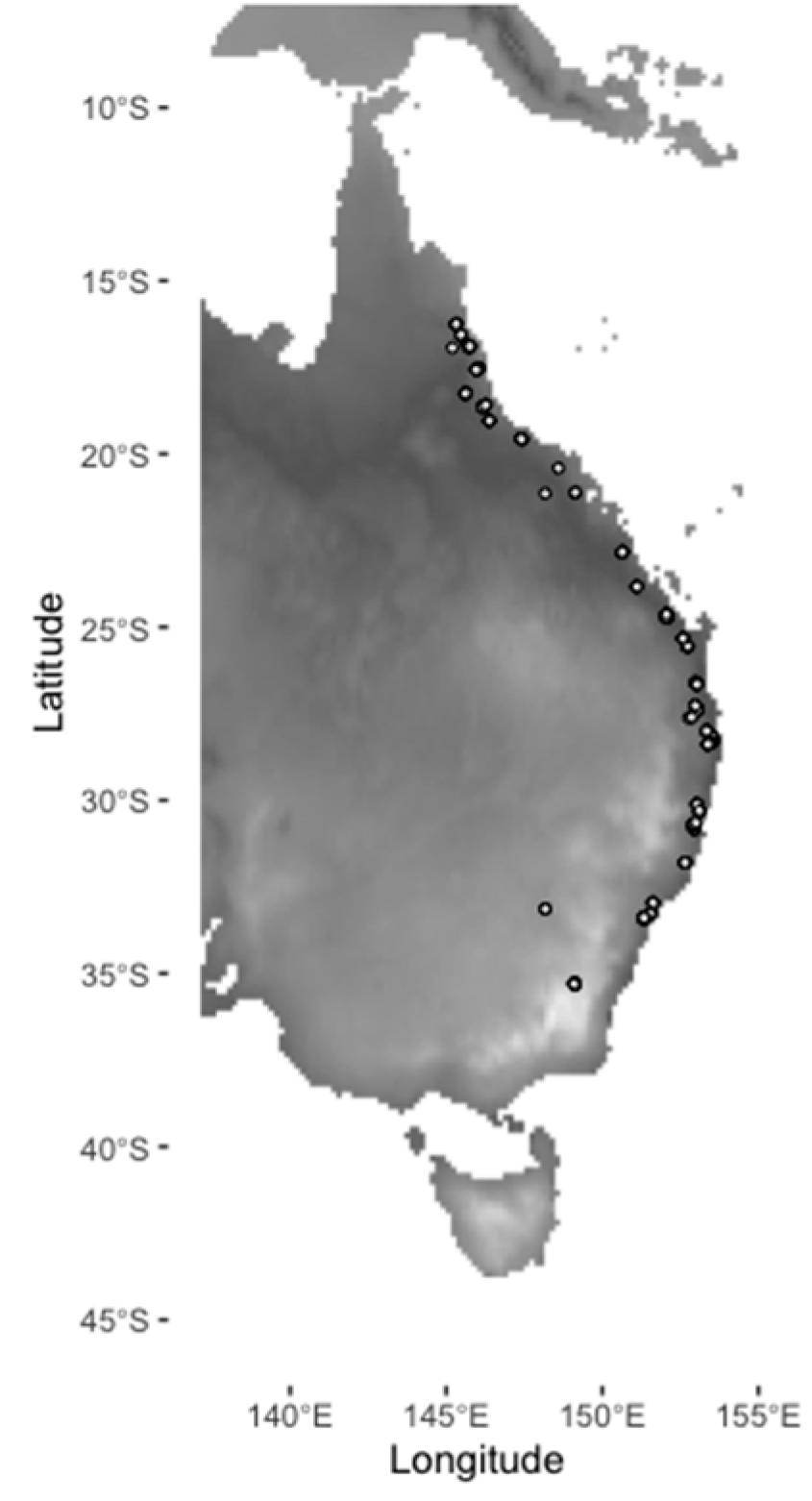

| Variable | ALA Layer Name [Layer ID] |
|---|---|
| Annual mean temperature | Temperature—annual mean (Bio01) (874) |
| Minimum temperature in the coldest month | Temperature—coldest month min (730) |
| Maximum temperature in the hottest month | Temperature—hottest month max (729) |
| Annual mean aridity | Aridity index—annual mean (715) |
| Precipitation seasonality | Precipitation—annual seasonality (772) |
| Precipitation in the driest period | Precipitation—driest period (Bio14) (872) |
| Precipitation in the wettest period | Precipitation—wettest period (Bio13) (866) |
| Annual mean relative humidity | Humidity—annual mean relative (728) |
| Body Size | ||||
| Explanatory Variable | β (Regression Coefficient) | Standard Error | 95% Confidence Interval | p |
| Sex | −0.090 | 0.015 | −0.12, −0.06 | <0.0001 |
| Annual mean temperature | −0.022 | 0.011 | −0.04, 0.001 | 0.0595 |
| Minimum temperature of the coldest month | 0.017 | 0.008 | 0.0005, 0.03 | <0.0001 |
| Precipitation seasonality | −0.002 | 0.001 | −0.003, −0.0002 | 0.0267 |
| Latitude | 0.006 | 0.001 | 0.003, 0.008 | <0.0001 |
| Wing Shape (AR) | ||||
| Explanatory Variable | β (Regression Coefficient) | Standard Error | 95% Confidence Interval | p |
| Sex | 0.029 | 0.009 | 0.01,0.05 | 0.0016 |
| Annual mean temperature | −0.013 | 0.003 | −0.02, −0.007 | <0.0001 |
| Maximum temperature in the hottest month | 0.025 | 0.005 | 0.02, 0.03 | <0.0001 |
| Wing Shape (Relative AR) | ||||
| Explanatory Variable | β (Regression Coefficient) | Standard Error | 95% Confidence Interval | p |
| Sex | 0.063 | 0.009 | 0.04, 0.08 | <0.0001 |
| Minimum temperature in the coldest month | −0.007 | 0.003 | −0.01, −0.002 | 0.0084 |
| Maximum temperature in the hottest month | 0.014 | 0.005 | 0.006, 0.02 | 0.0015 |
| Precipitation seasonality | 0.001 | 0.0004 | 0.0002, 0.002 | 0.0120 |
| Fluctuating Asymmetry | ||||
| Explanatory Variable | β (Regression Coefficient) | Standard Error | 95% Confidence Interval | p |
| Minimum temperature in the coldest month | −0.003 | 0.001 | −0.005, −3.95 × 10−4 | 0.0207 |
| Annual mean aridity | −0.023 | 0.012 | −0.046, −3.48 × 10−4 | 0.0475 |
| Precipitation seasonality | −0.0004 | 0.0002 | −0.0007, 8.63 × 10−6 | 0.0560 |
| Precipitation in the wettest period | 0.0006 | 0.0002 | 0.0003, 9.61 × 10−4 | 0.0001 |
| Annual mean humidity | 0.002 | 0.001 | −0.004, 4.63 × 10−4 | 0.1265 |
| Fluctuating Asymmetry (FA < M + 3 SD Subset) | ||||
| Explanatory Variable | β (Regression Coefficient) | Standard Error | 95% Confidence Interval | p |
| Minimum temperature in the coldest month | −0.002 | 0.001 | −0.003, 4.47 × 10−5 | 0.0573 |
| Annual mean aridity | −0.017 | 0.010 | −0.037, 2.27 × 10−3 | 0.0838 |
| Precipitation seasonality | −0.0003 | 0.0006 | −0.0007, 6.37 × 10−5 | 0.1101 |
| Precipitation in the wettest period | 0.0004 | 0.0002 | 0.0003, 7.04 × 10−4 | 0.0022 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Rodriguez, J.; Fisher, N.; Catullo, R.A. Ecological Drivers and Sex-Based Variation in Body Size and Shape in the Queensland Fruit Fly, Bactrocera tryoni (Diptera: Tephritidae). Insects 2020, 11, 390. https://doi.org/10.3390/insects11060390
Zhou Y, Rodriguez J, Fisher N, Catullo RA. Ecological Drivers and Sex-Based Variation in Body Size and Shape in the Queensland Fruit Fly, Bactrocera tryoni (Diptera: Tephritidae). Insects. 2020; 11(6):390. https://doi.org/10.3390/insects11060390
Chicago/Turabian StyleZhou, Yufei, Juanita Rodriguez, Nicole Fisher, and Renee A. Catullo. 2020. "Ecological Drivers and Sex-Based Variation in Body Size and Shape in the Queensland Fruit Fly, Bactrocera tryoni (Diptera: Tephritidae)" Insects 11, no. 6: 390. https://doi.org/10.3390/insects11060390
APA StyleZhou, Y., Rodriguez, J., Fisher, N., & Catullo, R. A. (2020). Ecological Drivers and Sex-Based Variation in Body Size and Shape in the Queensland Fruit Fly, Bactrocera tryoni (Diptera: Tephritidae). Insects, 11(6), 390. https://doi.org/10.3390/insects11060390





